Abstract
HER2/neu overexpression is a driving force in the carcinogenesis of several human cancers. In breast cancer the prognostic influence of HER2/neu was shown to be at least partly based on increased metastatic potential mediated by the chemokine–chemokine receptor pair SDF-1(CXCL12)/CXCR4. We wanted to evaluate the influence of HER2/neu on ovarian cancer prognosis and to investigate whether compromised survival would correlate with CXCR4 expression and/or SDF-1 abundance. Therefore, we analysed HER2/neu, CXCR4, and SDF-1 in 148 ovarian tumour samples by means of immunohistochemistry on tissue microarrays. Overexpression of HER2/neu was found in 27.6% of ovarian cancer tissues and in 15% of ovarian borderline tumours. In ovarian cancer patients, overexpression of HER2/neu correlated closely with overall survival (univariate hazard ratio (HR) 2.59, P=0.005; multiple corrected HR 1.92, P=0.074). In contrast, CXCR4 expression and SDF-1 abundance had no impact on overall survival, and both parameters were not correlated with HER2/neu expression. As expected, cytoplasmic CXCR4 expression and SDF-1 abundance correlated closely (P<0.0001). Our results confirm a univariate influence of HER2/neu expression on overall survival, which was completely independent of the expression of CXCR4 and the abundance of SDF-1, implying significant differences between the HER2/neu downstream pathways in ovarian cancer compared with breast cancer.
Similar content being viewed by others
Main
Epithelial ovarian cancer is the most lethal gynaecologic malignancy and with about 6% the fourth most frequent cause of cancer-related death of women in Western countries. Estimates indicate that one in 70 women will develop ovarian cancer in her lifetime, with a median survival rate of 4.5 years. A recent cancer statistic reported an estimated 22 200 new cases and 16 210 deaths per year in the United States (Jemal et al, 2005). Early diagnosis is a major challenge, as more than three-quarter of cases are diagnosed in late stages. Ovarian cancer metastasises preferentially to the local lymph nodes and the peritoneum, and in contrast to breast cancer, only rarely in other organs like liver, lung, and bones.
The majority of hereditary ovarian cancer cases are caused by germline mutations in BRCA1 and BRCA2, the so-called breast/ovarian cancer syndrome. Mutations in these caretaker genes set the scene for further genomic and epigenomic aberrations, which ultimately transform healthy cells into cells with perturbed cell cycle control and metastatic potential. The starting point of sporadic cases may differ but the sequence of events is not well described to date, for ovarian cancer in particular.
Ovarian cancer and breast cancer share the overexpression of HER2/neu, a member of the HER family of receptor tyrosine kinases triggering signalling pathways which control cell growth, differentiation, motility, and adhesion. In breast cancer, the prognostic value of HER2/neu expression is well established and the therapeutic modulation of this oncogene by antibodies or small molecules is a classic example of targeted therapy. The situation in ovarian cancer is less clear (recently reviewed in Serrano-Olvera et al, 2006), with contradicting results of HER2/neu expression in the prognosis of the disease and very little available data on its potential for therapeutic manipulations, prompting us to investigate HER2/neu expression and its potential consequences in ovarian cancer.
For breast cancer, it was shown that HER2/neu mediated tumour metastasis, and survival prognosis is essentially driven by upregulation of the chemokine receptor CXCR4, a membrane-bound G-protein-coupled receptor (Li et al, 2004). The chemokine was first described in its function as a key regulator of the homing process of lymphocytes to inflammatory tissues (Bleul et al, 1997). In previous reports dealing with CXCR4 expression in neoplastic diseases nuclear, cytoplasmic, and membrane staining was found by means of immunohistochemistry. Recently, for breast cancer, it was shown that only cytoplasmic staining of CXCR4 had significant impact on prognosis, but not nuclear staining – using the same antibody as we used for our study (Salvucci et al, 2005). Three previous studies identified nuclear localisation of CXCR4 in hepatocellular carcinoma (Shibuta et al, 2002), invasive ductal mammary carcinoma (Kato et al, 2003), and non-small-cell lung cancer (NSCLC) (Spano et al, 2004). Strong CXCR4 nuclear staining was associated with significantly better outcome in early-stage NSCLC (Spano et al, 2004). The natural ligand of CXCR4, the stromal cell-derived factor (SDF-1 or CXCL12), is highly expressed in lung, liver, and lymph nodes (Phillips et al, 2003), the preferred organs for metastasis of several tumours.
