Abstract
The estrogen-responsive B box protein (EBBP) and Pyrin belong to a family of structurally related proteins. While mutations in the pyrin gene cause an autoinflammatory disease, the biological function of EBBP is unknown. In this study, we identified the proinflammatory cytokine interleukin-1β (IL-1β) as an EBBP-binding partner. Furthermore, caspase-1 and NACHT, LRR and Pyrin domain containing protein (NALP) 1, two components of the recently identified inflammasome, a platform for the activation of caspase-1, also interact with EBBP. These proteins bind to the RFP domain of EBBP, suggesting that this domain of so far unknown function is an important protein-binding domain. EBBP was secreted in a caspase-1-dependent manner from cultured cells, and its secretion was enhanced by IL-1β. Vice versa, endogenous and overerexpressed EBBP increased IL-1β secretion. These results provide evidence for a role of EBBP in innate immunity by enhancing the alternative secretion pathway of IL-1β.
Similar content being viewed by others
Introduction
Interleukin-1β (IL-1β) is a proinflammatory cytokine, which elicits systemic as well as local responses to infection. It activates lymphocytes, recruits leukocytes and is involved in the generation of fever. Based on these activities, it functions as a central mediator in various acute and chronic inflammatory diseases, thus representing a potential target for therapeutic intervention (reviewed by Dinarello1, 2).
Initial synthesis of this cytokine occurs as an inactive precursor (proIL-1β), which lacks a signal sequence. Nevertheless, active IL-1β and proIL-1β are released by a poorly characterized mechanism, particularly from activated macrophages (reviewed by Dinarello1). Secretion is independent of the Golgi apparatus and can be stimulated by exogenous ATP via the P2X7 receptor.3, 4, 5 Processing and activation of proIL-1β is strictly dependent on the protease caspase-1, which cleaves proIL-1β after the aspartic acid residue at position 116, generating active IL-1β.6, 7 Consequently, macrophages from caspase-1 knockout mice cannot produce mature IL-1β.8 Caspase-1 itself is expressed as an inactive precursor. Recently, it was shown that activation of procaspase-1 and subsequent processing of proIL-1β in a cell-free system from macrophages is dependent on a protein complex called the inflammasome.9 It consists of procaspases-1 and -5, Nacht, LRR and Pyrin domain containing protein (NALP)110, 11 and apoptosis-associated speck-like protein containing a card (Asc).12 The assembly of this complex relies only on homotypic interactions of the death domain fold family member caspase recruitment domain (CARD) and the recently identified pyrin domain.11, 13, 14 The NALP1 protein contains an amino-terminal pyrin domain and a carboxy-terminal CARD (Figure 2a). The latter allows binding to the CARD of procaspase-5, whereas the pyrin domain binds via the bipartite adaptor protein Asc, which consists only of a pyrin domain and a CARD, to the CARD of procaspase-1 (Figure 2a). Thereby, procaspase-1 and -5 come in close proximity, allowing their activation.9 All components of the inflammasome are constitutively expressed in macrophages, but the signals, which stimulate the assembly and activation of the inflammasome, are as yet unknown.9 In addition, it is unclear if there is a link between procaspase-1 activation and IL-1β secretion. However, caspase-1 and the procaspase-1 activator Asc are secreted together with IL-1β from activated macrophages,9, 15 suggesting that procaspase-1 and proIL-1β activation and secretion are coupled.5
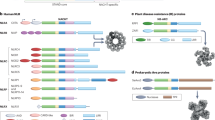
The RFP and BBCC domains of EBBP mediate the interactions with IL-1β and inflammasome components. (a) Domain structures of EBBP, proIL-1β, NALP1 and procaspase-1. Acidic domain: AD; B box and coiled-coil: BBCC; ret finger protein: RFP; pyrin domain: PYD; NAIP, CIITA, HET-E and TP1: NACHT; leucine-rich repeats: LRR; caspase recruitment domain: CARD. (b–e) Interaction of individual EBBP domains with IL-1β, procaspase-1 and NALP1. HA- and Myc-tagged proteins and individual domains of EBBP and its interaction partners were coexpressed in COS-1 cells. HA- and Myc-tagged immunoprecipitates (IP) were analyzed for the presence of Myc- and HA-tagged constructs (co-IP). Transfection of one tagged protein together with the empty vector was used as a control. The interacting domains are indicated by the black color. PAS: protein A sepharose. Unspecific bands are labeled by asterisks. (b) The RFP domain of EBBP interacts with the prodomain and the mature part of proIL-1β. Interaction of HA-tagged EBBP or RFP with Myc-tagged IL-1β could not be detected. However, they interacted with untagged IL-1β. Untagged IL-1β was precipitated with an anti-IL-1β antibody directed against the carboxy-terminus and detected via Western blot with an antibody directed against the amino-terminus of IL-1β. (c) The RFP domain of EBBP interacts with the p20 and p10 subunits of procaspase-1. (d) The BBCC domain of EBBP interacts with the p20 subunit of procaspase-1. (e) The RFP domain of EBBP interacts with the NACHT domain of NALP1
Several inhibitors of the inflammasome are known. COP and ICEBERG contain CARDs, which allow direct binding to procaspase-1, thereby inhibiting its activation.16, 17 Pyrin is also a potential inflammasome inhibitor. Mutations in the pyrin gene cause the recessive inherited disease Familial Mediterranean Fever. These patients suffer from self-limited episodes of fever and inflammation affecting the skin, joints and serosal membranes.18 Activated macrophages from mice with a truncated Pyrin protein produce more active caspase-1 and secrete increased amounts of IL-1β.19 A possible molecular explanation for these phenotypes is an interaction of Pyrin with the inflammasome component Asc via their pyrin domains. This could prevent binding of procaspase-1 to Asc and its activation.19, 20 However, the strength and significance of a binding of Pyrin to Asc is controversial.21 It is not known how the disease-causing mutations, which locate mainly to the carboxy-terminal RFP (SPRY; B30.2) domain, influence the amino-terminal pyrin domain.22, 23 Furthermore, recent results suggest that Pyrin activates caspase-1 via Asc oligomerization,24 suggesting that it is an inflammasome activator. Apart from its pyrin domain, Pyrin is a typical member of the TRIM (tripartite motif) family.25 These proteins share at least two domains, a B box and a coiled-coil domain, allowing self-interaction and the generation of large protein complexes.25
The estrogen-responsive B box protein (EBBP, TRIM16) is a close homolog of Pyrin (TRIM20), but it lacks the pyrin domain.25, 26 We have recently characterized EBBP and we showed that it accelerates differentiation of cultured keratinocytes.27 To elucidate the function of this ubiquitously expressed protein at the molecular level we performed a yeast two-hybrid screen with the RFP domain and identified proIL-1β as an EBBP-binding protein. Our results reveal an important role of EBBP in the secretion of IL-1β, indicating a novel and important function of EBBP in inflammation.
