Abstract
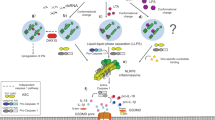
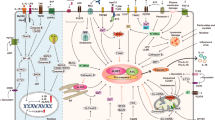
The mammalian NLR gene family was first reported over 20 years ago, although several genes that were later grouped into the family were already known at that time. Although it is widely known that NLRs include inflammasome receptors and/or sensors that promote the maturation of caspase 1, IL-1β, IL-18 and gasdermin D to drive inflammation and cell death, the other functions of NLR family members are less well appreciated by the scientific community. Examples include MHC class II transactivator (CIITA), a master transcriptional activator of MHC class II genes, which was the first mammalian NBD–LRR-containing protein to be identified, and NLRC5, which regulates the expression of MHC class I genes. Other NLRs govern key inflammatory signalling pathways or interferon responses, and several NLR family members serve as negative regulators of innate immune responses. Multiple NLRs regulate the balance of cell death, cell survival, autophagy, mitophagy and even cellular metabolism. Perhaps the least discussed group of NLRs are those with functions in the mammalian reproductive system. The focus of this Review is to provide a synopsis of the NLR family, including both the intensively studied and the underappreciated members. We focus on the function, structure and disease relevance of NLRs and highlight issues that have received less attention in the NLR field. We hope this may serve as an impetus for future research on the conventional and non-conventional roles of NLRs within and beyond the immune system.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
17 May 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41577-023-00891-9
References
Baker, B., Zambryski, P., Staskawicz, B. & Dinesh-Kumar, S. P. Signaling in plant-microbe interactions. Science 276, 726–733 (1997).
McHale, L., Tan, X., Koehl, P. & Michelmore, R. W. Plant NBS-LRR proteins: adaptable guards. Genome Biol. 7, 212 (2006).
Barbary, A., Djian-Caporalino, C., Palloix, A. & Castagnone-Sereno, P. Host genetic resistance to root-knot nematodes, Meloidogyne spp., in Solanaceae: from genes to the field. Pest Manag. Sci. 71, 1591–1598 (2015).
Steimle, V., Otten, L. A., Zufferey, M. & Mach, B. Complementation cloning of an MHC class II transactivator mutated in hereditary MHC class II deficiency (or bare lymphocyte syndrome). Cell 75, 135–146 (1993). This paper describes identification of the first described NLR protein, CIITA, by complementation cloning of a B cell line lacking MHC class II expression.
Harton, J. A. & Ting, J. P. Class II transactivator: mastering the art of major histocompatibility complex expression. Mol. Cell Biol. 20, 6185–6194 (2000).
Harton, J. A., Linhoff, M. W., Zhang, J. & Ting, J. P. Cutting edge: CATERPILLER: a large family of mammalian genes containing CARD, pyrin, nucleotide-binding, and leucine-rich repeat domains. J. Immunol. 169, 4088–4093 (2002). This paper represents the first description of the entire human NLR gene family.
Roy, N. et al. The gene for neuronal apoptosis inhibitory protein is partially deleted in individuals with spinal muscular atrophy. Cell 80, 167–178 (1995).
Liston, P. et al. Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature 379, 349–353 (1996).
Koonin, E. V. & Aravind, L. The NACHT family — a new group of predicted NTPases implicated in apoptosis and MHC transcription activation. Trends Biochem. Sci. 25, 223–224 (2000).
Inohara, N. et al. Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-κB. J. Biol. Chem. 274, 14560–14567 (1999).
Bertin, J. et al. Human CARD4 protein is a novel CED-4/Apaf-1 cell death family member that activates NF-κB. J. Biol. Chem. 274, 12955–12958 (1999). This paper, together with Inohara et al. (1999), describes the identification of NOD1 and its ability to activate NF-κB.
Ogura, Y. et al. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-κB. J. Biol. Chem. 276, 4812–4818 (2001).
Girardin, S. E. et al. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science 300, 1584–1587 (2003).
Chamaillard, M. et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 4, 702–707 (2003).
Inohara, N. et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2: implications for Crohn’s disease. J. Biol. Chem. 278, 5509–5512 (2003).
Girardin, S. E. et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 278, 8869–8872 (2003).
Hugot, J. P. et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 411, 599–603 (2001).
Ogura, Y. et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 411, 603–606 (2001). This paper, together with Hugot et al. (2001), provides the first description of NLR mutations associated with human disease by showing that NOD2 mutations increase susceptibility to Crohn’s disease.
Inohara, N. & Nuñez, G. The NOD: a signaling module that regulates apoptosis and host defense against pathogens. Oncogene 20, 6473–6481 (2001).
Poyet, J. L. et al. Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J. Biol. Chem. 276, 28309–28313 (2001).
Martinon, F., Burns, K. & Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 10, 417–426 (2002). This paper provides the first biochemical analysis of the inflammasome describing the molecular components and mechanistic roles they play in inflammatory caspase activation.
Tschopp, J., Martinon, F. & Burns, K. NALPs: a novel protein family involved in inflammation. Nat. Rev. Mol. Cell Biol. 4, 95–104 (2003).
Wang, L. et al. PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-κ B and caspase-1-dependent cytokine processing. J. Biol. Chem. 277, 29874–29880 (2002).
Grenier, J. M. et al. Functional screening of five PYPAF family members identifies PYPAF5 as a novel regulator of NF-κB and caspase-1. FEBS Lett. 530, 73–78 (2002).
Hoffman, H. M., Mueller, J. L., Broide, D. H., Wanderer, A. A. & Kolodner, R. D. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat. Genet. 29, 301–305 (2001). This paper represents the first description of an inflammasomopathy by using genetic mapping to identify NLRP3 mutations in patients with autoinflammatory disease.
Aganna, E. et al. Association of mutations in the NALP3/CIAS1/PYPAF1 gene with a broad phenotype including recurrent fever, cold sensitivity, sensorineural deafness, and AA amyloidosis. Arthritis Rheum. 46, 2445–2452 (2002).
Aksentijevich, I. et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 46, 3340–3348 (2002).
Agostini, L. et al. NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 20, 319–325 (2004).
Gao, L. A. et al. Prokaryotic innate immunity through pattern recognition of conserved viral proteins. Science 377, eabm4096 (2022).
Zhao, Y. & Shao, F. Diverse mechanisms for inflammasome sensing of cytosolic bacteria and bacterial virulence. Curr. Opin. Microbiol. 29, 37–42 (2016).
Sharma, B. R. & Kanneganti, T. D. NLRP3 inflammasome in cancer and metabolic diseases. Nat. Immunol. 22, 550–559 (2021).
Cressman, D. E., Chin, K. C., Taxman, D. J. & Ting, J. P. A defect in the nuclear translocation of CIITA causes a form of type II bare lymphocyte syndrome. Immunity 10, 163–171 (1999).
Steimle, V., Siegrist, C. A., Mottet, A., Lisowska-Grospierre, B. & Mach, B. Regulation of MHC class II expression by interferon-γ mediated by the transactivator gene CIITA. Science 265, 106–109 (1994).
Chang, C. H., Fontes, J. D., Peterlin, M. & Flavell, R. A. Class II transactivator (CIITA) is sufficient for the inducible expression of major histocompatibility complex class II genes. J. Exp. Med. 180, 1367–1374 (1994).
Harton, J. A., Cressman, D. E., Chin, K. C., Der, C. J. & Ting, J. P. GTP binding by class II transactivator: role in nuclear import. Science 285, 1402–1405 (1999).
Masternak, K. et al. CIITA is a transcriptional coactivator that is recruited to MHC class II promoters by multiple synergistic interactions with an enhanceosome complex. Genes Dev. 14, 1156–1166 (2000).
Choi, N. M., Majumder, P. & Boss, J. M. Regulation of major histocompatibility complex class II genes. Curr. Opin. Immunol. 23, 81–87 (2011).
Armstrong, T. D., Clements, V. K., Martin, B. K., Ting, J. P. & Ostrand-Rosenberg, S. Major histocompatibility complex class II-transfected tumor cells present endogenous antigen and are potent inducers of tumor-specific immunity. Proc. Natl Acad. Sci. USA 94, 6886–6891 (1997).
