Abstract
Previous studies have shown that under certain conditions some thiol-containing compounds can cause apoptosis in a number of different cell lines. Herein, we investigated the apoptotic pathways in HL-60 cells triggered by dithiothreitol (DTT), used as a model thiol compound, and tested the hypothesis that thiols cause apoptosis via production of hydrogen peroxide (H2O2) during thiol oxidation. The results show that, unlike H2O2, DTT does not induce apoptosis via a mitochondrial pathway. This is demonstrated by the absence of early cytochrome c release from mitochondria into the cytosol, the lack of mitochondrial membrane depolarization at early times, and the minor role of caspase 9 in DTT-induced apoptosis. The first caspase activity detectable in DTT-treated cells is caspase 3, which is increased significantly 1–2 h after the start of DTT treatment. This was shown by following the cleavage of both a natural substrate, DFF-45/ICAD, and a synthetic fluorescent substrate, z-DEVD-AFC. Cleavage of substrates of caspases 2 and 8, known as initiator caspases, does not start until 3–4 h after DTT exposure, well after caspase 3 has become active and at a time when apoptosis is in late stages, as shown by the occurrence of DNA fragmentation to oligonucleosomal-sized pieces. Although oxidizing DTT can produce H2O2, data presented here indicate that DTT-induced apoptosis is not mediated by production of H2O2 and occurs via a novel pathway that involves activation of caspase 3 at early stages, prior to activation of the common ‘initiator’ caspases 2, 8 and 9. Cell Death and Differentiation (2000) 7, 1002–1010
Similar content being viewed by others
Introduction
Thiol-containing compounds are effective radiation protectors and anti-oxidants that decrease cell injury from various oxidative stresses. In contrast to that protective effect, we and others have shown that, under certain circumstances, thiols can cause apoptosis. Our initial studies demonstrated that thiols such as dithiothreitol, cysteine, cysteamine, lipoic acid and WR-1065 cause apoptosis in HL-60 human promyelocytic leukemia cells in a fashion dependent on drug concentration and exposure time.1 Studies by others have shown that some of the same thiols, i.e., cysteine, cysteamine and WR-1065, cause apoptosis in mouse hybridoma TB8.3 cells2 and that N-acetylcysteine (NAC) causes apoptosis in mouse embryo fibroblasts, but only if the cells are transformed and express wild-type p53.3 However, little is known about the pathways involved in thiol-induced apoptosis.
Two general, major pathways for apoptosis have been identified, both requiring active caspase proteins either in the early steps (initiator caspases) or in the later stages (effector caspases) of apoptosis.4,5,6 Caspases are present in cells as inactive zymogens that are activated via action of other proteases, including caspases, into heterotetramers formed from two large and two small subunits. One of the apoptotic pathways is induced through death receptors (such as Fas/CD95, TNFα) which directly activate caspases 8/10 and subsequently trigger caspase executioners (activation of caspases 1/4/5 and then caspases 3/6/7) and cell death. In this pathway, mitochondrial signaling seems to be unnecessary but may contribute to apoptosis at a later time as an amplification mechanism.7,8,9,10,11 The second general apoptotic pathway is induced by a variety of stimuli including chemotherapeutic drugs, ionizing radiation, etc. In this case, apoptosis occurs through a mitochondria-dependent mechanism in which one of the key hallmarks is the release into the cytosol of apoptotic activating factors such as cytochrome c and AIF, Apoptosis Inducing Factor.4,5,12,13,14,15,16,17 Studies with cell free systems have shown the ability of cytosolic cytochrome c, in interaction with the Apaf-1 and dATP, to process the central initiator caspase 9, then the downstream executioners such as caspases 3, 6 and 7.18,19,20,21 In some instances the death receptor/caspase 8 pathway and the mitochondria/caspase 9 pathway seem to be separate pathways until they converge at caspase 3. However, in other instances there can be crosstalk between the caspase 8 and caspase 9 pathways, e.g., caspase 8 can activate caspase 9 via BID cleavage,8 or feedback amplification loops, e.g., caspase 3 can activate caspase 9.22 Activated caspases lead to the proteolysis of a number of cellular substrates (including PARP, DNA-PK, lamins, and DFF-45/ICAD).23 At least one endonuclease, the CAD/DFF-40 (Caspase Activated DNase), is known to be activated directly by the caspase pathway.24
Thiols are able to act as antioxidants by reacting with reactive oxygen species (ROS: hydrogen peroxide, •OH, etc.) at relatively high drug concentrations. However, thiols also can be pro-oxidants producing ROS in vitro, particularly H2O2 via copper-catalyzed thiol oxidation and •OH via the Fenton reaction.25,26 Several studies have suggested involvement of hydrogen peroxide in thiol-induced cell death in attached cell lines,1,27,28,29,30 although the role of ROS in thiol-induced apoptosis has not been investigated.