Results
EBBP interacts with proIL-1β
TRIM proteins form large protein complexes through self-interaction, which is most likely mediated via the B box and coiled-coil (BBCC) domain. However, they also bind to several other proteins (e.g. Kim et al.28 and El-Husseini et al.29). To elucidate the function of EBBP we performed a yeast two-hybrid screen to search for proteins, which bind to its RFP domain. We used a HaCaT keratinocyte library, since we originally identified and characterized EBBP in this cell type.27 Several positive clones coded for proIL-1β (data not shown), and this interaction was verified with expression vectors encoding the full-length proteins (Figure 1a, left picture and not shown). No autoactivation was observed after transformation of yeast strain AH109 with these constructs (not shown). The interaction of the SV40 large T antigen and p53 or lamin C served as positive or negative controls, respectively (Figure 1a, middle and right picture). The binding of proIL-1β to the RFP domain of EBBP was verified by glutathione S-transferase (GST) pull-down experiments (data not shown).
EBBP interacts with proIL-1β, procaspase-1 and NALP1. (a) Interaction of EBBP with proIL-1β in yeast. The yeast strain AH 109 was cotransformed with pGBK-EBBP and pGAD-proIL-1β and plated on high selection medium. Cotransformation of pGAD-T and pGBK-p53 served as a positive control, pGAD-T and pGBK-lamin as a negative control. (b) Co-IP of EBBP and proIL-1β. HA-tagged EBBP and Myc-tagged proIL-1β were coexpressed in COS-1 cells. HA- or Myc-tagged immunoprecipitates (IP) were analyzed for the presence of Myc- or HA-tagged proteins (co-IP). As a control plasmids encoding HA-tagged EBBP and Myc-tagged proIL-1β were transfected with the empty expression vector. PAS: protein A sepharose. Unspecific bands are labeled by asterisks. (c) EBBP does not interact with Asc. HA-tagged EBBP and VSV-tagged Asc were coexpressed in COS-1 cells. As a control a plasmid coding for VSV-tagged Asc was transfected with the empty expression vector. PAS: protein A sepharose. Unspecific bands are labeled by asterisks. (d) EBBP interacts with procaspase-1. Plasmids encoding HA-tagged EBBP and Myc-tagged procaspase-1 or Myc-tagged procaspase-1 with the empty vector were coexpressed in COS-1 cells, and their interaction was analyzed by co-IPs. Note the higher expression and degradation of procaspase-1 in the lysate obtained after coexpression of EBBP, in contrast to the lysate obtained after expression of procaspase-1 alone. PAS: protein A sepharose. Unspecific bands are labeled by asterisks. (e) EBBP interacts with NALP1-Nter. FLAG-tagged NALP1-Nter (pyrin and NACHT domains) and HA-tagged EBBP were coexpressed in COS-1 cells and analyzed for interaction by co-IP. As a control the plasmid coding for Flag-tagged NALP1-Nter was transfected together with the empty expression vector. PAS: protein A sepharose. Unspecific bands are labeled by asterisks
To determine whether endogenous EBBP and proIL-1β have the possibility to interact in vivo, we performed immunofluorescence analysis of primary human keratinocytes and of the macrophage cell line U937 with antibodies directed against the endogenous proteins. As previously published,27 EBBP was predominantly found in the cytoplasm and to a lesser extent in the nucleus. Most importantly, EBBP and IL-1β colocalized at the cell membrane and in the perinuclear region of keratinocytes and macrophages (results not shown).
To verify the EBBP/proIL-1β interaction in COS-1 cells via co-immunoprecipitation (co-IP) we expressed full-length EBBP with an amino-terminal epitope of the influenza virus hemagglutinin epitope (HA). The latter does not modify the intracellular localization of EBBP.27 ProIL-1β was expressed as a fusion protein with amino- and carboxy-terminal Myc epitope tags. COS-1 cells were cotransfected with both expression plasmids, and co-IPs were performed in both directions. Cotransfections with only one expression vector and an empty vector served as controls. Strong bands were seen upon co-IP of HA-EBBP and Myc-proIL-1β-Myc in both directions (Figure 1b). These results demonstrate that EBBP can also interact with proIL-1β in mammalian cells.
EBBP does not interact with Asc but with procaspase-1 and NALP1
As the inflammasome is responsible for proIL-1β processing, we wondered whether EBBP is also associated with this complex and whether the binding to proIL-1β is functionally important. The inflammasome component Asc has been described as an activator of procaspase-121 or as a modulator, leading to activation of procaspase-1 at low and inhibition at higher concentrations.30 As the EBBP homolog Pyrin binds to Asc,20 we tested if this is also the case for EBBP. Therefore, we performed co-IPs of HA-tagged EBBP and Asc fusion proteins tagged with an epitope of the vesicular stomatitis virus (VSV) or with a Flag tag. However, interaction of EBBP with Asc could not be detected (Figure 1c and not shown).
To determine whether EBBP interacts with procaspase-1, co-IPs of procaspase-1-Myc with HA-EBBP were performed in both directions. Indeed, procaspase-1 interacted with EBBP (Figure 1d). Moreover, cotransfection of COS-1 cells with procaspase-1 and EBBP expression vectors resulted in much higher expression of the protease and in its degradation (Figure 1d, compare lysates in lower panel), suggesting that this interaction has functional consequences. EBBP and procaspase-1 also interacted in yeast as determined by the yeast two-hybrid system (not shown). Using the same approach, a possible interaction of EBBP with procaspase-5 was examined, however, with a negative result (data not shown).
NALP1 is the large backbone protein of the inflammasome, which binds to Asc and procaspase-5.9 To determine whether EBBP is able to bind to NALP1 a co-IP was performed with HA-tagged EBBP and the Flag-tagged amino-terminal part of NALP1, encoding the pyrin and NACHT domains.9 EBBP was shown to interact with NALP1 (Figure 1e), and this result was verified in yeast (not shown).
Identification of the interaction domains of EBBP and its binding partners
To map the interaction domains of EBBP and its binding partners, HA-tagged EBBP domains and Myc-tagged domains of the interacting proteins (Figure 2a) were used in co-IP experiments. In EBBP, the BBCC domain most likely represents a functional unit (BBCC), because B boxes occur only together with coiled-coils. In addition, BBCC is most likely responsible for self-interaction of all TRIMs.25 We also expressed the RFP domain of as yet unknown function, which is present in different types of proteins.31
ProIL-1β consists of the prosequence, which inhibits receptor binding, and of the mature part. For the interaction of EBBP with proIL-1β the RFP domain of EBBP and the prodomain of proIL-1β were necessary and sufficient. The RFP domain and full-length EBBP interacted with the isolated prodomain and with proIL-1β, but not with mature IL-1β (Figure 2b and data not shown). The BBCC domain interacted neither with proIL-1β nor with its prodomain. Surprisingly, however, EBBP also interacted with mature IL-1β in yeast (data not shown). Therefore, we speculated that the carboxy-terminal tag inhibits the interaction of EBBP with IL-1β. As HA or Myc tags attached to the amino-terminus of IL-1β were not recognized by the corresponding antibodies in IP experiments (not shown), we used mature IL-1β for co-IPs together with an antibody, which recognizes the carboxy-terminus of IL-1β. In these experiments, we found an interaction of EBBP and the RFP domain with mature IL-1β (Figure 2b, right panel, and data not shown).