Yazawa, T. et al. Lack of class II transactivator causes severe deficiency of HLA-DR expression in small cell lung cancer. J. Pathol. 187, 191–199 (1999).
Accolla, R. S., Ramia, E., Tedeschi, A. & Forlani, G. CIITA-driven MHC class II expressing tumor cells as antigen presenting cell performers: toward the construction of an optimal anti-tumor vaccine. Front. Immunol. 10, 1806 (2019).
Steidl, C. et al. MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nature 471, 377–381 (2011).
Meissner, T. B. et al. NLR family member NLRC5 is a transcriptional regulator of MHC class I genes. Proc. Natl Acad. Sci. USA 107, 13794–13799 (2010).
Robbins, G. R. et al. Regulation of class I major histocompatibility complex (MHC) by nucleotide-binding domain, leucine-rich repeat-containing (NLR) proteins. J. Biol. Chem. 287, 24294–24303 (2012).
Biswas, A., Meissner, T. B., Kawai, T. & Kobayashi, K. S. Cutting edge: impaired MHC class I expression in mice deficient for Nlrc5/class I transactivator. J. Immunol. 189, 516–520 (2012).
Staehli, F. et al. NLRC5 deficiency selectively impairs MHC class I- dependent lymphocyte killing by cytotoxic T cells. J. Immunol. 188, 3820–3828 (2012).
Yoshihama, S. et al. NLRC5/MHC class I transactivator is a target for immune evasion in cancer. Proc. Natl Acad. Sci. USA 113, 5999–6004 (2016).
Cui, J. et al. NLRC5 negatively regulates the NF-κB and type I interferon signaling pathways. Cell 141, 483–496 (2010).
Benko, S., Kovacs, E. G., Hezel, F. & Kufer, T. A. NLRC5 functions beyond MHC I regulation-what do we know so far? Front. Immunol. 8, 150 (2017).
Booshehri, L. M. & Hoffman, H. M. CAPS and NLRP3. J. Clin. Immunol. 39, 277–286 (2019).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
Kayagaki, N. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015). This paper, together with Shi et al. (2015), identifies GSDMD cleavage as a central event in pyroptosis.
Sborgi, L. et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 35, 1766–1778 (2016).
Ding, J. et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116 (2016).
Liu, X. et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158 (2016).
Aglietti, R. A. et al. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc. Natl Acad. Sci. USA 113, 7858–7863 (2016).
Feldmann, J. et al. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am. J. Hum. Genet. 71, 198–203 (2002).
Swanson, K. V., Deng, M. & Ting, J. P. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 19, 477–489 (2019).
Bauernfeind, F. G. et al. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787–791 (2009).
Franchi, L. et al. Calcium-independent phospholipase A2β is dispensable in inflammasome activation and its inhibition by bromoenol lactone. J. Innate Immun. 1, 607–617 (2009).
Xing, Y. et al. Cutting edge: TRAF6 mediates TLR/IL-1R signaling-induced nontranscriptional priming of the NLRP3 inflammasome. J. Immunol. 199, 1561–1566 (2017).
Song, N. et al. NLRP3 phosphorylation is an essential priming event for inflammasome activation. Mol. Cell 68, 185–197.e6 (2017).
Juliana, C. et al. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J. Biol. Chem. 287, 36617–36622 (2012).
Py, B. F., Kim, M. S., Vakifahmetoglu-Norberg, H. & Yuan, J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol. Cell 49, 331–338 (2013).
Song, H. et al. The E3 ubiquitin ligase TRIM31 attenuates NLRP3 inflammasome activation by promoting proteasomal degradation of NLRP3. Nat. Commun. 7, 13727 (2016).
Barry, R. et al. SUMO-mediated regulation of NLRP3 modulates inflammasome activity. Nat. Commun. 9, 3001 (2018).
Lu, A. et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 156, 1193–1206 (2014).
Cai, X. et al. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell 156, 1207–1222 (2014).
Boucher, D. et al. Caspase-1 self-cleavage is an intrinsic mechanism to terminate inflammasome activity. J. Exp. Med. 215, 827–840 (2018).
Schmid-Burgk, J. L. et al. A genome-wide CRISPR (clustered regularly interspaced short palindromic repeats) screen identifies NEK7 as an essential component of NLRP3 inflammasome activation. J. Biol. Chem. 291, 103–109 (2016).
He, Y., Zeng, M. Y., Yang, D., Motro, B. & Nuñez, G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 530, 354–357 (2016).
Shi, H. et al. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat. Immunol. 17, 250–258 (2016). This paper, along with Schmid-Burgk et al. (2016) and He et al. (2016), identifies NEK7 as a protein that is a critical component of the NLRP3 inflammasome activation pathway.
Sharif, H. et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature 570, 338–343 (2019).
Kayagaki, N. et al. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 (2011).
Broz, P. et al. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 490, 288–291 (2012).
Rathinam, V. A. et al. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell 150, 606–619 (2012).
Hagar, J. A., Powell, D. A., Aachoui, Y., Ernst, R. K. & Miao, E. A. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341, 1250–1253 (2013).
Shi, J. et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192 (2014).
Ganesan, S. et al. Caspase-8 modulates dectin-1 and complement receptor 3-driven IL-1β production in response to β-glucans and the fungal pathogen, Candida albicans. J. Immunol. 193, 2519–2530 (2014).
Gurung, P. et al. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J. Immunol. 192, 1835–1846 (2014).
Allam, R. et al. Mitochondrial apoptosis is dispensable for NLRP3 inflammasome activation but non-apoptotic caspase-8 is required for inflammasome priming. EMBO Rep. 15, 982–990 (2014).
Gringhuis, S. I. et al. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1β via a noncanonical caspase-8 inflammasome. Nat. Immunol. 13, 246–254 (2012).
Broz, P. et al. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J. Exp. Med. 207, 1745–1755 (2010).
Man, S. M. et al. Inflammasome activation causes dual recruitment of NLRC4 and NLRP3 to the same macromolecular complex. Proc. Natl Acad. Sci. USA 111, 7403–7408 (2014).
Freeman, L. et al. NLR members NLRC4 and NLRP3 mediate sterile inflammasome activation in microglia and astrocytes. J. Exp. Med. 214, 1351–1370 (2017).
Kalantari, P. et al. Dual engagement of the NLRP3 and AIM2 inflammasomes by Plasmodium-derived hemozoin and DNA during malaria. Cell Rep. 6, 196–210 (2014).
Karki, R. et al. Concerted activation of the AIM2 and NLRP3 inflammasomes orchestrates host protection against Aspergillus infection. Cell Host Microbe 17, 357–368 (2015).
Swanson, K. V. et al. A noncanonical function of cGAMP in inflammasome priming and activation. J. Exp. Med. 214, 3611–3626 (2017).
Sanman, L. E. et al. Disruption of glycolytic flux is a signal for inflammasome signaling and pyroptotic cell death. eLife 5, e13663 (2016).
Duewell, P. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010).
Wen, H. et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 12, 408–415 (2011).
Zhou, R., Yazdi, A. S., Menu, P. & Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 (2011).
Shimada, K. et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36, 401–414 (2012).
Kanneganti, T. D. et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 440, 233–236 (2006).
Subramanian, N., Natarajan, K., Clatworthy, M. R., Wang, Z. & Germain, R. N. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell 153, 348–361 (2013).
Park, S. et al. The mitochondrial antiviral protein MAVS associates with NLRP3 and regulates its inflammasome activity. J. Immunol. 191, 4358–4366 (2013).
Franchi, L. et al. Cytosolic double-stranded RNA activates the NLRP3 inflammasome via MAVS-induced membrane permeabilization and K+ efflux. J. Immunol. 193, 4214–4222 (2014).
Munoz-Planillo, R. et al. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38, 1142–1153 (2013).
Wolf, A. J. et al. Hexokinase is an innate immune receptor for the detection of bacterial peptidoglycan. Cell 166, 624–636 (2016).