Hence, the goals of the present study are twofold. The first is to investigate the apoptotic pathway(s) triggered by thiols in HL-60 cells by assessing the involvement of specific caspases and mitochondria. Potential mitochondrial involvement is of particular interest because mitochondria have been implicated in apoptosis induction by numerous stimuli, especially oxidants.31,32 The second goal is to compare thiol-induced apoptosis with that induced by H2O2 and determine whether thiols cause apoptosis through production of H2O2. In these studies we have used the dithiol DTT as a model compound because our previous work has shown that it yields similar results to those obtained using cysteine, cysteamine, lipoic acid and WR1065, yet it is somewhat easier to use because of its slower oxidation rate.1,29 Human leukemia HL-60 cells are used in these studies because they are sensitive to a large panel of apoptotic stimuli including anticancer drugs such as etoposide and camptothecin and pro-oxidant agents such as hydrogen peroxide.33,34
Results
Apoptotic DNA fragmentation is induced by both DTT and H2O2 but on different time scales
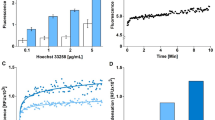
Exposure of HL-60 cells to DTT or H2O2 led to a time-dependent apoptotic cell death as determined by the DNA fragmentation assay (Figure 1A). The 2 mM DTT concentration was used in these experiments as it induces the highest apoptosis level in this cell line (Tartier et al, in preparation). Apoptotic DNA ladders are visualized on agarose gels with ethidium bromide (Figure 1B,C). DNA fragmentation was increased substantially after 2 h and was maximum at 4–5 h after treatment with H2O2 (100 μM). With DTT (2 mM), DNA fragmentation starts at 2–3 h and is maximum at 5–6 h after the start of DTT exposure. Hence, DTT-induced apoptosis is delayed 1–2 h relative to that caused by H2O2. The DNA fragmentation is caused by the reduced form of DTT as treatment with oxidized DTT does not induce DNA fragmentation (data not shown).
Apoptotic DNA fragmentation in HL-60 cells treated by DTT or H2O2. (A) HL-60 cells, at a concentration of 5×105 cells/ml, were treated with 2 mM DTT (▪) or 100 μM H2O2(○) at 37°C. The drugs were washed off after 2 h exposure and cells were reincubated at 37°C until assay at the indicated time. The percentage of DNA fragmentation was assayed as described in the Materials and Methods. Values are means±standard errors (S.E.) of four experiments. (B, C) Agarose gel electrophoresis of DNA extracted from HL-60 cells 0 to 6 h after treatment with 2 mM DTT (B) or 100 μM H2O2 (C)
DTT-treated cells show a low but prolonged production of intracellular hydrogen peroxide
DCFH-DA passively diffuses through cell membranes, where the acetate is cleaved off by intracellular esterases to form DCFH. DCFH is commonly used to detect the generation of reactive oxygen intermediates.35,36 Results shown in Figure 2A indicate that cells treated with 2 mM DTT start to show generation of ROS within 5 min of the addition of the drug, the ROS generation appears maximal at 15 min, then generation decreases to control, untreated levels by 2 h. By comparison, cells treated with H2O2 show the maximal DCF fluorescence at the earliest measurable time point, then detectable ROS decrease with time to reach control levels in about 1 h (Figure 2B). Also, although the H2O2-treated cells reach the same maximal ROS levels as menadione treated cells, the DTT-treated cells never reach that maximal level of ROS. These data suggest DTT produces ROS in a slower fashion than occurs when a bolus of H2O2 is added to cells, and are consistent with our earlier data showing that the half-life for DTT oxidation (i.e., production of H2O2) is about 1.3 h in medium29 and slightly less than an hour in the presence of cells (Held et al, unpublished).
Intracellular hydrogen peroxide measured using DCFH-DA in HL-60 cells treated with 2 mM DTT (A) or 100 μM H2O2 (B) for varying times. The positive control is cells treated with 1 mM menadione for 15 min (A). DCFH-DA was added to cells 30 min prior to analysis by flow cytometry. Result shown is typical of three replicate experiments
DTT-induced apoptotic DNA fragmentation is not prevented by removal of H2O2 using catalase or pyruvate
Catalase reacts enzymatically with H2O2 to produce water, but exogenously added catalase can not penetrate most cell types. Pyruvate reacts rapidly with H2O2 and can act both intracellularly and extracellularly.37 The data in Table 1 show that DTT-induced apoptosis is not prevented by removal of H2O2 by either catalase or pyruvate, although both agents prevent apoptotic DNA fragmentation caused by H2O2. Such a result suggests that, even though DTT is capable of generating H2O2 intracellularly (Figure 2A), the H2O2 is not responsible for the DTT-induced apoptosis.