From procaspase-1 the cDNA sequences encoding the CARD and the p20 and p10 subunits were expressed. For the interaction with procaspase-1, the RFP domain of EBBP was sufficient, but not necessary. The RFP domain or full-length EBBP interacted with procaspase-1, the p20 and the p10 subunit, but not with the CARD domain (Figure 2c and data not shown). Interestingly, the BBCC domain also interacted with procaspase-1 and the p20 subunit, but not with p10 or the CARD domain (Figure 2d and data not shown).
In NALP1 the amino-terminal pyrin domain and the carboxy-terminal CARD domain most likely interact in a homotypic manner. Therefore, we cloned only the cDNA encoding the NACHT domain, which is responsible for self-interaction of NALPs (reviewed by Tschopp et al.32), and the leucine-rich repeats (LRR). EBBP or the RFP domain, but not the BBCC domain, interacted with the NACHT domain, but not with the LRR domain of NALP1 (Figure 2e and results not shown).
In summary, the RFP domain of EBBP is responsible for the interaction with proIL-1β, procaspase-1 and NALP1. It binds to the inhibitory prosequence and the mature part of proIL-1β, to the p20 and p10 subunits of caspase-1 and to the NACHT domain of NALP1. In addition, the BBCC domain of EBBP can independently bind to the p20 subunit of caspase-1.
Endogenous EBBP interacts with procaspase-1, NALP1 and proIL-1β
To analyze whether endogenous EBBP interacts with proIL-1β, procaspase-1 or NALP1, we used protein lysates of phorbol ester-differentiated and lipopolysaccharide-activated U937 cells, which express high levels of inflammasome components and which secrete IL-1β. A new antibody was generated against full-length native EBBP (see Materials and Methods), affinity purified, and bound to anti-IgG agarose. After application of the lysate to the columns and elution of bound proteins via a pH shift, EBBP was detected in the elution fraction of the anti-EBBP column but not of the control column with anti-IgG agarose, demonstrating that the antibody binds to native EBBP (Figure 3, upper panel). Procaspase-1 could also be eluted (Figure 3, second panel). The second band that we observed most likely represents Igs, because it was also obtained with the second antibody alone (Figure 3, third panel). Vice versa, EBBP could be eluted from a column with immobilized caspase-1-specific antibody using the same lysate (data not shown). This demonstrates that endogenous EBBP and procaspase-1 interact in macrophages. The same is true for endogenous EBBP and NALP1 (Figure 3, fourth panel). In contrast, binding of proIL-1β to EBBP could not be shown under these conditions (not shown), suggesting that EBBP and proIL-1β interact only transiently in activated macrophages. However, when we used protein lysates from lipopolysacharide (LPS)-activated but nondifferentiated U937 cells, where proIL-1β is highly expressed but inefficiently secreted (data not shown), interaction of endogenous EBBP and proIL-1β could be detected (Figure 3, lower panels). This interaction was also verified using IP with the EBBP antibody, followed by Western blotting with the IL-1β antibody (data not shown).
Interaction of endogenous EBBP with inflammasome components. Lysates of TPA-differentiated and LPS-activated (upper three panels) or LPS-activated, nondifferentiated U937 cells (lower two panels) were incubated with anti-rabbit IgG agarose (agarose) or with the EBBP antibody bound to agarose (IP). Bound proteins were eluted and analyzed by Western blotting. Unspecific bands are labeled by asterisks. In the lysate lane, 1% (upper panels) or 2% (lower panels) of the input used for the IPs/co-IPs is loaded . For the IP/co-IP of the lower panels we precipitated IL-1β and IL-1β-binding proteins with an antibody against the cytokine
EBBP enhances secretion of IL-1β from transfected COS-1 cells
To determine whether the binding of EBBP to proIL-1β and the inflammasome components is functionally important we first established an in vitro model system. As COS-1 cells can be efficiently transfected and have been successfully used for the analysis of proteins, which influence proIL-1β activation and secretion,33 we overexpressed the proteins of interest in this cell line and determined the consequences with regard to secretion. Procaspase-1 is essential for proIL-1β activation, and this enzyme is able to autoactivate by oligomerization at higher concentrations, independent of components of the inflammasome.34 Therefore, there was no need to overexpress the latter. EBBP itself is ubiquitously expressed, and COS-1 cells also produce high levels of this protein (Figure 4a).
EBBP enhances IL-1β secretion from transfected COS-1 cells. COS-1 cells were transfected with plasmids coding for different proteins as indicated in the top panel. In all, 1 μg plasmid DNA coding for procaspase-1 was used, 1.5 μg for proIL-1β or mature IL-1β and 2.5 μg for EBBP, ADBBCC or RFP. When needed, empty vector was used to increase the total amount of DNA to 5 μg. After 30 h, cells were lysed and 50 μg of total cellular proteins were used for Western blots. Supernatants were concentrated 10-fold by precipitation with acetone. The three upper and lower blots were developed for the same time, respectively, allowing comparison of the individual lysates or supernatants. Results are a representative of three independent experiments. (a) Procaspase-1 and proIL-1β enhance EBBP secretion. Protein lysates (upper panel) and supernatants (lower panel) of transfected COS-1 cells were analyzed for EBBP expression. Note the secretion of endogenous EBBP. The antibody binds to the RFP domain of EBBP. As a loading control the blots with the lysates were also stained for β-actin expression. (b) Expression and secretion of procaspase-1. Protein lysates (upper panel) and supernatants (lower panel) of transfected COS-1 cells were analyzed for procaspase-1 expression. The procaspase-1 specific antibody binds to the CARD domain. (c) EBBP enhances IL-1β secretion. Protein lysates (upper panel) and supernatants (lower panel) of transfected COS-1 cells were analyzed for IL-1β expression. The IL-1β antibody binds to the mature part of the cytokine, allowing detection of pro- and mature IL-1β. (d) IL-1β secretion measured with an IL-1β specific ELISA. Given are means±S.E. from three measurements. (e) LDH activity assay of the supernatants. LDH activity correlates with cell lysis. Given are means±S.E. from two measurements
Transfection of COS-1 cells with low amounts of proIL-1β (1.5 μg) and procaspase-1 (1 μg) plasmids allowed the secretion of detectable levels of pro- and mature IL-1β into the supernatant (Figure 4c and d, lane 13). To enhance the levels of EBBP we transfected COS-1 cells with 2.5 μg of plasmid DNA coding for EBBP. Instead of proIL-1β (Figure 4, lanes 5–8, 13–16), mature IL-1β (lanes 17–24) was also used, as well as the amino- and carboxy-terminal parts of EBBP, ADBBCC (lanes 3, 7, 11, 15, 19, 23) and RFP (lanes 4, 8, 12, 16, 20, 24), instead of full-length EBBP (lanes 2, 6, 10, 14, 18, 22). All proteins were expressed without tag. The EBBP antibody used binds to the RFP domain and cannot detect ADBBCC.27
Surprisingly, endogenous (lanes 1–8) and also low levels of transfected EBBP (lane 2, 6) and RFP (lane 4, 8) were detected in the supernatant (Figure 4a). Cotransfection of proIL-1β and, to a lesser extent, mature IL-1β increased the intracellular levels of recombinant EBBP (lanes 6 and 18) and RFP (lane 8 and 20). Cotransfection of procaspase-1 resulted in higher levels of secreted EBBP (lane 10, supernatant, compare with lane 2) and RFP (lane 12, supernatant, compare with lane 4). The additional expression of proIL-1β further enhanced EBBP expression and in particular secretion (compare lanes 10 and 12 with 14 and 16), and EBBP was degraded (lane 14, supernatant). The effect of mature IL-1β was weaker compared to that of proIL-1β. These results demonstrate that COS-1 cells can secrete EBBP. Procaspase-1, in particular together with proIL-1β and to a lesser extent with mature IL-1β enhanced this secretion.