Coll, R. C. et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 21, 248–255 (2015). This paper links the anti-inflammatory activity of the small-molecule drug MCC950 with specific inhibition of the NLRP3 inflammasome.
Coll, R. C., Schroder, K. & Pelegrin, P. NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol. Sci. 43, 653–668 (2022).
Bertin, J. & DiStefano, P. S. The PYRIN domain: a novel motif found in apoptosis and inflammation proteins. Cell Death Differ. 7, 1273–1274 (2000).
Chu, Z. L. et al. A novel enhancer of the Apaf1 apoptosome involved in cytochrome c-dependent caspase activation and apoptosis. J. Biol. Chem. 276, 9239–9245 (2001).
Hlaing, T. et al. Molecular cloning and characterization of DEFCAP-L and -S, two isoforms of a novel member of the mammalian Ced-4 family of apoptosis proteins. J. Biol. Chem. 276, 9230–9238 (2001).
Fenini, G., Karakaya, T., Hennig, P., Di Filippo, M. & Beer, H. D. The NLRP1 inflammasome in human skin and beyond. Int. J. Mol. Sci. 21, 4788 (2020).
Jin, Y. et al. NALP1 in vitiligo-associated multiple autoimmune disease. N. Engl. J. Med. 356, 1216–1225 (2007).
Levandowski, C. B. et al. NLRP1 haplotypes associated with vitiligo and autoimmunity increase interleukin-1β processing via the NLRP1 inflammasome. Proc. Natl Acad. Sci. USA 110, 2952–2956 (2013).
Ekman, A. K., Verma, D., Fredrikson, M., Bivik, C. & Enerback, C. Genetic variations of NLRP1: susceptibility in psoriasis. Br. J. Dermatol. 171, 1517–1520 (2014).
Zhong, F. L. et al. Germline NLRP1 mutations cause skin inflammatory and cancer susceptibility syndromes via inflammasome activation. Cell 167, 187–202.e17 (2016).
Grandemange, S. et al. A new autoinflammatory and autoimmune syndrome associated with NLRP1 mutations: NAIAD (NLRP1-associated autoinflammation with arthritis and dyskeratosis). Ann. Rheum. Dis. 76, 1191–1198 (2017).
Ozretic, P. et al. Association of NLRP1 coding polymorphism with lung function and serum IL-1β concentration in patients diagnosed with chronic obstructive pulmonary disease (COPD). Genes 10, 783 (2019).
Drutman, S. B. et al. Homozygous NLRP1 gain-of-function mutation in siblings with a syndromic form of recurrent respiratory papillomatosis. Proc. Natl Acad. Sci. USA 116, 19055–19063 (2019).
Sastalla, I. et al. Transcriptional analysis of the three Nlrp1 paralogs in mice. BMC Genomics 14, 188 (2013).
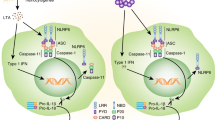
Zhong, F. L. et al. Human DPP9 represses NLRP1 inflammasome and protects against autoinflammatory diseases via both peptidase activity and FIIND domain binding. J. Biol. Chem. 293, 18864–18878 (2018). This paper uses a proteomics screen to identify DPP9 as an NLRP1-interacting protein that represses inflammasome activation by binding FIIND and maintaining NLRP1 in an inactive state.
Chavarria-Smith, J., Mitchell, P. S., Ho, A. M., Daugherty, M. D. & Vance, R. E. Functional and evolutionary analyses identify proteolysis as a general mechanism for NLRP1 inflammasome activation. PLoS Pathog. 12, e1006052 (2016).
Finger, J. N. et al. Autolytic proteolysis within the function to find domain (FIIND) is required for NLRP1 inflammasome activity. J. Biol. Chem. 287, 25030–25037 (2012).
Frew, B. C., Joag, V. R. & Mogridge, J. Proteolytic processing of Nlrp1b is required for inflammasome activity. PLoS Pathog. 8, e1002659 (2012).
Boyden, E. D. & Dietrich, W. F. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat. Genet. 38, 240–244 (2006).
Newman, Z. L. et al. Susceptibility to anthrax lethal toxin-induced rat death is controlled by a single chromosome 10 locus that includes rNlrp1. PLoS Pathog. 6, e1000906 (2010).
Levinsohn, J. L. et al. Anthrax lethal factor cleavage of Nlrp1 is required for activation of the inflammasome. PLoS Pathog. 8, e1002638 (2012).
Hellmich, K. A. et al. Anthrax lethal factor cleaves mouse nlrp1b in both toxin-sensitive and toxin-resistant macrophages. PLoS ONE 7, e49741 (2012).
Chavarria-Smith, J. & Vance, R. E. Direct proteolytic cleavage of NLRP1B is necessary and sufficient for inflammasome activation by anthrax lethal factor. PLoS Pathog. 9, e1003452 (2013).
Chui, A. J. et al. N-terminal degradation activates the NLRP1B inflammasome. Science 364, 82–85 (2019).
Sandstrom, A. et al. Functional degradation: a mechanism of NLRP1 inflammasome activation by diverse pathogen enzymes. Science 364, eaau1330 (2019).
Xu, H. et al. The N-end rule ubiquitin ligase UBR2 mediates NLRP1B inflammasome activation by anthrax lethal toxin. EMBO J. 38, e101996 (2019).
Robinson, K. S. et al. Enteroviral 3C protease activates the human NLRP1 inflammasome in airway epithelia. Science 370, eaay2002 (2020).
de Vasconcelos, N. M. et al. DPP8/DPP9 inhibition elicits canonical Nlrp1b inflammasome hallmarks in murine macrophages. Life Sci. Alliance 2, e201900313 (2019).
Okondo, M. C. et al. Inhibition of Dpp8/9 activates the Nlrp1b inflammasome. Cell Chem. Biol. 25, 262–267.e5 (2018).
Gai, K. et al. DPP8/9 inhibitors are universal activators of functional NLRP1 alleles. Cell Death Dis. 10, 587 (2019).
D’Osualdo, A. et al. CARD8 and NLRP1 undergo autoproteolytic processing through a ZU5-like domain. PLoS ONE 6, e27396 (2011).
Hollingsworth, L. R. et al. DPP9 sequesters the C terminus of NLRP1 to repress inflammasome activation. Nature 592, 778–783 (2021).
Huang, M. et al. Structural and biochemical mechanisms of NLRP1 inhibition by DPP9. Nature 592, 773–777 (2021).
Neiman-Zenevich, J., Stuart, S., Abdel-Nour, M., Girardin, S. E. & Mogridge, J. Listeria monocytogenes and Shigella flexneri activate the NLRP1B inflammasome. Infect. Immun. 85, e00338-17 (2017).
Ewald, S. E., Chavarria-Smith, J. & Boothroyd, J. C. NLRP1 is an inflammasome sensor for Toxoplasma gondii. Infect. Immun. 82, 460–468 (2014).
Okondo, M. C. et al. DPP8 and DPP9 inhibition induces pro-caspase-1-dependent monocyte and macrophage pyroptosis. Nat. Chem. Biol. 13, 46–53 (2017).
Gorfu, G. et al. Dual role for inflammasome sensors NLRP1 and NLRP3 in murine resistance to Toxoplasma gondii. mBio 5, e01117-13 (2014).
Bauernfried, S., Scherr, M. J., Pichlmair, A., Duderstadt, K. E. & Hornung, V. Human NLRP1 is a sensor for double-stranded RNA. Science 371, eabd0811 (2021).
Liao, K. C. & Mogridge, J. Activation of the Nlrp1b inflammasome by reduction of cytosolic ATP. Infect. Immun. 81, 570–579 (2013).
Neiman-Zenevich, J., Liao, K. C. & Mogridge, J. Distinct regions of NLRP1B are required to respond to anthrax lethal toxin and metabolic inhibition. Infect. Immun. 82, 3697–3703 (2014).
Ball, D. P. et al. Oxidized thioredoxin-1 restrains the NLRP1 inflammasome. Sci. Immunol. 7, eabm7200 (2022).