Activation of caspase 3 is required for DTT-induced apoptosis
Processing of the so-called ‘executioner’ caspase 3 protein into its active form and subsequent cleavage of various substrates by the active caspase 3 is associated with apoptosis induced by a broad range of agents.6,7,10 Therefore, both caspase processing and caspase activity were measured in DTT-treated cells, the former by Western blot analysis of procaspase cleavage and the later by testing the ability of caspases to cleave natural or synthetic fluorescent peptide substrates.
Caspase 3 cleavage into 19- and 17-kDa products is evident after 2 h DTT-treatment (Figure 3A). The 17- and 11-kDa fragments (the latter not recognized by the antibody used) are the active subunits of caspase 3.10,15,38,39 The ability of activated caspase 3 to cleave a natural substrate, DFF-45/ICAD (Figure 3B), and the specific synthetic substrate, z-DEVD-AFC (Figure 3C), are also shown. Cleavage of both substrates was detectable 1–2 h after the start of DTT-treatment, and maximum activity with the fluorescent substrate, about 0.7 nmoles AFC cleaved/h/106 cells, was reached at 4–5 h (Figure 3C). Cleavage of the natural substrate DFF-45/ICAD is specific for caspase 3-like activity as the addition of z-DEVD-cmk, an irreversible inhibitor of caspase 3, prevents its cleavage (Figure 3B). Once cleaved, DFF-45/ICAD releases the protein DFF-40, also known as Caspase Activated DNase (CAD), which is responsible for at least part of the apoptotic DNA fragmentation,24 such as that seen in Figure 1.40,41
Caspase 3 activity after 2 mM DTT-treatment. (A) Western blot of procaspase 3 (p32) processed to its active peptides p19 and p17. (B) Western blot of DFF-45/ICAD, a natural substrate of caspase 3. The last lane corresponds to 6 h DTT treatment plus 200 μM z-DEVD-cmk, a caspase 3 inhibitor. (C) Caspase activities measured as cleavage of fluorescent peptide-AFC: z-DEVD-AFC for caspase 3 (•), z-VDVAD-AFC for caspase 2 (▪), z-LEHD-AFC for caspase 9 (X) and Ac-IETD-AFC for caspase 8 (▴). Values are means±S.E. of four experiments. T=0 is the cellular extract without DTT treatment. Note that the DTT (5 mM) included in the caspase reaction buffer is not able to activate caspases in the control samples
Caspases 2, 8, 9 and 1 play minor roles, if any, in DTT-induced apoptosis
Generally, caspase 3 must be processed by other caspases or proteases in order to be activated. Therefore, we next investigated the possible involvement in thiol-induced apoptosis of caspases 2, 8, 9 and 1/4, all of which are known to activate caspase 3 in other circumstances.6,15 The effects of irreversible cell permeable caspase inhibitors were studied first. z-DEVD-cmk, z-LEHD-fmk and Ac-YVAD-cmk, inhibitors of caspases 3, 9 and 1/4, respectively, were tested, as well as the pan-caspase inhibitor z-VAD-fmk (Figure 4). DTT-induced DNA fragmentation is decreased significantly by z-VAD-fmk (3.5-fold decrease at 50 μM; total inhibition with 100 μM – data not shown), but is decreased only partially by z-DEVD-cmk (twofold decrease at 200 μM). This is consistent with a partial decrease of PARP cleavage by z-DEVD-cmk (data not shown). On the other hand, z-DEVD-cmk prevents cleavage of DFF-45 (Figure 3B). z-VAD-fmk also completely prevents DFF-45 cleavage, but only partially prevents cleavage of PARP (data not shown). The data in Figure 4A, showing only partial protection by z-DEVD-cmk, together with the PARP cleavage observations, suggest that a protease in addition to caspase 3 is involved in the thiol-induced pathway. The inhibitors Ac-YVAD-cmk and z-LEHD-fmk fail to inhibit DNA fragmentation induced by DTT-treatment, indicating caspases 1/4 and 9 are not involved in this pathway. However, a low cleavage activity of caspase 9 toward its synthetic substrate was observed (Figure 3C). The cleavage started 4 h after treatment and reached maximum activity (about 0.1 nmoles AFC/h/106 cells) after 5 h.
Effect of caspase inhibitors on DTT- and H2O2- induced-apoptosis. HL-60 cells were pretreated with the indicated amount of caspase inhibitor for 1 h, and then the cells were treated with 2 mM DTT (A) or 100 μM H2O2 (B) for 2 h with assay 4 h after the removal of the agent. Values are means±S.E. of 3–4 experiments. Inhibitors do not have significant effects on cells by themselves (except for z-LEHD-fmk inducing 10% apoptosis)
In contrast to the significant protection against DTT-induced apoptosis afforded by z-DEVD-cmk (Figure 4A), this caspase 3 inhibitor has no effect on H2O2-induced apoptosis (Figure 4B). Furthermore, although the caspase 9 inhibitor z-LEHD-fmk has no effect on DTT-induced apoptosis, it decreases H2O2-induced apoptosis about 25% (Figure 4B). These observations suggest that, at least in part, the relative importance of different caspases varies for apoptosis induced by DTT and H2O2.