Endogenous procaspase-1 was not detectable in COS-1 cells by the anti-caspase-1 antibody, which binds to the CARD (Figure 4b, lanes 1–8, 17–20). Recombinant procaspase-1 was detected in the supernatant, but particularly in the cell lysate (lane 9); cotransfection of plasmids encoding EBBP or individual EBBP domains slightly increased the secretion (lanes 10–12, supernatant). This secretion-promoting effect of EBBP for caspase-1 was much stronger after cotransfection of pro- and mature IL-1β expression plasmids (lanes 13–16, 21–24, supernatant). Highest secretion was observed after coexpression of the cytokine with either EBBP or individual EBBP domains (lanes 14–16, 22–24). Surprisingly, forced expression of EBBP and pro- and to a lesser extent mature IL-1β resulted in degradation of procaspase-1 (lanes 14, 15, 22, 23, lysate and supernatant). This degradation most likely does not reflect procaspase-1 autoactivation, because it was also detected in the presence of a specific caspase-1 inhibitor or a broad range caspase inhibitor. Reduced secretion of mature IL-1β demonstrated the activity of the inhibitors (results not shown).
For the detection of IL-1β an antibody was used that recognizes the pro- and mature form (Figure 4c); an IL-1β specific ELISA allowed the quantification of secreted IL-1β (Figure 4d). This ELISA detects predominantly mature IL-1β, but only 10–15% of total proIL-1β. Pro- and qmature IL-1β were only detectable after transfection (Figure 4c and d, lanes 5–8, 13–16, 17–24), demonstrating that COS-1 cells express no or low levels of the cytokine. After overexpression of proIL-1β alone (lane 5), low levels of secreted IL-1β were detected in the supernatant by ELISA but not by Western blotting, suggesting that COS-1 cells express low levels of endogenous procaspase-1. Cotransfection of plasmids encoding EBBP or individual EBBP domains approximately doubled the secretion (lanes 6–8). As expected, processing of proIL-1β was strongly enhanced by coexpression of procaspase-1 (Figure 4c, lysate, lane 13), leading to about 10-fold higher levels of IL-1β in the supernatant (Figure 4d, lane 13). Additional EBBP or ADBBCC expression further enhanced the levels of secreted IL-1β by a factor of approximately 4 to more than 8000 pg/ml (Figure 4d, lanes 14 and 15), whereas the RFP domain was less effective (lane 16).
Expression of mature IL-1β alone resulted in high levels of the cytokine in the supernatant (Figure 4c, d and e, lane 17), confirming that the prodomain inhibits its secretion. Nevertheless, EBBP (lane 18) or individual EBBP domains (lanes 19 and 20) as well as procaspase-1 (lane 21) enhanced the secreted amount of mature IL-1β by a factor of 2, both together by a factor of 4 (lanes 22–24). As a control for cell lysis, the activity of the intracellular enzyme lactate dehydrogenase (LDH) was measured in the supernatant (Figure 4e). The percentage of total LDH activity detected in the supernatant (up to about 2.5% compared to the activity in the total cell lysate) was much lower than the transfection rate, demonstrating that the enhanced secretion is not due to cell lysis. In fact, additional overexpression of EBBP or individual EBBP domains together with procaspase-1 lowered the extracellular LDH activity, whereas IL-1β secretion was enhanced (Figure 4e, compare lane 13 with 14–16 and lane 20 with 21–24).
To investigate whether the EBBP-induced increase in IL-1β secretion is a general effect, a similar experiment was performed with human keratinocytes. EBBP or individual EBBP domains enhanced the secretion of the cytokine by the same factor as seen in COS-1 cells (results not shown).
Knockdown of endogenous EBBP reduces IL-1β secretion
Finally, we investigated whether endogenous EBBP is also involved in proIL-1β maturation. For this purpose EBBP expression was suppressed by transfection of siRNA specific for the BBCC domain. As a control an EBBP-specific siRNA with two mismatches was used. ProIL-1β and procaspase-1 were coexpressed in COS-1 cells, allowing monitoring of proIL-1β maturation. EBBP-specific siRNA reduced the level of overexpressed EBBP in comparison to control siRNA (Figure 5, lanes 3 and 7). In contrast, RFP expression was not affected (lanes 2 and 6), demonstrating that the siRNA specifically reduced full-length EBBP expression. Reduction of endogenous EBBP was only detected in the supernatant (lanes 5 and 6) and was rescued by overexpression of EBBP or ADBBCC (lanes 7 and 8). Secretion of IL-1β was reduced by EBBP-specific siRNA (compare lanes 1 and 5). This effect was rescued by overexpression of EBBP (lanes 3 and 7) and ADBBCC (lanes 4 and 8) but not RFP (lanes 2 and 6).