To contribute to the controversial discussion about the influence of HER2/neu overexpression on ovarian cancer prognosis and whether the chemokine receptor system SDF-1/CXCR4 is significantly involved in this process, we examined the expression of these three potential oncoproteins by means of immunohistochemistry on tissue microarrays comprising 148 ovarian cancer patients.
Materials and methods
Ovarian tissue microarray and immunohistochemistry
For immunohistochemical studies, paraffin material available from primary diagnosis was used. Patients gave informed consent according to the criteria of the Medical University of Vienna. Relevant clinical information was collected and tissue samples and clinical data anonymised. A tissue microarray was composed by taking core needle ‘biopsies’ from specific locations in the preexisting paraffin-embedded tissue blocks and re-embedding them in an arrayed master block, using techniques and an apparatus developed by Beecher Instruments Inc., Micro-Array Technology (Sun Prairie, WI, USA). To achieve good representation of the tumour, three biopsies of tumour material were selected from each patient. Using this technology, each tissue sample was treated in an identical manner and the entire cohort was analysed in one batch on three slides. Reagent conditions, incubation times and temperatures, wash conditions, and antigen retrieval (if necessary) were held identical for each case. A 4–5 μm paraffin section of the tissue microarray was deparaffinised (xylene) and rehydrated (incubation in serial dilutions of ethanol), and, subsequently, the sections were treated with 0.2% H2O2/PBS (pH 7.4) to quench endogenous peroxidases. After blocking with 2% normal serum (from the animal in which the secondary antibody was raised) for 30 min, the sections were incubated at 4°C overnight with primary antibodies (CXCR4, mouse monoclonal anti-human CXCR4 (MAB172) (R&D Systems, Minneapolis, MN, USA); SDF-1, mouse monoclonal anti-human/mouse SDF-1/CXCL12 antibody (MAB350) (R&D Systems); HER2/neu (DAKO, Glostrup, Denmark); and HercepTest (DAKO, Glostrup, Denmark). As secondary antibody for CXCR4 and SDF-1, an anti-mouse Ig, horseradish peroxidase-linked whole antibody from sheep (NA 931, Amersham, Buckinghamshire, UK) was utilised. Staining was performed using a staining kit from DAKO: DAKO Cytomation Liquid DAB+substrate (Glostrup, Denmark). DAKO HercepTest was carried out and stained as described by the manufacturer. Positive and negative control slides (as appropriate) were stained within the same batch with the tissue microarrays and examined before evaluation of the tissue microarrays.
Data analyses and statistics
Staining of the tissue microarrays was interpreted by two independent pathologists. Classification of all three tissue microarrays was performed at once to ensure reliability and reproducibility. Staining for CXCR4 was classified in ‘1’ (missing or very low cytoplasmic expression), ‘2’ (medium cytoplasmic expression), and ‘3’ (high cytoplasmic expression), and in addition ‘−’ (no) for negative nuclear staining or ‘+’ (yes) for positive nuclear staining. Staining of SDF-1 was classified in ‘1’ (missing or very low membrane staining), ‘2’ (medium membrane staining), and ‘3’ (high membrane staining). HercepTest was classified according to the standard procedures and translated to our classification system as follows: HercepTest score ‘0’ and ‘1+’ as ‘1’ (negative for Her2/neu expression), score ‘2+’ as ‘2’ (weak positive), and score ‘3+’ as ‘3’ (strong positive). Results of both independent interpretations (already the average of the three biopsies per patient on the microarrays) were averaged and newly classified (1.00–1.66=‘1’, 1.67–2.33=‘2’, and 2.34–3.00=‘3’). Data were analysed statistically with SPSS 13 (SPSS, Chicago, IL, USA). P-values below 0.05 were considered statistically significant. P-values above 0.1 were signed simply as NS (not significant). P-values in between were signed NS, with the corresponding P-value in parentheses. Correlations among clinicopathologic parameters were calculated using the Pearson's χ2 or Fisher's exact test as appropriate and were corrected for multiple testing (Bonferroni–Holmes). Correlation of staining intensities of the putative oncoproteins among each other and with International Federation of Gynecology and Obstetrics (FIGO) stages was calculated using the Spearman's Correlation Test and corrected for multiple testing (Bonferroni–Holmes).