Jia, J., Zhang, X., Xu, G., Zeng, X. & Li, L. Thioredoxin-1 inhibits amyloid-β25-35-induced activation of NLRP1/caspase-1/GSDMD pyroptotic pathway in PC12 cells. Mol. Biol. Rep. 49, 3445–3452 (2022).
Robinson, K. S. et al. ZAKα-driven ribotoxic stress response activates the human NLRP1 inflammasome. Science 377, 328–335 (2022).
Mariathasan, S. et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430, 213–218 (2004).
Miao, E. A. et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nat. Immunol. 7, 569–575 (2006).
Miao, E. A. et al. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc. Natl Acad. Sci. USA 107, 3076–3080 (2010).
Zhao, Y. et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477, 596–600 (2011). This paper discovers NAIPs as the PAMP sensors that recognize bacterial flagellin and rod proteins, resulting in interaction with NLRC4 and inflammasome activation.
Rayamajhi, M., Zak, D. E., Chavarria-Smith, J., Vance, R. E. & Miao, E. A. Cutting edge: mouse NAIP1 detects the type III secretion system needle protein. J. Immunol. 191, 3986–3989 (2013).
Yang, J., Zhao, Y., Shi, J. & Shao, F. Human NAIP and mouse NAIP1 recognize bacterial type III secretion needle protein for inflammasome activation. Proc. Natl Acad. Sci. USA 110, 14408–14413 (2013).
Amer, A. et al. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J. Biol. Chem. 281, 35217–35223 (2006).
Franchi, L. et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in salmonella-infected macrophages. Nat. Immunol. 7, 576–582 (2006).
Romberg, N., Vogel, T. P. & Canna, S. W. NLRC4 inflammasomopathies. Curr. Opin. Allergy Clin. Immunol. 17, 398–404 (2017).
Wang, S. B. et al. DDX17 is an essential mediator of sterile NLRC4 inflammasome activation by retrotransposon RNAs. Sci. Immunol. 6, eabi4493 (2021).
Normand, S. et al. Nod-like receptor pyrin domain-containing protein 6 (NLRP6) controls epithelial self-renewal and colorectal carcinogenesis upon injury. Proc. Natl Acad. Sci. USA 108, 9601–9606 (2011).
Elinav, E. et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145, 745–757 (2011).
Mukherjee, S. et al. Deubiquitination of NLRP6 inflammasome by Cyld critically regulates intestinal inflammation. Nat. Immunol. 21, 626–635 (2020).
Chen, G. Y., Liu, M., Wang, F., Bertin, J. & Nuñez, G. A functional role for Nlrp6 in intestinal inflammation and tumorigenesis. J. Immunol. 186, 7187–7194 (2011).
Chiang, H. Y. et al. IL-22 initiates an IL-18-dependent epithelial response circuit to enforce intestinal host defence. Nat. Commun. 13, 874 (2022).
Wlodarska, M. et al. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell 156, 1045–1059 (2014).
Mamantopoulos, M. et al. Nlrp6- and ASC-dependent inflammasomes do not shape the commensal gut microbiota composition. Immunity 47, 339–348.e4 (2017).
Lemire, P. et al. The NLR protein NLRP6 does not impact gut microbiota composition. Cell Rep. 21, 3653–3661 (2017).
Henao-Mejia, J. et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482, 179–185 (2012).
Ji, X. et al. NLRP6 exerts a protective role via NF-kB with involvement of CCL20 in a mouse model of alcoholic hepatitis. Biochem. Biophys. Res. Commun. 528, 485–492 (2020).
Schneider, K. M. et al. Imbalanced gut microbiota fuels hepatocellular carcinoma development by shaping the hepatic inflammatory microenvironment. Nat. Commun. 13, 3964 (2022).
Liu, Z. et al. Intestinal Candida albicans promotes hepatocarcinogenesis by up-regulating NLRP6. Front. Microbiol. 13, 812771 (2022).
Levy, M. et al. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell 163, 1428–1443 (2015).
Hara, H. et al. The NLRP6 inflammasome recognizes lipoteichoic acid and regulates gram-positive pathogen infection. Cell 175, 1651–1664.e14 (2018).
Wang, P. et al. Nlrp6 regulates intestinal antiviral innate immunity. Science 350, 826–830 (2015).
Shen, C. et al. Phase separation drives RNA virus-induced activation of the NLRP6 inflammasome. Cell 184, 5759–5774.e20 (2021).
Anand, P. K. et al. NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature 488, 389–393 (2012).
Ghimire, L. et al. NLRP6 negatively regulates pulmonary host defense in Gram-positive bacterial infection through modulating neutrophil recruitment and function. PLoS Pathog. 14, e1007308 (2018).
Lu, W. L. et al. NLRP6 suppresses the inflammatory response of human periodontal ligament cells by inhibiting NF-κB and ERK signal pathways. Int. Endod. J. 52, 999–1009 (2019).
Caruso, R., Warner, N., Inohara, N. & Nuñez, G. NOD1 and NOD2: signaling, host defense, and inflammatory disease. Immunity 41, 898–908 (2014).
Trindade, B. C. & Chen, G. Y. NOD1 and NOD2 in inflammatory and infectious diseases. Immunol. Rev. 297, 139–161 (2020).
Stafford, C. A. et al. Phosphorylation of muramyl peptides by NAGK is required for NOD2 activation. Nature 609, 590–596 (2022).
Inohara, N. et al. An induced proximity model for NF-κB activation in the Nod1/RICK and RIP signaling pathways. J. Biol. Chem. 275, 27823–27831 (2000).
da Silva Correia, J., Miranda, Y., Leonard, N., Hsu, J. & Ulevitch, R. J. Regulation of Nod1-mediated signaling pathways. Cell Death Differ. 14, 830–839 (2007).
Miceli-Richard, C. et al. CARD15 mutations in Blau syndrome. Nat. Genet. 29, 19–20 (2001).
Maekawa, S., Ohto, U., Shibata, T., Miyake, K. & Shimizu, T. Crystal structure of NOD2 and its implications in human disease. Nat. Commun. 7, 11813 (2016).
Irving, A. T. et al. The immune receptor NOD1 and kinase RIP2 interact with bacterial peptidoglycan on early endosomes to promote autophagy and inflammatory signaling. Cell Host Microbe 15, 623–635 (2014).
Nakamura, N. et al. Endosomes are specialized platforms for bacterial sensing and NOD2 signalling. Nature 509, 240–244 (2014).
Lu, Y. et al. Palmitoylation of NOD1 and NOD2 is required for bacterial sensing. Science 366, 460–467 (2019).
Moore, C. B. et al. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature 451, 573–577 (2008). This paper identifies NLRX1 as an NLR sensor that localizes to mitochondria and regulates antiviral immunity.
Tattoli, I. et al. NLRX1 is a mitochondrial NOD-like receptor that amplifies NF-κB and JNK pathways by inducing reactive oxygen species production. EMBO Rep. 9, 293–300 (2008).
Hong, M., Yoon, S. I. & Wilson, I. A. Structure and functional characterization of the RNA-binding element of the NLRX1 innate immune modulator. Immunity 36, 337–347 (2012).
Lu, P. et al. Modeling-enabled characterization of novel NLRX1 ligands. PLoS ONE 10, e0145420 (2015).
Li, X. et al. Viral DNA binding to NLRC3, an inhibitory nucleic acid sensor, unleashes STING, a cyclic dinucleotide receptor that activates type I interferon. Immunity 50, 591–599.e6 (2019).
Arnoult, D. et al. An N-terminal addressing sequence targets NLRX1 to the mitochondrial matrix. J. Cell Sci. 122, 3161–3168 (2009).
Fekete, T., Bencze, D., Biro, E., Benko, S. & Pazmandi, K. Focusing on the cell type specific regulatory actions of NLRX1. Int. J. Mol. Sci. 22, 1316 (2021).
Allen, I. C. et al. NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I-MAVS and TRAF6-NF-κB signaling pathways. Immunity 34, 854–865 (2011).