Knockdown of endogenous EBBP in COS-1 cells reduces IL-1β secretion. COS-1 cells were transfected with 2.5 μg plasmid DNA encoding a specific siRNA against the BBCC domain of EBBP or siRNA with two mismatches as indicated in the table. In addition, 0.5 μg DNA encoding procaspase-1, 0.75 μg DNA encoding proIL-1β and 1.25 μg DNA encoding RFP, EBBP or ADBBCC were used. At 12 h after transfection, the medium was changed to DMEM with 10% FCS, after additional 24 h it was replaced by OptimemI and cells and supernatants were harvested after additional 24 h. Western blots were performed as described in Figure 4 using 40 μg total protein lysate. For quantification of IL-1β secretion an ELISA was performed and as a control for cell lysis a LDH activity assay. EBBP knockdown correlates with reduced IL-1β secretion and can be rescued by overexpression of EBBP or ADBBCC
To avoid unspecific effects due to proIL-1β and caspase-1 overexpression we used EBBP-specific siRNA to reduce expression of endogenous EBBP in primary keratinocytes, which can be transfected with a much higher efficency than macrophages or monocytes. These epithelial cells secrete IL-1β after UV-B irradiation.35 In this experiment we used two different siRNAs, which strongly reduced expression of EBBP in primary keratinocytes (Figure 6, upper panels). 24 h after UV-B irradiation, cells transfected with EBBP-specific siRNAs secreted approximately 30% less IL-1β than cells transfected with control siRNA (Figure 6, lower panels). As a control, we verified the maturation of proIL-1β to mature IL-1β in the cell lysate as well as in the supernatant by Western blotting (data not shown). These findings demonstrate that reduction of endogenous EBBP reduces IL-1β secretion.
Knockdown of endogenous EBBP in primary human keratinocytes reduces IL-1β secretion. Human primary keratinocytes were transfected with two different EBBP-specific siRNAs and one randomized oligonucleotide as a control. At 72 h after transfection, the EBBP knockdown was verified by Western blotting of the cell lysate using an antibody against EBBP (upper panel); staining with an antibody against β-actin served as a control (lower panel). At the same time point the medium was changed and the cells were irradiated with 50 mJ/cm2 UV-B or mock treated. After 24 h, IL-1β concentrations and LDH activities in the supernatants were measured. The LDH activity in the supernatant was calculated as % of total activity after lysis of all cells. The error bars indicate the standard deviation of two individual experiments performed at the same time and measured in the same assays
Discussion
In this study, we show by genetic and biochemical methods that the as yet poorly characterized EBBP interacts with proIL-1β, procaspase-1 and NALP1 (Figure 1). Coexpression of the latter proteins together with EBBP but not with different control proteins enhanced their expression (Figure 1b–e, and results not shown). This finding suggests that proIL-1β, procaspase-1, and NALP1 are stabilized by EBBP, and further supports the hypothesis that EBBP is a binding partner of these proteins. Vice versa, EBBP expression was also higher in the presence of its binding partners (Figure 1b and results not shown).
Interestingly, the RFP domain of EBBP was sufficient for the binding to proIL-1β and the inflammasome components NALP1 and procaspase-1 (Figure 2). This binding is most likely not due to unspecific ‘stickyness’ of the RFP domain, because interaction with the CARD of procaspase-1, the LRR of NALP1, or with other control proteins could not be detected (results not shown). The RFP domain is present in several other TRIM proteins as well as in unrelated proteins.36, 37 Recently, it was shown that the RFP domain of TRIM5 is responsible for HIV-1 restriction in rhesus monkeys.38 This finding and our results suggest that this domain is a crucial protein-binding domain. In addition to the RFP domain, EBBP binds also with the BBCC domain to caspase-1, however, only to the p20 subunit.
The interactions that we identified with the overexpression approach are also relevant for the endogenous proteins. Thus, we identified caspase-1, NALP-1 and proIL-1β as binding partners of endogenous EBBP in macrophages (Figure 3). Interestingly, we only detected the EBBP-IL-1β interaction in nondifferentiated monocytes, which express proIL-1β but do not secrete it efficiently, whereas the interaction was not detectable in activated macrophages. As the latter efficiently secrete IL-1β, this finding suggests that the interaction of EBBP with IL-1β under these conditions is only transient or that the secretion occurs very rapidly.
What is the biological function of EBBP? To address this question we overexpressed EBBP or individual EBBP domains with procaspase-1 and proIL-1β or mature IL-1β in COS-1 cells (Figure 4). Surprisingly, EBBP was not only detected in the lysate but also in the cell supernatant, although it lacks a signal peptide. IL-1β and procaspase-1 were also secreted and each protein enhanced the secretion of the others. These effects are not due to unspecific cell lysis because the secretion levels did not correlate with the amount of the intracellular enzyme LDH in the supernatant. The strong increase in IL-1β secretion after EBBP coexpression could be due to the activation of procaspase-1. In fact, procaspase-1 was degraded upon expression of EBBP and proIL-1β, and this could reflect its activation. However, degradation products of the same size were also seen in the presence of caspase inhibitors (results not shown), suggesting that a different protease is responsible. In addition, caspase-1 activation occurs through a so-called induced proximity mechanism34 via amino-terminal CARD interactions, and only CARD proteins have been identified as procaspase-1 activators, for example, RIP2/RICK/CARDIAK,39, 40, 41 CARD12/CLAN/Ipaf42, 43, 44 and Asc.9, 21 Thus, it seems unlikely that a protein without a CARD, such as EBBP, is a caspase-1 activator. The third argument against caspase-1 activation by EBBP is the fact that EBBP also enhances secretion of mature IL-1β, which does not need activation by caspase-1. The other possible reason for the enhancement of proIL-1β maturation and secretion via EBBP is a direct involvement of EBBP in the secretion process.
The pathway of leaderless secretion is only partially characterized, but involves most likely lysosomal exocytosis.5 The hypothesis that EBBP directly plays a role in secretion is supported by the cosecretion of the leaderless proteins EBBP, caspase-1 and IL-1β. In addition, we have identified other EBBP-binding proteins, which are secreted by an alternative pathway, for example, galectin-1 (unpublished results). It will be interesting to determine whether EBBP is also able to influence the secretion of these proteins.
Surprisingly, we also found that the ADBBCC domain of EBBP enhanced IL-1β secretion, although it did not directly bind to the cytokine. The reason for this positive effect of ADBBCC on IL-1β secretion is as yet unclear. As ADBBCC can interact with full-length EBBP (data not shown), it may stabilize endogenous EBBP. Alternatively, ADBBCC could interact with another TRIM protein, which is involved in IL-1β secretion, and preliminary results from our laboratory support this possibility.
Although most of our experiments were performed with transfected cells, several findings suggest that these results are also relevant for the endogenous proteins: (i) Asc is an essential activator of procaspase-1 in macrophages,15 and Asc is also expressed by COS-1 cells and human primary keratinocytes (our own unpublished results); (ii) NALP1 belongs to a family of 14 members32 and at least NALP1 is expressed by human primary keratinocytes (results not shown); (iii) proIL-1β secretion and activation is strictly dependent on the expression of procaspase-1 in vivo8 and also in our experiments; (iv) endogenous EBBP is also secreted by primary keratinocytes and macrophages (results not shown); and (v) endogenous EBBP and IL-1β colocalize in keratinocytes and macrophages (results not shown) and interact with each other, at least in monocytes (Figure 3). Finally, downregulation of endogenous EBBP in COS-1 cells or human primary keratinocytes using siRNA reduced the level of secreted IL-1β (Figures 5 and 6), demonstrating that also endogenous EBBP is involved in proIL-1β maturation.