The roles of caspases 2 and 8 were assessed by observing the cleavage kinetics of their synthetic substrates (Figure 3C). Activity of both caspases increases 3–4 h after the start of DTT treatment with a maximum activity reached at 5 h (about 0.2 nmoles AFC/h/106 cells). Thus, this increase occurs later than the increase in caspase 3 activity. For caspase 8, this late activation was confirmed by Western blot, which shows that a significant cleavage of procaspase 8 is seen only after 4 and 6 h DTT-treatment (Figure 5A), well after the activation of caspase 3. The addition of z-DEVD-cmk, an inhibitor selective for caspase 3, to DTT-treated cells causes a significant decrease of procaspase 8 cleavage (Figure 5B). This finding, together with the slow and small activation of caspase 8 compared with caspase 3 (Figure 3C), suggests caspase 8 is activated downstream of caspase 3. In summary, the data suggest caspases 2, 8, 9 and 1/4 are not good candidates to be upstream of caspase 3, but they are activated later in the thiol-induced apoptotic process as part of a feedback loop to amplify the apoptosis signal. This activation occurs at a time when cells are already undergoing extensive DNA fragmentation (Figure 1A).
Processing of procaspase-8. (A) Western blot of procaspase-8 (p55) after the indicated times of DTT treatment. Caspase 8 activation is seen as the loss of the band at 55 kDa (pro-form). (B) Effect of an inhibitor of caspase 3 activation on DTT-induced processing of procaspase 8. Cells were treated with 2 mM DTT±200 μM z-DEVD-cmk for 6 h. No activation of caspase-8 is seen when caspase 3 is inhibited
DTT-induced apoptosis does not involve mitochondrial signaling
The opening of the mitochondrial permeability transition (PT) pore appears to be a common event in apoptosis induced by several stimuli triggering oxidative stress, e.g., hydrogen peroxide or t-butylhydroperoxide.32,42 It has been shown that when PT is happening, the opening of the pores and the subsequent collapse of the mitochondrial trans-membrane potential (ΔΨm) constitute an irreversible step leading to apoptosis.43,44,45 Mitochondrial cytochrome c release may also occur and can either precede the drop in ΔΨm12,13,20 or occur concomitant with the PT pore opening.46,47 With DTT treatment, there is a slight and slow decrease of the ΔΨm (Figure 6), which follows approximately the same time course as that seen for apoptotic DNA fragmentation in this cell line, starting at 3 h and reaching a maximum 6 h after treatment (Figure 1A). In contrast, the mitochondrial uncoupler mClCCP causes a rapid decrease in ΔΨm to low levels. The small decrease in ΔΨm caused by DTT may only reflect non-specific changes in mitochondria at relatively late times in the apoptotic pathway. Such an assumption is confirmed by adding cyclosporin A (a ligand of cyclophilin D that is a constituent of the PT pore known for inhibiting its opening),15,48,49 to DTT treated-cells. Cyclosporin A has no effect on the slow decrease of ΔΨm induced by DTT (Figure 6).
Measurement of mitochondrial trans-membrane potential ΔΨm. Uptake (percentage of maximum control) of the fluorochrome DiOC6(3) into HL-60 cells treated with 2 mM DTT (•) or 2 mM DTT plus 10 μM CSA (○). 100 μM mClCCP (▪) was used as a positive control. A decrease of DiOC6(3) uptake by cells is indicative of disruption of the mitochondrial trans-membrane potential ΔΨm. Values are means±S.E. of 3–6 experiments
Cytochrome c level was also analyzed by Western blot in both cytosolic and mitochondrial fractions of DTT or H2O2 treated cells. Results with DTT treatment show no significant change in cytochrome c levels in either fraction (Figure 7A), except a slight cytosolic increase of cytochrome c after 6 h. In contrast, HL-60 cells treated with H2O2 demonstrate rapid accumulation of cytochrome c in the cytosol (starting at 30 min) with a parallel decrease in the mitochondrial compartment where the cytochrome c becomes undetectable at 4–6 h treatment (Figure 7B). No cytochrome oxidase (subunit II) was detected in the cytosolic fraction, indicating there was negligible mitochondrial contamination in the preparations. As cytosolic cytochrome c is indispensible for caspase 9 activation,15,21 the late and small cytosolic increase observed here is in accordance with the late activation of caspase 9 seen in the study of cleavage of its fluorescent substrate in DTT-treated cells (Figure 3C). The data imply that mitochondrial signaling does not have a major role in DTT-induced apoptosis, but seems rather to contribute at a later time, perhaps as part of an amplification mechanism. However, the H2O2 results show that HL-60 cells activate the cytochrome c pathway to apoptosis early in response to at least some stimuli.