Guo, H. et al. NLRX1 sequesters STING to negatively regulate the interferon response, thereby facilitating the replication of HIV-1 and DNA viruses. Cell Host Microbe 19, 515–528 (2016).
Xia, X. et al. NLRX1 negatively regulates TLR-induced NF-κB signaling by targeting TRAF6 and IKK. Immunity 34, 843–853 (2011).
Koblansky, A. A. et al. The innate immune receptor NLRX1 functions as a tumor suppressor by reducing colon tumorigenesis and key tumor-promoting signals. Cell Rep. 14, 2562–2575 (2016).
Coutermarsh-Ott, S. et al. NLRX1 suppresses tumorigenesis and attenuates histiocytic sarcoma through the negative regulation of NF-κB signaling. Oncotarget 7, 33096–33110 (2016).
Feng, H. et al. NLRX1 promotes immediate IRF1-directed antiviral responses by limiting dsRNA-activated translational inhibition mediated by PKR. Nat. Immunol. 18, 1299–1309 (2017).
Lei, Y. et al. The mitochondrial proteins NLRX1 and TUFM form a complex that regulates type I interferon and autophagy. Immunity 36, 933–946 (2012).
Luo, X. et al. HPV16 drives cancer immune escape via NLRX1-mediated degradation of STING. J. Clin. Invest. 130, 1635–1652 (2020).
Lei, Y. et al. EGFR-targeted mAb therapy modulates autophagy in head and neck squamous cell carcinoma through NLRX1-TUFM protein complex. Oncogene 35, 4698–4707 (2016).
Huang, J. H. et al. NLRX1 facilitates Histoplasma capsulatum-induced LC3-associated phagocytosis for cytokine production in macrophages. Front. Immunol. 9, 2761 (2018).
Zhang, Y. et al. Listeria hijacks host mitophagy through a novel mitophagy receptor to evade killing. Nat. Immunol. 20, 433–446 (2019).
Singh, K. et al. NLRX1 regulates TNF-α-induced mitochondria-lysosomal crosstalk to maintain the invasive and metastatic potential of breast cancer cells. Biochim. Biophys. Acta Mol. Basis Dis. 1865, 1460–1476 (2019).
Li, S., Zhou, Y., Gu, X., Zhang, X. & Jia, Z. NLRX1/FUNDC1/NIPSNAP1-2 axis regulates mitophagy and alleviates intestinal ischaemia/reperfusion injury. Cell Prolif. 54, e12986 (2021).
Peng, J. et al. Morphine-induced microglial immunosuppression via activation of insufficient mitophagy regulated by NLRX1. J. Neuroinflammation 19, 87 (2022).
Aikawa, C. et al. NLRX1 negatively regulates group A streptococcus invasion and autophagy induction by interacting with the beclin 1-UVRAG complex. Front. Cell Infect. Microbiol. 8, 403 (2018).
Leber, A. et al. Activation of NLRX1 by NX-13 alleviates inflammatory bowel disease through immunometabolic mechanisms in CD4+ T cells. J. Immunol. 203, 3407–3415 (2019).
Conti, B. J. et al. CATERPILLER 16.2 (CLR16.2), a novel NBD/LRR family member that negatively regulates T cell function. J. Biol. Chem. 280, 18375–18385 (2005).
Uchimura, T. et al. The innate immune sensor NLRC3 acts as a rheostat that fine-tunes T cell responses in infection and autoimmunity. Immunity 49, 1049–1061.e6 (2018).
Hu, S. et al. NLRC3 negatively regulates CD4+ T cells and impacts protective immunity during Mycobacterium tuberculosis infection. PLoS Pathog. 14, e1007266 (2018).
Schneider, M. et al. The innate immune sensor NLRC3 attenuates toll-like receptor signaling via modification of the signaling adaptor TRAF6 and transcription factor NF-κB. Nat. Immunol. 13, 823–831 (2012).
Zhang, L. et al. NLRC3, a member of the NLR family of proteins, is a negative regulator of innate immune signaling induced by the DNA sensor STING. Immunity 40, 329–341 (2014).
Tocker, A. M. et al. The Scaffolding protein IQGAP1 interacts with NLRC3 and inhibits type I IFN production. J. Immunol. 199, 2896–2909 (2017).
Wang, C. et al. NLRC3 high expression represents a novel predictor for positive overall survival correlated with CCL5 and CXCL9 in HCC patients. Front. Oncol. 12, 815326 (2022).
Liu, R. et al. Expression profile of innate immune receptors, NLRs and AIM2, in human colorectal cancer: correlation with cancer stages and inflammasome components. Oncotarget 6, 33456–33469 (2015).
Karki, R. et al. NLRC3 is an inhibitory sensor of PI3K-mTOR pathways in cancer. Nature 540, 583–587 (2016).
Williams, K. L., Taxman, D. J., Linhoff, M. W., Reed, W. & Ting, J. P. Cutting edge: Monarch-1: a pyrin/nucleotide-binding domain/leucine-rich repeat protein that controls classical and nonclassical MHC class I genes. J. Immunol. 170, 5354–5358 (2003).
Williams, K. L. et al. The CATERPILLER protein monarch-1 is an antagonist of Toll-like receptor-, tumor necrosis factor α-, and Mycobacterium tuberculosis-induced pro-inflammatory signals. J. Biol. Chem. 280, 39914–39924 (2005).
Lich, J. D. et al. Monarch-1 suppresses non-canonical NF-κB activation and p52-dependent chemokine expression in monocytes. J. Immunol. 178, 1256–1260 (2007).
Vladimer, G. I. et al. The NLRP12 inflammasome recognizes Yersinia pestis. Immunity 37, 96–107 (2012).
Ataide, M. A. et al. Malaria-induced NLRP12/NLRP3-dependent caspase-1 activation mediates inflammation and hypersensitivity to bacterial superinfection. PLoS Pathog. 10, e1003885 (2014).
Allen, I. C. et al. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-κB signaling. Immunity 36, 742–754 (2012).
Zaki, M. H. et al. The NOD-like receptor NLRP12 attenuates colon inflammation and tumorigenesis. Cancer Cell 20, 649–660 (2011).
Chen, L. et al. NLRP12 attenuates colon inflammation by maintaining colonic microbial diversity and promoting protective commensal bacterial growth. Nat. Immunol. 18, 541–551 (2017).
Sharma, N. et al. Differential expression profile of NLRs and AIM2 in glioma and implications for NLRP12 in glioblastoma. Sci. Rep. 9, 8480 (2019).
Zamoshnikova, A. et al. NLRP12 is a neutrophil-specific, negative regulator of in vitro cell migration but does not modulate LPS- or infection-induced NF-κB or ERK signalling. Immunobiology 221, 341–346 (2016).
Jeru, I. et al. Mutations in NALP12 cause hereditary periodic fever syndromes. Proc. Natl Acad. Sci. USA 105, 1614–1619 (2008).
Jeru, I. et al. Role of interleukin-1β in NLRP12-associated autoinflammatory disorders and resistance to anti-interleukin-1 therapy. Arthritis Rheum. 63, 2142–2148 (2011).
Wang, Y. et al. PYNOD, a novel Apaf-1/CED4-like protein is an inhibitor of ASC and caspase-1. Int. Immunol. 16, 777–786 (2004).
Imamura, R. et al. Anti-inflammatory activity of PYNOD and its mechanism in humans and mice. J. Immunol. 184, 5874–5884 (2010).
Eisenbarth, S. C. et al. NLRP10 is a NOD-like receptor essential to initiate adaptive immunity by dendritic cells. Nature 484, 510–513 (2012).
Krishnaswamy, J. K. et al. Coincidental loss of DOCK8 function in NLRP10-deficient and C3H/HeJ mice results in defective dendritic cell migration. Proc. Natl Acad. Sci. USA 112, 3056–3061 (2015).
Clay, G. M. et al. An anti-inflammatory role for NLRP10 in murine cutaneous leishmaniasis. J. Immunol. 199, 2823–2833 (2017).