Univariate Cox models were used to demonstrate the influence of known prognostic factors and the four potential new prognostic factors. For each of the new factors, a multiple Cox model with known prognostic factors as adjustment variables was calculated.
Results
Description of patient cohort
Clinical and histopathological characteristics of patients included in this study show a typical ovarian cancer population and are presented in Table 1. Mean age of patients at first diagnosis was 58.6 years (range 27.6–87.2 years). 54.7 of patients with malignant tumours had serous adenocarcinomas and 62.5% had stage III/IV disease. Of the 128 patients with malignant tumours, 81 patients (67.5%) received carboplatin–paclitaxel-based standard chemotherapy, nine patients (7.5%) received cisplatin–cyclophosphamid-based chemotherapy, 14 patients (11.7%) another regimen, and 16 patients (13.3%) no systemic therapy at all. For eight patients, no information about systemic treatment was available.
Median follow-up for patients with malignant tumours was 43.7 months (range 0.4–168.7 months), and 39 patients (26.4%) had already died. None of the patients with borderline tumours died during the follow-up time of median 45.7 months (range 0.6–120.9 months).
HER2/neu overexpression in ovarian cancer samples
HER2/neu protein was stained with the DAKO HercepTest and interpreted following the standard procedures for breast cancer diagnosis. 35 out of 127 cancer tissues (27.6%) of patients with malignant tumours were found to overexpress the HER2/neu gene product including four tissues with high HER2/neu expression (3+) (Table 2). Only three out of 20 tissues (15.0%) of patients with borderline tumours showed overexpression of HER2/neu, none of them with high expression. Table 2 shows the prevalence of tumour staining scores with respect to histology, FIGO stage, and grade. There was no difference between HER2/neu staining regarding these clinical characteristics.
CXCR4 expression in ovarian cancer samples
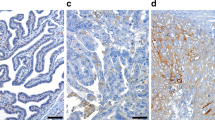
We examined expression of CXCR4 in the same tissues as described above. CXCR4 expression was found independently in the cytoplasm and/or in the nucleus (Salvucci et al, 2005). In positive cases, membrane staining of CXCR4 was not distinguishable from high cytoplasmic staining. This is in accordance with similar experiments with breast cancer tissues and the same primary antibody (Salvucci et al, 2005). Cytoplasmatic staining was classified as 1 (missing or weak expression), 2 (medium expression), and 3 (high expression). Nuclear expression was classified as ‘Yes’ (visible staining) and ‘No’ (no visible staining of the nucleus) (Figure 1, Table 2). The relatively low standard deviations within the three corresponding tumour cores from different positions of the tumour on the tissue microarray (the mean of the standard deviations over all patients equals 0.22) points to a low variability of cytoplasmic CXCR4 expression within one tumour. In 53.8% of malignant tumours, cytoplasmatic CXCR4 expression was medium or high (2/3) and 21.8% showed CXCR4 staining of the nucleus. There was no correlation of CXCR4 staining in the cytoplasm and the nucleus (Table 3). No significant different expression of nuclear and cytoplasmic staining regarding, histology, FIGO stage, or grade could be found; only the nuclear expression appeared indirectly correlated to grading (but not significant after correction for multiple testing), starting at 37.5% positive tumours for grade 1, 30.0% positive tumours for grade 2, and ending with 12.7% positive tumours for grade 3 (Table 2). Neither the cytoplasmatic nor the nuclear CXCR4 expression was correlated with the HER2/neu expression in ovarian cancer tissues (data not shown). CXCR4 stained high or medium in the cytoplasm of 50% borderline tumours and in 33.3% of borderline tumour nuclei, which was not different from the staining frequencies in malignant tumours (Table 2).