Several inflammatory diseases are characterized by enhanced IL-1β secretion and are responsive to IL-1 inhibition.2, 18, 22, 45 It will be interesting to determine if activated macrophages from patients suffering from these diseases secrete not only higher levels of IL-1β but also express more EBBP. Vice versa, the regulation of EBBP by estrogen in vitro26 raises the possibility that this is also the case in vivo, which may contribute to the higher incidence of autoimmune diseases in women.
In summary, we showed that EBBP interacts with the inflammasome components procaspase-1 and NALP1 and with the ‘substrate’ of this protein complex, proIL-1β, resulting in enhanced IL-1β secretion. These results suggest an important role of EBBP in innate immunity by enhancing the secretion of IL-1β. In addition, it may play a general role in the leaderless secretion pathway(s).
Materials and Methods
Plasmids
Plasmids coding for HA-EBBP, Asc-VSV and the amino-terminus of NALP1 (NALP-1-Nter) have been described.9, 27 For the yeast two-hybrid screen the open reading frames were amplified by PCR, sequenced and cloned into pGBKT7 or pGADT7 (Clontech, Paolo Alto, CA, USA). All other full-length and truncated proteins were expressed via pCG vectors with or without Myc or HA epitope tags. The prosequence of proIL-1β is nt 88-435, the mature part nt 436–894 (GenBank™ accession number NM000576); the prosequence of procaspase-1 nt 18–374, p20 nt 375–965 and p10 nt 966–1228 (NM_033292); NACHT of NALP1 nt 1482–2048, LRR nt 2916–3488 (AF310105); ADBBCC of EBBP nt 228–1343, BBCC nt 437–1343 and RFP nt 1335–1919 (NM006470).
Cell culture
COS-1 cells were cultured in DMEM (Sigma, Munich, Germany), supplemented with 10% FCS (Amimed-BioConcept, Allschwil, Switzerland) and 1% penicillin/streptomycin. For transfection 4 × 105 cells were seeded into a 3 cm plate and transfected using 7.5 μl Lipofectamine 2000 (Invitrogen, Basel, Switzerland) and 5 μg plasmid DNA in Optimem I (Invitrogen), and harvested 30 h after transfection. For quantitative studies, plasmids used for transfection were purified using the Endo-Free kit (Qiagen, Hilden, Germany). For co-IP cells were transfected in 5 cm dishes with 10 μg plasmid DNA and 15 μl Lipofectamine 2000 overnight. Then the medium was changed to DMEM with 10% FCS and cells were harvested after 24 h in 400 μl co-IP buffer (50 mM Tris pH 7.5, 15 mM EDTA, 100 mM NaCl, 0.1% (w/v) Triton X-100, 1% Aprotinin (Sigma), 0.5 mM AEBSF (Sigma)). Human primary keratinocytes were isolated from foreskin and cultured in keratinocyte-SFM (Invitrogen) with epidermal growth factor/bovine pituitary extract and 1% penicillin/streptomycin. U937 cells were cultured in RPMI 1640 (Sigma) with 10% FCS, 1% penicillin/streptomycin, 1 mM sodium pyruvate and 20 mM HEPES. 5 × 105 cells/ml were differentiated with 20 ng/ml 12-O-tetradecanoylphorbol-13-acetate (Sigma) for 3 days and activated after the addition of new medium with 1 μg/ml LPS (Sigma) for 14 h.
Co-IP and antibodies
After sonication and centrifugation of cells the supernatant was incubated with antibodies against the HA epitope (rabbit, 1 : 20 diluted; Santa Cruz, CA, USA), the Myc epitope (mouse, 1 : 50 diluted, Clontech), IL-1β (goat, 1 : 20, Santa Cruz) or the VSV epitope (mouse, 1 : 50 diluted, Sigma) for 90 min at 4°C (100 μl lysate for each antibody and for protein A control, respectively, 2–10 μl were applied for the transfection control). After centrifugation, antigen–antibody complexes were precipitated with protein A sepharose slurry (Amersham Biosciences, Braunschweig, Germany) for 1 h at 4°C and subsequently pelleted. Pellets were washed with co-IP buffer and incubated for 5 min at 95°C in Laemmli sample buffer. Eluates were analyzed by Western blotting (50% each were applied for the IP and co-IP, respectively). Antibodies used for Western blotting were against the Myc, HA or VSV epitope, or proIL-1β, procaspase-1 (both from Santa Cruz), EBBP,27 or the amino-terminus of IL-1β (Cell Signaling; Beverly, MA, USA or R&D Systems, Minneapolis, MN, USA). Development of the Western blot was performed with alkaline phosphatase- or horseradish peroxidase-conjugated secondary antibodies (Promega, Madison, WI, USA).
Co-IP of endogenous proteins
For the IP of endogenous EBBP, His-tagged EBBP was expressed in Escherichia coli, purified and used for the immunization of rabbits. The antiserum was purified by affinity chromatography using the purified and immobilized antigen. Of this antibody, 120 μg was immobilized on 280 μl anti-rabbit IgG agarose slurry, the untreated agarose served as a control. Lysate of 1.25 × 108 TPA-differentiated and LPS-activated U937 cells was preincubated with 1 ml of protein A sepharose and subsequently with anti-EBBP or control agarose for 14 h at 4°C. Bound proteins were eluted via pH shift and immediately neutralized. After dialysis fractions were freeze-dried and solubilized in Laemmli buffer. To obtain nondifferentiated U937 cells 200 ml suspension of 1.5 × 106 cells was treated with 1 μg/ml LPS. After 24 h cells were harvested by centrifugation. For the co-IP we used 20 μg IL-1β antibody (R&D Systems, Minneapolis, USA).
Yeast two-hybrid screen
The Matchmaker 3 System was used according to the manufacturer's instructions (Clontech Laboratories) using the yeast strain AH109 and a human HaCaT keratinocyte pACT2 library.
IL-1β ELISA
Coating of Maxisorb plates (Nalge Nunc, Hereford, UK) and ELISA were performed as described in the instruction manual (AMS Biotechnology, Bioggio, Switzerland). This ELISA detects mainly IL-1β and only about 10–15% of proIL-1β as estimated by Western blotting of lysates and supernatants of pro- and IL-1β-transfected COS-1 cells.
Preparation of protein lysates and concentration of cell supernatants
After transfection, proteins were harvested in 100 μl co-IP buffer, sonicated and pelleted by centrifugation. The protein concentration in the supernatant was determined with the BCA kit (Pierce, Rockford, IL, USA). The medium of the transfected cells was cleared by centifugation at 2000 r.p.m. in a microcentrifuge and frozen. Alternatively, proteins were precipitated with 2.5 volumes acetone and harvested by centrifugation. Pellets were resuspended in Laemmli buffer, which allowed 10-fold concentration of proteins.