Damm, A., Giebeler, N., Zamek, J., Zigrino, P. & Kufer, T. A. Epidermal NLRP10 contributes to contact hypersensitivity responses in mice. Eur. J. Immunol. 46, 1959–1969 (2016).
Nakajima, S. et al. Characterization of innate and adaptive immune responses in PYNOD-deficient mice. Immunohorizons 2, 129–141 (2018).
Zhang, P. et al. Expression analysis of the NLRP gene family suggests a role in human preimplantation development. PLoS ONE 3, e2755 (2008).
Fiorentino, L. et al. A novel PAAD-containing protein that modulates NF-κ B induction by cytokines tumor necrosis factor-α and interleukin-1β. J. Biol. Chem. 277, 35333–35340 (2002).
Cui, J. et al. NLRP4 negatively regulates type I interferon signaling by targeting the kinase TBK1 for degradation via the ubiquitin ligase DTX4. Nat. Immunol. 13, 387–395 (2012).
Eibl, C. et al. Structural and functional analysis of the NLRP4 pyrin domain. Biochemistry 51, 7330–7341 (2012).
Jounai, N. et al. NLRP4 negatively regulates autophagic processes through an association with beclin1. J. Immunol. 186, 1646–1655 (2011).
Nozawa, T., Aikawa, C., Minowa-Nozawa, A. & Nakagawa, I. The intracellular microbial sensor NLRP4 directs Rho-actin signaling to facilitate group A Streptococcus-containing autophagosome-like vacuole formation. Autophagy 13, 1841–1854 (2017).
Wu, C. et al. NLRP11 attenuates Toll-like receptor signalling by targeting TRAF6 for degradation via the ubiquitin ligase RNF19A. Nat. Commun. 8, 1977 (2017).
Khare, S. et al. An NLRP7-containing inflammasome mediates recognition of microbial lipopeptides in human macrophages. Immunity 36, 464–476 (2012).
Tian, X., Pascal, G. & Monget, P. Evolution and functional divergence of NLRP genes in mammalian reproductive systems. BMC Evol. Biol. 9, 202 (2009).
Ellwanger, K. et al. The NLR family pyrin domain-containing 11 protein contributes to the regulation of inflammatory signaling. J. Biol. Chem. 293, 2701–2710 (2018).
Qin, Y. et al. NLRP11 disrupts MAVS signalosome to inhibit type I interferon signaling and virus-induced apoptosis. EMBO Rep. 18, 2160–2171 (2017).
Kienes, I. et al. DDX3X Links NLRP11 to the regulation of type I interferon responses and NLRP3 inflammasome activation. Front. Immunol. 12, 653883 (2021).
Oshiumi, H., Sakai, K., Matsumoto, M. & Seya, T. DEAD/H BOX 3 (DDX3) helicase binds the RIG-I adaptor IPS-1 to up-regulate IFN-β-inducing potential. Eur. J. Immunol. 40, 940–948 (2010).
Gangopadhyay, A. et al. NLRP3 licenses NLRP11 for inflammasome activation in human macrophages. Nat. Immunol. 23, 892–903 (2022).
Hruz, T. et al. Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. Adv. Bioinformatics 2008, 420747 (2008).
Van Gorp, H., Kuchmiy, A., Van Hauwermeiren, F. & Lamkanfi, M. NOD-like receptors interfacing the immune and reproductive systems. FEBS J. 281, 4568–4582 (2014).
Westerveld, G. H. et al. Mutations in the testis-specific NALP14 gene in men suffering from spermatogenic failure. Hum. Reprod. 21, 3178–3184 (2006).
Abe, T. et al. Germ-cell-specific inflammasome component NLRP14 negatively regulates cytosolic nucleic acid sensing to promote fertilization. Immunity 46, 621–634 (2017).
Yin, Y. et al. A noncanonical role of NOD-like receptor NLRP14 in PGCLC differentiation and spermatogenesis. Proc. Natl Acad. Sci. USA 117, 22237–22248 (2020).
Bruey, J. M. et al. PAN1/NALP2/PYPAF2, an inducible inflammatory mediator that regulates NF-κB and caspase-1 activation in macrophages. J. Biol. Chem. 279, 51897–51907 (2004).
Fontalba, A., Gutierrez, O. & Fernandez-Luna, J. L. NLRP2, an inhibitor of the NF-κB pathway, is transcriptionally activated by NF-κB and exhibits a nonfunctional allelic variant. J. Immunol. 179, 8519–8524 (2007).
Li, C., Liu, Q. & Xie, L. Suppressing NLRP2 expression accelerates hepatic steatosis: a mechanism involving inflammation and oxidative stress. Biochem. Biophys. Res. Commun. 507, 22–29 (2018).
Tilburgs, T. et al. NLRP2 is a suppressor of NF-kB signaling and HLA-C expression in human trophoblasts. Biol. Reprod. 96, 831–842 (2017).
Li, T. et al. NLRP2 inhibits cell proliferation and migration by regulating EMT in lung adenocarcinoma cells. Cell Biol. Int. 46, 588–598 (2022).
Yang, Y. et al. NLRP2 negatively regulates antiviral immunity by interacting with TBK1. Eur. J. Immunol. 48, 1817–1825 (2018).
Peng, H. et al. Nlrp2, a maternal effect gene required for early embryonic development in the mouse. PLoS ONE 7, e30344 (2012).
Mu, J. et al. Mutations in NLRP2 and NLRP5 cause female infertility characterised by early embryonic arrest. J. Med. Genet. 56, 471–480 (2019).
Peng, H. et al. NLRP2 and FAF1 deficiency blocks early embryogenesis in the mouse. Reproduction 154, 245–251 (2017).
Meyer, E. et al. Germline mutation in NLRP2 (NALP2) in a familial imprinting disorder (Beckwith-Wiedemann syndrome. PLoS Genet. 5, e1000423 (2017).
Begemann, M. et al. Maternal variants in NLRP and other maternal effect proteins are associated with multilocus imprinting disturbance in offspring. J. Med. Genet. 55, 497–504 (2018).
Zhang, X., Lu, X., Yu, L., Gu, Y. & Qu, F. Downregulation of NLRP2 inhibits HUVEC viability by inhibiting the MAPK signaling pathway. Mol. Med. Rep. 19, 85–92 (2019).
Tong, Z. B. et al. Mater, a maternal effect gene required for early embryonic development in mice. Nat. Genet. 26, 267–268 (2000). This paper identifies Mater (now known as Nlrp5) as a maternal-effect gene necessary for embryonic development, providing the first description of a reproductive system-associated NLR.
Tong, Z. B. et al. Developmental expression and subcellular localization of mouse MATER, an oocyte-specific protein essential for early development. Endocrinology 145, 1427–1434 (2004).
Li, L., Baibakov, B. & Dean, J. A subcortical maternal complex essential for preimplantation mouse embryogenesis. Dev. Cell 15, 416–425 (2008).
Fernandes, R. et al. NLRP5 mediates mitochondrial function in mouse oocytes and embryos. Biol. Reprod. 86, 138 (2012).
Tong, Z. B., Bondy, C. A., Zhou, J. & Nelson, L. M. A human homologue of mouse Mater, a maternal effect gene essential for early embryonic development. Hum. Reprod. 17, 903–911 (2002).
Sena, P. et al. Human MATER localization in specific cell domains of oocytes and follicular cells. Reprod. Biomed. Online 18, 226–234 (2009).
Alimohammadi, M. et al. Autoimmune polyendocrine syndrome type 1 and NALP5, a parathyroid autoantigen. N. Engl. J. Med. 358, 1018–1028 (2008).
Docherty, L. E. et al. Mutations in NLRP5 are associated with reproductive wastage and multilocus imprinting disorders in humans. Nat. Commun. 6, 8086 (2015).
Kinoshita, T., Wang, Y., Hasegawa, M., Imamura, R. & Suda, T. PYPAF3, a PYRIN-containing APAF-1-like protein, is a feedback regulator of caspase-1-dependent interleukin-1β secretion. J. Biol. Chem. 280, 21720–21725 (2005).