Immunohistochemistry staining of CXCR4 on different malignant ovarian cancer tissues. In the left-hand panel, representative tissues with low nuclear and low (1), medium (2), or high (3) cytoplasmic CXCR4 stainings are shown. In the right-hand panel, representative tissues with high nuclear and low (1), medium (2), or high (3) cytoplasmic CXCR4 stainings are shown.
SDF-1 abundance in ovarian cancer tissues and stroma
To get some insight into the functionality of CXCR4 receptors on the surface of ovarian cancer cells, we included an analysis of SDF-1 on the cell membrane – the only known soluble ligand of CXCR4.
For SDF-1, 32.0% of membranes of malignant tumour samples stained medium or high (2/3) (Figure 2). Positive staining did not correlate with any clinicopathologic characteristics like histology, FIGO stage, or grade (Table 2). Membranous SDF-1 staining correlated significantly with the cytoplasmatic CXCR4 expression, as expected (correlation r=0.373, P< 0.001; Table 3), but, interestingly, – indirectly, also as a trend with the nuclear CXCR4 expression (Spearman correlation rr=−0.244; P=0.084 after correction; Table 3). No correlation of SDF-1 abundance and HER2/neu expression was found (data not shown). Medium or high (2/3) SDF-1 protein levels were found in 31.5% of membranes of borderline tumours, which is the same frequency as for malignant tumour samples. As expected, SDF-1 was expressed at low level in the stroma of all ovarian cancer cases (data not shown).
Immunohistochemistry staining of SDF-1 (CXCL12) of different malignant ovarian cancer tissues. The representative stainings of tissues with low (virtually missing) (1), medium (2), and high (3) SDF-1 abundance are shown. Notice prevalent SDF-1 staining of endothelium in the right upper corner of the middle picture.
Overall survival analysis
For Kaplan–Meier plots with patients with malignant tumours, the parameters were dichotomised in two groups, one with low (1) HER2/neu or cytoplasmic CXCR4 expression or SDF-1 abundance and one with medium or high (2/3) expression/abundance of the corresponding protein (Figure 3). The only parameter with significant univariate influence on prognosis was HER2/neu (P=0.004; Figure 3A). The medium overall survival for patients with HER2/neu overexpression was 40.3 months compared with 168.7 months for the HER2/neu-negative patients. Type of systemic chemotherapy (carboplatin–paclitaxel based vs other) had no significant influence on the prognostic value of the HER2/neu overexpression status (data not shown).
Plots of Kaplan–Meier estimates for overall survival of patients with tumour tissues with low (1) or high (2/3) HER2/neu (A), low (1) or high (2/3) SDF-1 (B), low (1) or high (2/3) cytoplasmic CXCR4 (C), and low (1) or high (2/3) nuclear CXCR4 (D) expression/abundance. P-values are from the log-rank test.
All other parameters – cytoplasmic or nuclear CXCR4 expression and SDF-1 abundance – had no influence on overall survival. The 75% percentile of overall survival for all groups with low or high expression of each of these three parameters was very similar and ranged from 28.6 to 33.4 months (Figure 3B–D). There was no subgroup, for example, histological, FIGO stage, or grade, which resulted in a significant influence of these three parameters under investigation on patient prognosis (data not shown). There was also no combination of variables, for example, only CXCR4 and SFD-1-positive tumours compared with others, which resulted in a significant influence on patient prognosis (data not shown). Relative risk of patients with positive CXCR4 expressing tumours but negative staining for SDF-1 was not significantly higher compared with patients with negative CXCR4 expressing tumours in the same background (relative risk of 1.57, P=0.281).
A multiple analysis revealed FIGO stage and grade as the only independent prognostic factors for overall survival in our cohort of 128 malignant ovarian cancer patients. Of all parameters under investigation, only HER2/neu showed negative trend, also, for overall survival (relative risk of 1.92, P=0.074; Table 4) after multiple corrections also a negative trend also for overall survival (relative risk of 1.92, P=0.074; Table 4).