LDH assay
100 μl supernatants was added to 20 μl freshly prepared lactate solution (26 mg/ml lactate in 10 mM Tris pH 8.5), 20 μl INT dye (2 mg/ml 2-p-iodophenyl-3-nitrophenyl tetrazolium chloride in DMSO) and 20 μl NAD+ solution (4.5 mg NAD+, 13.5 U/ml diaphorase, 0.03% BSA, 1.2% sucrose in PBS pH 8.2) in a microtiter plate. After 20 min the reaction was stopped by addition of 20 μl oxymate solution (16.6 mg/ml), and the OD 490 nm was determined. Values were normalized to those of the supernatant of vector-transfected cells lysed by the addition of Triton X-100 (end concentration 10% (v/v)) (Current Protocols in Toxicology, Chapter 2, UNIT 2.6, Alternate Protocol 3).
siRNA knockdown of EBBP
Five different siRNAs were tested by transient transfection of HaCaT cells. EBBP mRNA was measured using RNase protection assay as described.27 The most efficient sequence (sense: ACCUGCAUGGUGAAUUACUTT; antisense: AGUAAUUCACCAUGCAGGUTT) was used for further experiments. As a control an EBBP-specific sequence with two mismatches was used. For EBBP knockdown in human primary keratinocytes siRNA (HS_TRIM16_1/2, ctr. siRNA non-sil.) was purchased from Qiagen (Hilden, Germany). 6.5 × 104 cells per well were seeded in a volume of 0.65 ml in 12-well plates. Transfection was performed with 2 μg/ml Profectin 65 (Atugen, Berlin, Germany) and 100 nM siRNA. At 72 h after transfection, cells were irradiated in new growth medium at 50 mJ/cm2 (Medisun HF-54, Schulze&Böhm, Hürth, Germany).
Accession codes
Abbreviations
- ASC:
-
apoptosis-associated speck-like protein containing a CARD
- CARD:
-
caspase recruitment domain
- co-IP:
-
co-immunoprecipitation
- EBBP:
-
estrogen-responsive B box protein
- IL-1β:
-
Interleukin-1β
- IP:
-
immunoprecipitation
- LDH:
-
lactate dehydrogenase
- LPS:
-
lipopolysacharide
- NALP:
-
Nacht, LRR and Pyrin domain containing protein
- TRIM:
-
tripartite motif
- VSV:
-
vesicular stomatitis virus
References
Dinarello CA (1998) Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann. NY Acad. Sci. 856: 1–11.
Dinarello CA (2004) Therapeutic strategies to reduce IL-1 activity in treating local and systemic inflammation. Curr. Opin. Pharmacol. 4: 378–385.
Perregaux D and Gabel CA (1994) Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J. Biol. Chem. 269: 15195–15203.
Ferrari D, Chiozzi P, Falzoni S, Hanau S and Di Virgilio F (1997) Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. J. Exp. Med. 185: 579–582.
Andrei C, Margiocco P, Poggi A, Lotti LV, Torrisi MR and Rubartelli A (2004) Phospholipases C and A2 control lysosome-mediated IL-1 beta secretion: implications for inflammatory processes. Proc. Natl. Acad. Sci. USA 101: 9745–9750.
Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Van Ness K, Greenstreet TA, March CJ, Kronheim SR, Druck T, Cannizzaro LA, Heubner K and Black RA (1992) Molecular cloning of the interleukin-1 beta converting enzyme. Science 256: 97–100.
Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J, Elliston KO, Ayala JM, Casano FJ, Chin J, Ding GJF, Egger LA, Gaffney EP, Limjuco G, Palyha OC, Raju SM, Rolando AM, Salley JP, Yamin TT, Lee TD, Shively JE, MacCross M, Mumford RA, Schmidt JA and Tocci MJ (1992) A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 356: 768–774.
Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J, Towne E, Tracey D, Wardwell S, Wei FY, Wong W, Kamen R and Seshadri T (1995) Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell 80: 401–411.
Martinon F, Burns K and Tschopp J (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 10: 417–426.
Bertin J and DiStefano PS (2000) The PYRIN domain: a novel motif found in apoptosis and inflammation proteins. Cell. Death Differ. 7: 1273–1274.
Martinon F, Hofmann K and Tschopp J (2001) The pyrin domain: a possible member of the death domain-fold family implicated in apoptosis and inflammation. Curr. Biol. 11: R118–R120.
Masumoto J, Taniguchi S, Ayukawa K, Sarvotham H, Kishino T, Niikawa N, Hidaka E, Katsuyama T, Higuchi T and Sagara J (1999) ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J. Biol. Chem. 274: 33835–33838.
Aravind L, Dixit VM and Koonin EV (1999) The domains of death: evolution of the apoptosis machinery. Trends Biochem. Sci. 24: 47–53.
Fairbrother WJ, Gordon NC, Humke EW, O'Rourke KM, Starovasnik MA, Yin JP and Dixit VM (2001) The PYRIN domain: a member of the death domain-fold superfamily. Protein Sci. 10: 1911–1918.
Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S and Dixit VM (2004) Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430: 213–218.
Lee SH, Stehlik C and Reed JC (2001) Cop, a caspase recruitment domain-containing protein and inhibitor of caspase-1 activation processing. J. Biol. Chem. 276: 34495–34500.
Humke EW, Shriver SK, Starovasnik MA, Fairbrother WJ and Dixit VM (2000) ICEBERG: a novel inhibitor of interleukin-1beta generation. Cell 103: 99–111.
Drenth JP and van der Meer JW (2001) Hereditary periodic fever. N. Engl. J. Med. 345: 1748–1757.
Chae JJ, Komarow HD, Cheng J, Wood G, Raben N, Liu PP and Kastner DL (2003) Targeted disruption of pyrin, the FMF protein, causes heightened sensitivity to endotoxin and a defect in macrophage apoptosis. Mol. Cell 11: 591–604.
Richards N, Schaner P, Diaz A, Stuckey J, Shelden E, Wadhwa A and Gumucio DL (2001) Interaction between pyrin and the apoptotic speck protein (ASC) modulates ASC-induced apoptosis. J. Biol. Chem. 276: 39320–39329.
Srinivasula SM, Poyet JL, Razmara M, Datta P, Zhang Z and Alnemri ES (2002) The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J. Biol. Chem. 277: 21119–21122.
The French FMF Consortium (1997) A candidate gene for familial Mediterranean fever. Nat. Genet. 17: 25–31.
Gumucio DL, Diaz A, Schaner P, Richards N, Babcock C, Schaller M and Cesena T (2002) Fire and ICE: the role of pyrin domain-containing proteins in inflammation and apoptosis. Clin. Exp. Rheumatol. 20: S45–S53.