Murdoch, S. et al. Mutations in NALP7 cause recurrent hydatidiform moles and reproductive wastage in humans. Nat. Genet. 38, 300–302 (2006).
Moglabey, Y. B. et al. Genetic mapping of a maternal locus responsible for familial hydatidiform moles. Hum. Mol. Genet. 8, 667–671 (1999).
Carriere, J., Dorfleutner, A. & Stehlik, C. NLRP7: from inflammasome regulation to human disease. Immunology 163, 363–376 (2021).
Bednash, J. S. et al. Targeting the deubiquitinase STAMBP inhibits NALP7 inflammasome activity. Nat. Commun. 8, 15203 (2017).
Li, B. et al. NLRP7 deubiquitination by USP10 promotes tumor progression and tumor-associated macrophage polarization in colorectal cancer. J. Exp. Clin. Cancer Res. 40, 126 (2021).
Mullins, B. & Chen, J. NLRP9 in innate immunity and inflammation. Immunology 162, 262–267 (2021).
Kanzaki, S. et al. Involvement of Nlrp9a/b/c in mouse preimplantation development. Reproduction 160, 181–191 (2020).
Amoushahi, M. et al. Maternally contributed Nlrp9b expressed in human and mouse ovarian follicles contributes to early murine preimplantation development. J. Assist. Reprod. Genet. 37, 1355–1365 (2020).
Zhu, S. et al. Nlrp9b inflammasome restricts rotavirus infection in intestinal epithelial cells. Nature 546, 667–670 (2017).
Marleaux, M., Anand, K., Latz, E. & Geyer, M. Crystal structure of the human NLRP9 pyrin domain suggests a distinct mode of inflammasome assembly. FEBS Lett. 594, 2383–2395 (2020).
Ha, H. J. & Park, H. H. Crystal structure of the human NLRP9 pyrin domain reveals a bent N-terminal loop that may regulate inflammasome assembly. FEBS Lett. 594, 2396–2405 (2020).
Yanling, Q. et al. Inhibition of NLRP9b attenuates acute lung injury through suppressing inflammation, apoptosis and oxidative stress in murine and cell models. Biochem. Biophys. Res. Commun. 503, 436–443 (2018).
Leipe, D. D., Koonin, E. V. & Aravind, L. STAND, a class of P-loop NTPases including animal and plant regulators of programmed cell death: multiple, complex domain architectures, unusual phyletic patterns, and evolution by horizontal gene transfer. J. Mol. Biol. 343, 1–28 (2004).
Hu, Z. et al. Crystal structure of NLRC4 reveals its autoinhibition mechanism. Science 341, 172–175 (2013). This paper describes the crystal structure of NLRC4 in the ADP-bound closed conformation, and structure-guided mutagenesis is used to analyse the interdomain interactions that contribute to the autoinhibited state.
Riedl, S. J., Li, W., Chao, Y., Schwarzenbacher, R. & Shi, Y. Structure of the apoptotic protease-activating factor 1 bound to ADP. Nature 434, 926–933 (2005).
Zhang, L. et al. Cryo-EM structure of the activated NAIP2-NLRC4 inflammasome reveals nucleated polymerization. Science 350, 404–409 (2015).
Hu, Z. et al. Structural and biochemical basis for induced self-propagation of NLRC4. Science 350, 399–404 (2015). This paper, along with Zhang et al. (2015), uses cryo-EM to solve the structure of the NAIP2–NLRC4 inflammasome, showing that activation of a single NAIP2 triggers a self-propagating cascade of NLRC4 activation.
Tenthorey, J. L. et al. The structural basis of flagellin detection by NAIP5: a strategy to limit pathogen immune evasion. Science 358, 888–893 (2017). This paper describes the cryo-EM structure of the NAIP5–NLRC4 inflammasome with bound flagellin, providing the first glimpse into how a bacterial PAMP interacts with an NLR sensor to initiate inflammasome formation.
Andreeva, L. et al. NLRP3 cages revealed by full-length mouse NLRP3 structure control pathway activation. Cell 184, 6299–6312.e22 (2021).
Hochheiser, I. V. et al. Structure of the NLRP3 decamer bound to the cytokine release inhibitor CRID3. Nature 604, 184–189 (2022).
Ohto, U. et al. Structural basis for the oligomerization-mediated regulation of NLRP3 inflammasome activation. Proc. Natl Acad. Sci. USA 119, e2121353119 (2022).
Arbore, G. et al. T helper 1 immunity requires complement-driven NLRP3 inflammasome activity in CD4+ T cells. Science 352, aad1210 (2016).
Chou, W. C., Rampanelli, E., Li, X. & Ting, J. P. Impact of intracellular innate immune receptors on immunometabolism. Cell Mol. Immunol. 19, 337–351 (2022).
Ozel, I., Akkaya, I., Oylumlu, E., Uzel, G. & Ciraci, C. Adenosine-induced NLRP11 in B lymphoblasts suppresses human CD4+ T helper cell responses. J. Immunol. Res. 2020, 1421795 (2020).
Guo, H. et al. Multi-omics analyses reveal that HIV-1 alters CD4+ T cell immunometabolism to fuel virus replication. Nat. Immunol. 22, 423–433 (2021).
Leber, A. et al. NLRX1 regulates effector and metabolic functions of CD4+ T cells. J. Immunol. 198, 2260–2268 (2017).
Chen, J. & Chen, Z. J. PtdIns4P on dispersed trans-Golgi network mediates NLRP3 inflammasome activation. Nature 564, 71–76 (2018).
Normand, S. et al. Proteasomal degradation of NOD2 by NLRP12 in monocytes promotes bacterial tolerance and colonization by enteropathogens. Nat. Commun. 9, 5338 (2018).
Swanberg, M. et al. MHC2TA is associated with differential MHC molecule expression and susceptibility to rheumatoid arthritis, multiple sclerosis and myocardial infarction. Nat. Genet. 37, 486–494 (2005).
Yao, Q. et al. A new category of autoinflammatory disease associated with NOD2 gene mutations. Arthritis Res. Ther. 13, R148 (2011).
Kabesch, M. et al. Association between polymorphisms in caspase recruitment domain containing protein 15 and allergy in two German populations. J. Allergy Clin. Immunol. 111, 813–817 (2003).
Canna, S. W. et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat. Genet. 46, 1140–1146 (2014).
Romberg, N. et al. Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nat. Genet. 46, 1135–1139 (2014).
Kitamura, A., Sasaki, Y., Abe, T., Kano, H. & Yasutomo, K. An inherited mutation in NLRC4 causes autoinflammation in human and mice. J. Exp. Med. 211, 2385–2396 (2014).
Steiner, A. et al. Recessive NLRC4-autoinflammatory disease reveals an ulcerative colitis locus. J. Clin. Immunol. 42, 325–335 (2022).
Zupin, L. et al. NLRC5 polymorphism is associated with susceptibility to chronic periodontitis. Immunobiology 222, 704–708 (2017).
Zhong, J. et al. NLRP3, NLRC4 and NLRC5 gene polymorphisms associate with susceptibility of pulmonary aspergillosis in non-neutropenic patients. J. Clin. Med. 11, 1870 (2022).
Soler, V. J. et al. Whole exome sequencing identifies a mutation for a novel form of corneal intraepithelial dyskeratosis. J. Med. Genet. 50, 246–254 (2013).
Pontillo, A., Catamo, E., Arosio, B., Mari, D. & Crovella, S. NALP1/NLRP1 genetic variants are associated with Alzheimer disease. Alzheimer Dis. Assoc. Disord. 26, 277–281 (2012).
Pontillo, A., Vendramin, A., Catamo, E., Fabris, A. & Crovella, S. The missense variation Q705K in CIAS1/NALP3/NLRP3 gene and an NLRP1 haplotype are associated with celiac disease. Am. J. Gastroenterol. 106, 539–544 (2011).
Magitta, N. F. et al. A coding polymorphism in NALP1 confers risk for autoimmune Addison’s disease and type 1 diabetes. Genes Immun. 10, 120–124 (2009).