A multiple analyses of the impact of HER2/neu expression on overall survival, using both CXCR4 expressions (nuclear and cytoplasmic) and the SDF-1 abundance as correcting variables showed that there was no influence of one of these three parameters on the prognostic value of HER2/neu expression (data not shown). Survival analysis was performed only with patients of malignant tumours, because patients with borderline tumours had a significant better prognosis, with no cases of death during follow-up.
Discussion
Hereditary ovarian and breast cancers are based on germline mutations in the same cancer susceptibility genes, BRCA1 and BRCA2, suggesting similar pathways of oncogenesis at least for a fraction of these diseases. Moreover, both cancer types show, in a comparable percentage, overexpression of the oncoprotein HER2/neu.
In breast cancer, the diagnostic and therapeutic possibilities of HER2/neu expression are well explored. Well known since decades as an adverse prognostic factor, the more recent insight that breast cancers expressing HER2/neu are more susceptible to anthracycline-based chemotherapy (Pritchard et al, 2006) as well as the introduction of the HER2/neu antibody trastuzumab into the adjuvant setting has had significant impact on the prognosis of this particular subgroup of breast cancer patients (Slamon et al, 2001).
In ovarian cancer, the prognostic influence of HER2/neu is still a matter of debate and the therapeutic capacity of the available drugs to target the HER2/neu pathway are insufficiently explored. Even the percentage of HER2/neu-positive patients varies considerably among individual studies. The lack of knowledge about the prognostic and therapeutic impact of HER2/neu expression in ovarian cancer may be partly explained by its lower prevalence in the general population resulting in slower patient recruitment and underpowered studies. Besides this simplistic view, the possibility of a less significant and/or different influence of HER2/neu expression in breast and ovarian cancer could be another and more challenging explanation.
Even in breast cancer where the functionality of HER2/neu has been extensively studied, its role in oncogenesis is still far from being understood. Recently, interesting functional data were generated, showing that HER2/neu enhances expression of the chemokine receptor CXCR4. CXCR4 is furthermore crucial for HER2/neu induced invasion, migration, and adhesion activities, and in vitro HER2/neu protects CXCR4 from ligand-induced protein degradation. CXCR4 is furthermore responsible for HER2/neu-induced lung metastases in vivo. These in vivo findings are corroborated by the observation that CXCR4 is upregulated in HER2/neu overexpressing primary breast tumour tissues and is correlated with poor patient survival (Li et al, 2004). The findings that cytoplasmic CXCR4 expression is elevated in breast cancer samples, and that higher expression of CXCR4 is associated with parameters of tumour aggressiveness, and a poor prognosis was later confirmed in an independent patient population (Salvucci et al, 2005).
In accordance with the majority of publications, we found HER2/neu to be overexpressed in about a quarter of malignant tumours. HER2/neu-positive patients had a significantly shorter overall survival. There was a trend that HER2/neu-positive patients were diagnosed with a higher FIGO stage, resulting in the fact that in a multivariate model HER2/neu positivity did not hold as an independent variable. HER2/neu-positive tumours did not show a higher expression of cytoplasmic CXCR4 staining, which was positive in over half of the cases and correlated closely with the expression of its ligand SDF-1 as expected. There was no impact of cytoplasmic and nuclear CXCR4 expression or SDF-1 abundance on overall survival (Figure 3 and Table 4).