Cytochrome c immunoblotting. Cytochrome c level of both cytosolic and mitochondrial compartments was assessed after different exposure times of HL-60 cells to 2 mM DTT (A) or 100 μM H2O2 (B). Actin protein is used as a loading control in the cytosolic fraction. Cytochrome oxidase subunit II (COX) is used as a mitochondrial loading control and as a mitochondrial marker. Its absence in the cytosolic fraction indicates there is no mitochondrial contamination in that fraction
Discussion
Caspase proteins with long prodomains allow caspase aggregation and subsequent autocatalytic activation (such as caspase 2, 8, 10, 1/4 and 9), and are commonly believed to act in initial steps of apoptosis. Caspases with short prodomains (such as caspase 3, 6 and 7) commonly act as executioner caspases.6,7,22,50 The sequential caspase processing appears to be strongly cell type- and stimulus-dependent, although caspase 3 is generally activated late in most systems studied. Strikingly, our data with DTT treatment of HL-60 cells show caspases 2, 8 and 9, usually initiator caspases, are activated later than caspase 3. Figure 5B, which shows that a caspase 3 inhibitor can prevent procaspase 8 activation, emphasizes this result. Additionally, data presented here show that the DTT-induced apoptosis pathway is independent of mitochondrial signaling: there are no early changes in either cytosolic cytochrome c levels or the ΔΨm (Figures 6 and 7A), and the inability of a caspase 9 inhibitor to inhibit DTT-induced apoptosis (Figure 4A) indicates caspase 9 does not have a key role in this pathway. Thus caspase 3 appears to be an initiator caspase in DTT-treated HL-60 cells, with caspases 2, 8 and 9 activated late and only in a feedback loop, probably due to cleavage by the activated caspase 3. There are other literature reports describing the ability of caspase 3 to cleave, in vitro and in vivo, upstream caspases, such as caspase 9, as part of a feedback apoptosis amplification loop.15,23,51,52
Although caspase 3 seems to be an initiator rather than an executioner caspase in thiol-treated HL-60 cells, the identity of the initial event triggering procaspase 3 processing is unclear. Studies from Suzuki et al38 and Han et al39 suggested the possible involvement of a serine protease in the second stage of the procaspase 3 maturation process (cleavage of the intermediate 19/20-kDa fragment into the active 17-kDa). Such a pathway could possibly be involved here, but we have been unable to test this hypothesis as all commonly-used serine protease inhibitors we have tested to date, e.g., TPCK and TLCK, cause rapid necrosis (total cell lysis within 1 h) in HL-60 cells or have no effect on thiol-induced apoptosis at the very low doses where they do not cause cell killing (data not shown). Other approaches to test for a potential role of serine proteases in this system are being investigated. Alternatively, since the caspase 3 inhibitor is unable to inhibit DTT-induced apoptosis totally, although the pan-caspase inhibitor does (Figure 4A), other as yet unidentified caspases may be involved in DTT-induced activation of caspase 3.
A number of instances have been reported in the literature of thiol-containing compounds protecting against apoptosis induced, for example, by ionizing radiation,2,53,54 etoposide55 and TNF-α.56 In those studies, the thiols were used under controlled conditions where cell killing by the thiols was minimal. On the other hand, our earlier data,1 as well as the data reported herein, have shown clearly, that thiols can cause apoptosis in HL-60 cells. Other examples of thiol-induced apoptosis or thiol enhancement of apoptosis have been reported in the literature.2,3,57 The paper by Liu et al3 is particularly interesting. They demonstrated that NAC-induced apoptosis in mouse embryo fibroblasts (MEFs) occurred only if the cells were transformed and wild-type p53; non-transformed MEFs or transformed, p53 null MEFs did not undergo apoptosis when treated with NAC. Those data are consistent with our observations that NAC does not cause apoptosis in HL-60 cells1 that are neoplastically transformed and functionally p53 null. On the other hand, in HL-60 cells NAC is not typical of many thiols such as WR-1065, cysteamine, cysteine and lipoic acid that cause apoptosis in a fashion analogous to that shown in this study with DTT1 (Held et al, unpublished data). Clearly, the mode of thiol-induced apoptosis depends on both the specific thiol and the cell type. Hence, it is important to elucidate these various actions when thiols are used as anti-oxidants and anti-apoptosis agents.