Alkhateeb, A., Jarun, Y. & Tashtoush, R. Polymorphisms in NLRP1 gene and susceptibility to autoimmune thyroid disease. Autoimmunity 46, 215–221 (2013).
Pontillo, A. et al. Polimorphisms in inflammasome genes are involved in the predisposition to systemic lupus erythematosus. Autoimmunity 45, 271–278 (2012).
Serrano, A. et al. Evidence of association of the NLRP1 gene with giant cell arteritis. Ann. Rheum. Dis. 72, 628–630 (2013).
Witola, W. H. et al. NALP1 influences susceptibility to human congenital toxoplasmosis, proinflammatory cytokine response, and fate of Toxoplasma gondii-infected monocytic cells. Infect. Immun. 79, 756–766 (2011).
Sui, J. et al. NLRP1 gene polymorphism influences gene transcription and is a risk factor for rheumatoid arthritis in Han Chinese. Arthritis Rheum. 64, 647–654 (2012).
Nakanishi, H. et al. NLRP3 mutation and cochlear autoinflammation cause syndromic and nonsyndromic hearing loss DFNA34 responsive to anakinra therapy. Proc. Natl Acad. Sci. USA 114, E7766–E7775 (2017).
Turunen, J. A. et al. Keratoendotheliitis fugax hereditaria: a novel cryopyrin-associated periodic syndrome caused by a mutation in the nucleotide-binding domain, Leucine-rich repeat family, pyrin domain-containing 3 (NLRP3) gene. Am. J. Ophthalmol. 188, 41–50 (2018).
Basiorka, A. A. et al. The NLRP3 inflammasome functions as a driver of the myelodysplastic syndrome phenotype. Blood 128, 2960–2975 (2016).
Uh, S. T. et al. Association of genetic variants of NLRP4 with exacerbation of asthma: the effect of smoking. DNA Cell Biol. 38, 76–84 (2019).
Lin, Y. & Luo, Z. NLRP6 facilitates the interaction between TAB2/3 and TRIM38 in rheumatoid arthritis fibroblast-like synoviocytes. FEBS Lett. 591, 1141–1149 (2017).
Tanaka, N. et al. Eight novel susceptibility loci and putative causal variants in atopic dermatitis. J. Allergy Clin. Immunol. 148, 1293–1306 (2021).
Macaluso, F. et al. Polymorphisms in NACHT-LRR (NLR) genes in atopic dermatitis. Exp. Dermatol. 16, 692–698 (2007).
Zhao, Q. et al. Rare inborn errors associated with chronic hepatitis B virus infection. Hepatology 56, 1661–1670 (2012).
Wang, J. et al. Reconstitution and structure of a plant NLR resistosome conferring immunity. Science 364, eaav5870 (2019).
Travassos, L. H. et al. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat. Immunol. 11, 55–62 (2010).
Xiao, L., Magupalli, V. G. & Wu, H. Cryo-EM structures of the active NLRP3 inflammasome disk. Nature 613, 595–600 (2023).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Acknowledgements
This work is supported by NIH grants R56 AI158314, R01 AI158314, AI029564, AI141333, DK094779, U19 AI067798 and R35 CA232109 to J.P.-Y.T.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to the article.
Corresponding authors
Ethics declarations
Competing interests
J.P-Y.T. is a co-founder of IMMvention Therapeutix. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks H. Wu and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Walker A and Walker B motifs: https://en.wikipedia.org/wiki/Walker_motifs
Glossary
- AIM2
-
An innate immune sensor that detects cytosolic double-stranded DNA, resulting in inflammasome formation. AIM2 is composed of an N-terminal pyrin domain (PYD) and a C-terminal double-stranded DNA-binding HIN domain distinguishing it from NLR inflammasome proteins.
- Anakinra
-
A short-acting human recombinant IL-1 receptor (IL-1R) antagonist that can competitively inhibit the binding of IL-1β and IL-1α to IL-1R and block IL-1 signal transduction.
- APAF1
-
A protein of the STAND class of P-loop ATPases that is central to initiating apoptosis upon mitochondrial cytochrome c release into the cytosol. In addition to the STAND ATPase module, APAF1 contains an N-terminal caspase recruitment domain (CARD) and C-terminal WD40 repeats. The formation of the apoptosome and activation of caspase 9 upon cytochrome c binding was a biochemical model that significantly influenced early NLR studies.
- ASC
-
(Also known as PYCARD and TMS1). Adaptor protein that contains a pyrin domain (PYD) and caspase recruitment domain (CARD) allowing for inflammasome recruitment of pro-caspase 1.
- Cryopyrin-induced autoinflammatory syndromes
-
(CAPS). Autoinflammatory diseases caused by gain-of-function mutations in NLRP3 (cryopyrin).
- Hydatidiform moles
-
A hydatidiform mole is a rare condition in which tissue around a fertilized egg that would normally have developed into the placenta instead develops as an abnormal mass of cells.
- Imprinting disorder
-
Diseases caused by genetic defects or epigenetic mutations affecting imprinted chromosomal regions or genes that are expressed in a parent-of-origin specific manner.
- Inflammasomopathies
-
Autoinflammatory diseases resulting from gain-of-function mutations in inflammasome-forming NLRs.
- Maternal-effect genes
-
Genes that are transcribed in the mother and influence the development of oocytes and embryos.
- MHC class II transactivator
-
(CIITA). The master transcriptional regulator of MHC class II expression.
- M2-like macrophages
-
M1 and M2 are classifications historically used to define macrophages activated in vitro as pro-inflammatory or anti-inflammatory, respectively. In vivo macrophages are highly specialized, transcriptomically dynamic and extremely heterogeneous. Therefore, the M1 or M2 classification is too simplistic to explain the true nature of in vivo macrophages, but these terms are still often used to indicate whether the macrophages in question are more pro-inflammatory or anti-inflammatory.
- NACHT domain
-
The NACHT domain is a subgroup of the STAND class of P-loop NTPases and is composed of four subdomains (NBD, HD1, WHD and HD2). This domain allows for nucleotide-binding-dependent conformational changes and oligomerization to influence diverse biological outcomes such as transcriptional activation, cytokine signalling and pyroptosis.
- NR100
-
N-terminal domain of rodent NLRP1 proteins, approximately 100 amino acids. Whereas human NLRP1 possesses an N-terminal PYD, mouse NLRP1 proteins contain this sequence of unknown function. AlphaFold predicts this region to be mostly disordered.
- STAND
-
A subgroup of the AAA+ ATPase superfamily that includes both apoptotic ATPases as well as NACHT ATPases. The model for STAND protein function involves ADP binding stabilizing a closed, inactive state, and exchange for ATP triggers a conformational change to the open, active state.
- SXY cis-elements
-
A regulatory module comprising four elements: S or W box, X1 box, X2 box, and the Y box. When bound by their cognate transcription factors, these sites allow for assembly of the MHC enhanceosome.
- Type III secretion system
-
(T3SS). A multiprotein membrane apparatus present in Gram-negative bacteria used to inject proteins into host cytosol. Components of this nanomachine trigger NAIP–NLRC4 inflammasome assembly.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chou, WC., Jha, S., Linhoff, M.W. et al. The NLR gene family: from discovery to present day. Nat Rev Immunol 23, 635–654 (2023). https://doi.org/10.1038/s41577-023-00849-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-023-00849-x
This article is cited by
-
The interaction of inflammasomes and gut microbiota: novel therapeutic insights
Cell Communication and Signaling (2024)
-
Mechanistic insights from inflammasome structures
Nature Reviews Immunology (2024)
-
Physiological and immunological barriers in the lung
Seminars in Immunopathology (2024)
-
The NLRP3 inflammasome: contributions to inflammation-related diseases
Cellular & Molecular Biology Letters (2023)
-
The predictive value of the neutrophil/platelet ratio on in-hospital adverse events and long-term prognosis in patients with coronary artery disease after percutaneous coronary intervention and its possible internal mechanism
Molecular and Cellular Biochemistry (2023)