This is in contrast to breast cancer and also other forms of cancer where CXCR4 has been shown to be of significant prognostic influence like oesophageal cancer (Kaifi et al, 2005), colon cancer (Kim et al, 2005), early-stage non-small-cell lung cancer (Spano et al, 2004), low-grade glioma (Salmaggi et al, 2005), malignant melanoma (Scala et al, 2005), osteosarcoma (Laverdiere et al, 2005), oral squamous cell carcinoma (Almofti et al, 2004), and adult acute myeloid leukaemia (Rombouts et al, 2004). One explanation might be the importance of distant metastases to SDF-1 expressing tissues on the cause of death in most of these other forms of cancer. Patients only rarely die of their primary cancer, but rather as a result of metastatic disease. In ovarian cancer, recurrences in the pelvis and, in most cases even within the peritoneum, which has a comparable microenvironment as the primary tumour, are the main causes for death. In our series, only 6.3% (eight out of 128) of patients had distant metastases at primary diagnosis (FIGO 4) and therefore a statistical analysis for CXCR4 overexpression from this group was fruitless. No difference in HER2/neu, CXCR4, and SDF1 expression (or combination) could be found in this small subgroup. Most patients with ovarian cancer die on local recurrences (transcoelomic metastases) within the peritoneum and not on distant metastases (haematogenous metastases) (Vaccarello et al, 1995). In fact, only about 15% of patients get distant metastases during their course of disease (Sood et al, 1999). Further support came from the more recent finding that intraperitoneal chemotherapy is equivalent and even superior to systemic therapy in this disease (Armstrong et al, 2006). Thus, the biology of ovarian cancer seems to be quite different from other epithelial cancers (Naora and Montell, 2005). As an example, normal cells of the ovarian surface epithelium express only little or even no E-cadherin and have both mesenchymal and epithelial features, whereas many primary ovarian carcinomas express higher levels of E-cadherin. The gain of E-cadherin expression, completely unexpected for tumour cells, may result in an advantage for ovarian cancer cells to colonise new sites in the peritoneum. The characteristic epithelia–mesenchymal transition — thought to be necessary for distant metastases, – as well as for the development of distant (haematogenous) metastases, seems to play a subordinate role in the course of ovarian cancer disease. Thus, our finding of the missing influence of CXCR4 expression (and SDF-1 abundance) on patient outcome is in line with the above-mentioned fact that haematogenetic metastases to organs with high SDF-1 expression is relatively rare in ovarian cancer. Molecular mechanisms for these differences are not well understood in detail (Tan et al, 2006) but are in the focus of increasing scientific endeavours.
In summary, no clear-cut relationship between HER2/neu, CXCR4, SDF1, and metastasis and/or prognosis as obvious for breast cancer was found in ovarian cancer. If HER2/neu expression is of biological relevance and not merely reflecting a more advanced stage of the disease, other than CXCR4-mediated HER2/neu activities have to be explored for ovarian cancer.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Almofti A, Uchida D, Begum NM, Tomizuka Y, Iga H, Yoshida H, Sato M (2004) The clinicopathological significance of the expression of CXCR4 protein in oral squamous cell carcinoma. Int J Oncol 25: 65–71
Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA (2006) Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 354: 34–43
Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR (1997) The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA 94: 1925–1930
Jemal A, Murray T, Ward E, Samuels A (2005) Cancer statistics, 2005. Ca-A Cancer J Clin 55: 10–30
Kaifi JT, Yekebas EF, Schurr P, Obonyo D, Wachowiak R, Busch P, Heinecke A, Pantel K, Izbicki JR (2005) Tumor-cell homing to lymph nodes and bone marrow and CXCR4 expression in esophageal cancer. J Natl Cancer Inst 97: 1840–1847
Kato M, Kitayama J, Kazama S, Nagawa H (2003) Expression pattern of CXC chemokine receptor-4 is correlated with lymph node metastasis in human invasive ductal carcinoma. Breast Cancer Res 5: R144–R150
Kim J, Takeuchi H, Lam ST, Turner RR, Wang HJ, Kuo C, Foshag L, Bilchik AJ, Hoon DS (2005) Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol 23: 2744–2753