In previous studies with DTT and other thiols, we had shown that hydrogen peroxide produced by copper-catalyzed thiol oxidation was involved in thiol-induced cell killing measured as loss of clonogenic ability in attached cell lines.1,29,30,58 Hence, the original postulate in the current work was that thiol-induced apoptosis in HL-60 cells would also be mediated by hydrogen peroxide production. Data presented in this paper show that hydrogen peroxide is effective in causing apoptosis in HL-60 cells (Figure 1), consistent with data of others,59 and that DTT can produce hydrogen peroxide in cells (Figure 2A). However, three sets of new data in this paper do not support the hypothesis that DTT-induced apoptosis is mediated by hydrogen peroxide production. First, pyruvate and catalase, which effectively remove hydrogen peroxide,35 do not protect against DTT-induced apoptosis (Table 1). Second, Figure 4 shows that the patterns of protection by caspase inhibitors are, at least in part, different for DTT- and H2O2-induced apoptosis. Third, Figure 7 shows that treating HL-60 cells with hydrogen peroxide causes rapid release of cytochrome c from mitochondria into the cytosol, although treating the cells with DTT did not cause any changes in cytochrome c. Hence, alternative, non-hydrogen peroxide mediated, mechanisms for activation of apoptosis by thiols in HL-60 cells need to be considered. One potential mechanism is a thiol-mediated reduction of critical disulfides in an enzyme(s) involved in the apoptosis pathway, e.g., a protease that cleaves caspase 3 to its active form. It is unlikely that DTT acts directly on caspase 3 because DTT is commonly included in the reaction buffer used in caspase activity assays and does not cause any activation itself (Figure 3C). Clearly, whole cells are required for the initiation of the apoptosis process by DTT. Alternatively, the thiol could be chelating zinc and thus preventing the apoptosis-inhibiting actions of zinc-containing IAP (inhibitor of apoptosis) proteins.60
In conclusion, we have demonstrated a novel pathway to apoptosis caused by thiols in HL-60 cells in which caspase 3, usually a downstream or ‘effector’ caspase, is activated early, and neither of the common upstream pathways via mitochondria/caspase 9 or death receptor/caspase 8 are involved until the late stages of apoptosis, when DNA fragmentation is well advanced. Although DTT can produce H2O2, DTT does not appear to be acting via an H2O2-mediated pathway to cause apoptosis.
Materials and Methods
Materials
HL-60 cells were obtained from ATCC (Rockville, MD, USA). DiOC6(3) and DCFH-DA were from Molecular Probes (Eugene, OR, USA). DTT, CSA, mClCCP, menadione and complete protease inhibitor cocktail were from Sigma (St. Louis, MO, USA). Kits for caspase substrates 3 and 8 were obtained from Clontech (Palo Alto, CA, USA). Substrates for caspase 9 [z-LEHD-AFC] and 2 [z-VDVAD-AFC], and inhibitors z-VAD-fmk, z-LEHD-fmk and Ac-YVAD-cmk were obtained from Calbiochem (La Jolla, CA, USA). z-DEVD-cmk was from Bachem Bioscience (King of Prussia, PA, USA).
Cell culture
Human leukemia HL-60 cells were cultured in RPMI 1640 medium supplemented with penicillin (100 μg/ml), streptomycin (100 units/ml) and 20% fetal bovine serum (FBS). The cells were maintain in log phase growth at 37°C under a humidified atmosphere of 5% CO2.
Agarose gel electrophoresis of DNA
DNA samples were prepared for agarose gel electrophoresis following the procedure of Hopcia et al.61 DNA fragments were visualized by ethidium bromide staining.
Flow cytometry analysis of cells by DCFH-DA staining
In order to determine the presence of intracellular oxidants, HL-60 cells were treated in complete medium at 37°C with 2 mM DTT or 100 μM H2O2 for various times. As a positive control, cells were treated with 1 mM menadione for 15 min. DCFH-DA (10 μM) was added 30 min prior to analysis by flow cytometry (Epics-Elite flow cytometer). A total of 10 000 events were analyzed for each sample. Data were analyzed using CellQuest software and are representative of three replicate experiments.
Western blotting for caspases and DFF-45
Control and treated cells were lysed in lysis buffer (10 mM HEPES pH 7.4, 42 mM KCl, 5 mM MgCl2, 0.1 mM EDTA, 0.1 mM EGTA, 5 mM DTT, 2 mM PMSF, 1×complete protease inhibitor cocktail) containing 0.5% CHAPS. Cellular debris were spun down at 14 000×g for 20 min, and the supernatants were used as whole cell protein extracts. Polyclonal Ab anti-caspase 3 (antiserum, Pharmingen, San Diego, CA, USA) was diluted at 1 : 2000, anti-caspase 8 (clone B9-2, Pharmingen) at 1 μg/ml and polyclonal anti-DFF-45 (C-19, Santa Cruz Biotech) at 1 : 1000. The appropriate horseradish peroxidase coupled secondary antibody was used, and bound antibodies were revealed with ECL-Plus (Amersham, Arlington, IL, USA).