Yu JW, Wu J, Zhang Z, Datta P, Ibrahimi I, Taniguchi S, Sagrada J, Fernandes-Alnemri T and Alnemri ES (2006) Cryopyrin and pyrin activate caspase-1, but not NF-kappaB, via ASC oligomerization. Cell. Death Differ. 13: 236–249.
Reymond A, Meroni G, Fantozzi A, Merla G, Cairo S, Luzi L, Riganelli D, Zanaria E, Messali S, Cainarca S, Guffanti A, Minucci S, Pelicci PG and Ballabio A (2001) The tripartite motif family identifies cell compartments. EMBO. J. 20: 2140–2151.
Liu HL, Golder-Novoselsky E, Seto MH, Webster L, McClary J and Zajchowski DA (1998) The novel estrogen-responsive B-box protein (EBBP) gene is tamoxifen-regulated in cells expressing an estrogen receptor DNA-binding domain mutant. Mol. Endocrinol. 12: 1733–1748.
Beer HD, Munding C, Dubois N, Mamie C, Hohl D and Werner S (2002) The estrogen-responsive B box protein: a novel regulator of keratinocyte differentiation. J. Biol. Chem. 277: 20740–20749.
Kim SS, Chen YM, O'Leary E, Witzgall R, Vidal M and Bonventre JV (1996) A novel member of the RING finger family, KRIP-1, associates with the KRAB-A transcriptional repressor domain of zinc finger proteins. Proc. Natl. Acad. Sci. USA 93: 15299–15304.
El-Husseini AE and Vincent SR (1999) Cloning and characterization of a novel RING finger protein that interacts with class V myosins. J. Biol. Chem. 274: 19771–19777.
Stehlik C, Lee SH, Dorfleutner A, Stassinopoulos A, Sagara J and Reed JC (2003) Apoptosis-associated speck-like protein containing a caspase recruitment domain is a regulator of procaspase-1 activation. J. Immunol. 171: 6154–6163.
Seto MH, Liu HL, Zajchowski DA and Whitlow M (1999) Protein fold analysis of the B30.2-like domain. Proteins 35: 235–249.
Tschopp J, Martinon F and Burns K (2003) NALPs: a novel protein family involved in inflammation. Nat. Rev. Mol. Cell. Biol. 4: 95–104.
Shoham NG, Centola M, Mansfield E, Hull KM, Wood G, Wise CA and Kastner DL (2003) Pyrin binds the PSTPIP1/CD2BP1 protein, defining familial Mediterranean fever and PAPA syndrome as disorders in the same pathway. Proc. Natl. Acad. Sci. USA 100: 13501–13506.
Salvesen GS and Dixit VM (1999) Caspase activation: the induced-proximity model. Proc. Natl. Acad. Sci. USA 96: 10964–10967.
Naderi-Hachtroudi L, Peters T, Brenneisen P, Meewes C, Hommel C, Razi-Wolf Z, Schneider LA, Schuller J, Wlaschek M and Scharffetter-Kochanek K (2002) Induction of manganese superoxide dismutase in human dermal fibroblasts: a UV-B-mediated paracrine mechanism with the release of epidermal interleukin 1α, interleukin 1 β, and tumor necrosis factor α. Arch. Dermatol. 138: 473–479.
Henry J, Ribouchon MT, Offer C and Pontarotti P (1997) B30.2-like domain proteins: a growing family. Biochem. Biophys. Res. Commun. 235: 162–165.
Henry J, Mather IH, McDermott MF and Pontarotti P (1998) B30.2-like domain proteins: update and new insights into a rapidly expanding family of proteins. Mol. Biol. Evol. 15: 1696–1705.
Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P and Sodorski J (2004) the cytoplasmic body component TRIM5α restricts HIV-1 infection in old world monkeys. Nature 427: 848–853.
McCarthy JV, Ni J and Dixit VM (1998) RIP2 is a novel NF-kappaB-activating and cell death-inducing kinase. J. Biol. Chem. 273: 16968–16975.
Inohara N, del Peso L, Koseki T, Chen S and Nunez G (1998) RICK, a novel protein kinase containing a caspase recruitment domain, interacts with CLARP and regulates CD95-mediated apoptosis. J. Biol. Chem. 273: 12296–12300.
Thome M, Hofmann K, Burns K, Martinon F, Bodmer JL, Mattmann C and Tschopp J (1998) Identification of CARDIAK, a RIP-like kinase that associates with caspase-1. Curr. Biol. 8: 885–888.
Geddes BJ, Wang L, Huang WJ, Lavellee M, Manji GA, Brown M, Jurman M, Cao J, Morgenstern J, Merriam S, Glucksmann MA, DiStefano PS and Bertin J (2001) Human CARD12 is a novel CED4/Apaf-1 family member that induces apoptosis. Biochem. Biophys. Res. Commun. 284: 77–82.
Damiano JS, Stehlik C, Pio F, Godzik A and Reed JC (2001) CLAN, a novel human CED-4-like gene. Genomics 75: 77–83.
Poyet JL, Srinivasula SM, Tnani M, Razmara M, Fernandes-Alnemri T and Alnemri ES (2001) Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J. Biol. Chem. 276: 28309–28313.
Pascual V, Allantaz F, Arce E, Punaro M and Banchereau J (2005) Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J. Exp. Med. 201: 1479–1486.
Acknowledgements
We thank Dr Anke Klippel, Atugen AG, Berlin for kindly providing Profectin 65 transfection reagent. This work was supported by grants from the Swiss National Science Foundation (3100A0-104170 to D.B.), and the ETH Research Foundation (to S.W. and D.B.).
Author information
Authors and Affiliations
Author notes
C Munding, M Keller: These authors have equally contributed to this work
Corresponding author
Additional information
Edited by R De Maria
Rights and permissions
About this article
Cite this article
Munding, C., Keller, M., Niklaus, G. et al. The estrogen-responsive B box protein: a novel enhancer of interleukin-1β secretion. Cell Death Differ 13, 1938–1949 (2006). https://doi.org/10.1038/sj.cdd.4401896
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4401896
Keywords
This article is cited by
-
Tripartite motif-containing protein 16 protects against myocardial ischemia/reperfusion injury by affecting the Keap1/Nrf2 axis
Cell and Tissue Research (2021)
-
Heterozygous loss of keratinocyte TRIM16 expression increases melanocytic cell lesions and lymph node metastasis
Journal of Cancer Research and Clinical Oncology (2019)
-
Estrogen suppresses hepatocellular carcinoma cells through ERβ-mediated upregulation of the NLRP3 inflammasome
Laboratory Investigation (2015)
-
TRIM16 overexpression induces apoptosis through activation of caspase-2 in cancer cells
Apoptosis (2013)
-
Regulation of inflammasome signaling
Nature Immunology (2012)