Laverdiere C, Hoang BH, Yang R, Sowers R, Qin J, Meyers PA, Huvos AG, Healey JH, Gorlick R (2005) Messenger RNA expression levels of CXCR4 correlate with metastatic behavior and outcome in patients with osteosarcoma. Clin Cancer Res 11: 2561–2567
Li YM, Pan Y, Wei Y, Cheng X, Zhou BP, Tan M, Zhou X, Xia W, Hortobagyi GN, Yu D, Hung MC (2004) Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell 6: 459–469
Naora H, Montell DJ (2005) Ovarian cancer metastasis: integrating insights from disparate model organisms. Nat Rev Cancer 5: 355–366
Phillips RJ, Burdick MD, Lutz M, Belperio JA, Keane MP, Strieter RM (2003) The stromal derived factor-1/CXCL12-CXC chemokine receptor 4 biological axis in non-small cell lung cancer metastases. Am J Respir Crit Care Med 167: 1676–1686
Pritchard KI, Shepherd LE, O'Malley FP, Andrulis IL, Tu D, Bramwell VH, Levine MN (2006) HER2 and responsiveness of breast cancer to adjuvant chemotherapy. N Engl J Med 354: 2103–2111
Rombouts EJ, Pavic B, Lowenberg B, Ploemacher RE (2004) Relation between CXCR-4 expression, Flt3 mutations, and unfavorable prognosis of adult acute myeloid leukemia. Blood 104: 550–557
Salmaggi A, Gelati M, Pollo B, Marras C, Silvani A, Balestrini MR, Eoli M, Fariselli L, Broggi G, Boiardi A (2005) CXCL12 expression is predictive of a shorter time to tumor progression in low-grade glioma: a single-institution study in 50 patients. J Neurooncol 74: 287–293
Salvucci O, Bouchard A, Baccarelli A, Deschenes J, Sauter G, Simon R, Bianchi R, Basik M (2005) The role of CXCR4 receptor expression in breast cancer: a large tissue microarray study. Breast Cancer Res Treat 97: 275–283
Scala S, Ottaiano A, Ascierto PA, Cavalli M, Simeone E, Giuliano P, Napolitano M, Franco R, Botti G, Castello G (2005) Expression of CXCR4 predicts poor prognosis in patients with malignant melanoma. Clin Cancer Res 11: 1835–1841
Serrano-Olvera A, Duenas-Gonzalez A, Gallardo-Rincon D, Candelaria M, De lG-S (2006) Prognostic, predictive and therapeutic implications of HER2 in invasive epithelial ovarian cancer. Cancer Treat Rev 32: 180–190
Shibuta K, Mori M, Shimoda K, Inoue H, Mitra P, Barnard GF (2002) Regional expression of CXCL12/CXCR4 in liver and hepatocellular carcinoma and cell-cycle variation during in vitro differentiation. Jpn J Cancer Res 93: 789–797
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344: 783–792
Sood AK, Sorosky JI, Dolan M, Anderson B, Buller RE (1999) Distant metastases in ovarian cancer: association with p53 mutations. Clin Cancer Res 5: 2485–2490
Spano JP, Andre F, Morat L, Sabatier L, Besse B, Combadiere C, Deterre P, Martin A, Azorin J, Valeyre D, Khayat D, Le CT, Soria JC (2004) Chemokine receptor CXCR4 and early-stage non-small cell lung cancer: pattern of expression and correlation with outcome. Ann Oncol 15: 613–617
Tan DS, Agarwal R, Kaye SB (2006) Mechanisms of transcoelomic metastasis in ovarian cancer. Lancet Oncol 7: 925–934
Vaccarello L, Rubin SC, Vlamis V, Wong G, Jones WB, Lewis JL, Hoskins WJ (1995) Cytoreductive surgery in ovarian carcinoma patients with a documented previously complete surgical response. Gynecol Oncol 57: 61–65
Acknowledgements
This work was supported by the Austrian Science Fund (FWF) grant no. P17891.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Pils, D., Pinter, A., Reibenwein, J. et al. In ovarian cancer the prognostic influence of HER2/neu is not dependent on the CXCR4/SDF-1 signalling pathway. Br J Cancer 96, 485–491 (2007). https://doi.org/10.1038/sj.bjc.6603581
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6603581
Keywords
This article is cited by
-
Cancer-associated fibroblasts contribute to cancer metastasis and apoptosis resistance in human ovarian cancer via paracrine SDF-1α
Clinical and Translational Oncology (2023)
-
Human epidermal growth factor receptor 2 (HER2)-specific chimeric antigen receptor (CAR) for tumor immunotherapy; recent progress
Stem Cell Research & Therapy (2022)
-
miR200-regulated CXCL12β promotes fibroblast heterogeneity and immunosuppression in ovarian cancers
Nature Communications (2018)
-
Evaluation of HER2-specific peptide ligand for its employment as radiolabeled imaging probe
Scientific Reports (2018)
-
A meta-analysis of CXCL12 expression for cancer prognosis
British Journal of Cancer (2017)