Preparation of cytosolic and mitochondrial extract for cytochrome c immunoblotting
Cytosolic and mitochondrial fractions were obtained as described by Han et al.17 Thirty μg of cytosolic protein extract or 10 μg of mitochondrial protein extract mixed with loading buffer were loaded onto each lane of an 8–16% gradient SDS–polyacrylamide gel (pre-cast gels, Bio-Rad, Hercules, CA, USA), electrophoresed and then blotted to PVDF membrane. Anti-cytochrome c mouse monoclonal antibody 7H82C12 (Pharmingen) was diluted at 1 : 5000, anti-cytochrome oxidase subunit II mouse monoclonal antibody 12C4.F12 (Molecular Probes, Eugene, OR, USA) at 1 : 2000, and anti-actin goat polyclonal antibody (Santa Cruz Biotech., CA, USA) at 1 : 2000. Bound antibodies were revealed as described previously.
Caspase assay
Caspase activities were assayed by release of AFC-fluorescent compound from synthetic peptides according to the procedures of the kit manufacturer (Clontech). Approximately 1×106 cells were spun at 2000 r.p.m. for 5 min, washed in PBS, and resuspended in 50 μl of chilled cell lysis buffer. The cell lysates were centrifuged at 12 000 r.p.m. for 3 min, the supernatants were transferred to new tubes, and 50 μl of 2×reaction buffer [100 mM HEPES pH 7.2, 0.2 M NaCl, 2 mM EDTA, 0.2% CHAPS, 20% sucrose] containing 5 mM DTT was added. The reactions were initiated by addition of 50 μM substrates-AFC and incubated 1 h at 37°C. The fluorescence was read on a Perkin-Elmer LS-5 fluorometer with excitation and emission wavelengths of 400 nm and 505 nm, respectively.
DNA fragmentation assay for apoptosis
DNA fragmentation characteristic of apoptosis was measured according to the method of Sellins and Cohen,62 with modification.61 Cellular DNA was labeled by growth of cells in medium containing 0.02 μCi/ml [14C]-thymidine for approximately 24 h, followed by a ‘chase’ in fresh medium lacking radiolabel for 4 h before drug treatments. After treatment, cells at a concentration of about 5×105 cells/sample were lysed (10 mM Tris, 1 mM EDTA and 0.2% Triton X-100, pH 7.5). The cell lysates were centrifuged at 13 000×g for 10 min at 4°C, and the DNA of the separated pellet and supernatant was hydrolyzed for 20 min at 90°C by the addition of HCl to a final concentration of 1 N. The samples were mixed with scintillation cocktail (Scintiverse II, Fisher Scientific) and counted in a Packard Tri Carb 4000 series liquid scintillation counter. The percentage of fragmented DNA in each sample was calculated as the amount of DNA in the supernatant divided by the total DNA for that sample (supernatant plus pellet).
Assessment of mitochondrial potential
Mitochondrial trans-membrane potential (ΔΨm) was evaluated using DiOC6(3), a fluorochrome which incorporates into cells dependent upon their ΔΨm. Cells (at about 5×105/ml) were pre-incubated with 60 nM DiOC6(3) for 30 min at 37°C in the dark, then washed once in media and drug added. At appropriate times after drug addition, cells were washed and resuspended in PBS. The fluorescence was measured in PBS on a Perkin-Elmer LS-5 spectrofluorometer (excitation 487 nm, emission 515 nm). Control experiments were performed in the presence of 100 μM mClCCP, an uncoupling agent that abolishes the ΔΨm.
Abbreviations
- AFC:
-
7-Amino-4-trifluoromethyl coumarin
- CHAPS:
-
3-[(3-cholamidopropyl)-dimethyl ammoniol]-1-propanesulfonate
- CSA:
-
cyclosporine A
- DCFH-DA:
-
2′,7′-dichlorofluorescin diacetate
- DiOC6(3):
-
3,3′-dihexyloxacarbocyanine iodide
- DTT:
-
dithiothreitol
- H2O2:
-
hydrogen peroxide
- mClCCP:
-
carbonyl cyanide m-chlorophenyl hydrazone
- NAC:
-
N-acetylcysteine
- PARP:
-
poly(ADP-ribose) polymerase
- PMSF:
-
phenylmethyl-sulfonyl fluoride
- ROS:
-
reactive oxygen species
- TPCK:
-
N-tosyl-L-phenylalanine chloromethyl ketone
- TLCK:
-
N-tosyl-Lys-chloromethyl ketone
- ΔΨm:
-
mitochondrial trans-membrane potential
References
Held KD, Sylvester FC, Hopcia KL and Biaglow JE . 1996 Role of Fenton chemistry in thiol-induced toxicity and apoptosis. Radiat. Res. 145: 542–553
Warters RL, Roberts JC, Wilmore BH and Kelley LL . 1997 Modulation of radiation-induced apoptosis by thiolamines. Int. J. Radiat. Biol. 72: 439–448
Liu M, Pelling JC, Ju J, Chu E and Brash DE . 1998 Antioxidant action via p53-mediated apoptosis. Cancer Res. 58: 1723–1729
Ashkenazi A and Dixit VM . 1998 Death receptors: Signaling and modulation. Science 281: 1305–1308
Green DR and Reed JC . 1998 Mitochondria and apoptosis. Science 281: 1309–1312
Thornberry NA and Lazebnik Y . 1998 Caspases: Enemies within. Science 281: 1312–1316
Salvesen GS and Dixit VM . 1997 Caspases: intracellular signaling by proteolysis. Cell 91: 443–446
Li H, Zhu H, Xu C and Yuan J . 1998 Cleavage of BID by Caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94: 491–501
Vercammen D, Brouchaert G, Denecker G, Van De Craen M, Declercq W, Friers W and Vandenabeele P . 1998 Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways. J. Exp. Med. 188: 919–930
Ferrari D, Stepczynska A, Los M, Wesselborg S and Schulze-Osthoff K . 1998 Differential regulation and ATP requirement for caspase-8 and caspase-3 activation during CD95- and anticancer drug-induced apoptosis. J. Exp. Med. 188: 979–984
Juo P, Kuo CJ, Yuan J and Blemis J . 1998 Essential requirement for caspase 8/FLICE in the initiation of the Fas-induced apoptotic cascade. Curr. Biol. 8: 1001–1008
Bossy-Wetzel E, Newmeyer DD and Green DR . 1998 Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J. 17: 37–49
Stridh M, Kimland M, Jones DP, Orrenius S and Hampton MB . 1998 Cytochrome c release and caspase activation in hydrogen peroxide- and tributyltin-induced apoptosis. FEBS Lett. 429: 351–355
Marchetti P, Castedo M, Susin SA, Zamzami N, Hirsch T, Macho A, Haeffner A, Hirsch F, Geuskens M and Kroemer G . 1996 Mitochondrial permeability transition is a central coordinating event of apoptosis. J. Exp. Med. 184: 1155–1160
Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, Wang H-G, Reed JC, Nicholson DW, Alnemri ES, Green DR and Martin SJ . 1999 Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase 9-dependent manner. J. Cell Biol. 144: 281–292
Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng T-I, Jones DP and Wang X . 1997 Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science 275: 1129–1132
Han Z, Bhalla K, Pantazis P, Hendrickson EA and Wyche JH . 1999 Cif(cytochrome c efflux-inducing factor) activity is regulated by Bcl-2 and caspases and correlates with the activation of Bid. Mol. Cell. Biol. 19: 1381–1389
Liu X, Kim CC, Yang J, Jemmerson R and Wang X . 1996 Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86: 147–157
Zou H, Henzel WJ, Liu X, Lutschg A and Wang X . 1997 Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 90: 405–413
Kluck RM, Bossy-Wetzel E, Green DR and Newmeyer DD . 1997 The release of cytochrome c from mitochondria: A primary site for Bcl-2 regulation of apoptosis. Science 275: 1132–1136
Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES and Wang X . 1997 Cytochrome c and dATP-dependent formation of apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91: 479–489
Green D and Kroemer G . 1998 The central executioners of apoptosis: caspases or mitochondria? Trends Cell Biol. 8: 267–271
Cohen GM . 1997 Caspases: the executioners of apoptosis. Biochem. J. 326: 1–16
Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A and Nagata SA . 1998 A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391: 43–50
Kachur AV, Held KD, Koch CJ and Biaglow JE . 1997 Mechanism of production of hydroxyl radicals in the copper-catalyzed oxidation of dithiothreitol. Radiat. Res. 147: 409–415
Biaglow JE, Manevich Y, Uckun F and Held KD . 1997 Quantitation of hydroxyl radicals produced by radiation and copper-linked oxidation of ascorbate by 2-deoxy-D-ribose method. Free Radic. Biol. Med. 22: 1129–1138
Takagi Y, Shikita M, Terasima T and Akaboshi S . 1974 Specificity of radioprotective and cytotoxic effects of cysteamine in HeLa S3 cells: Generation of peroxide as the mechanism of paradoxical toxicity. Radiat. Res. 60: 292–301
Biaglow JE, Issels RD, Gerweck LE, Varnes ME, Jacobson B, Mitchell JB and Russo A . 1984 Factors influencing the oxidation of cysteamine and other thiols: Implications for hyperthermic sensitization and radiation protection. Radiat. Res. 100: 298–312
Held KD and Biaglow JE . 1993 Role of copper in the oxygen radical-mediated toxicity of the thiol-containing radioprotector dithiothreitol in mammalian cells. Radiat. Res. 134: 375–382
Held KD and Biaglow JE . 1994 Mechanisms for the oxygen radical-mediated toxicity of various thiol-containing compounds in cultured mammalian cells. Radiat. Res. 139: 15–23
Zoratti M and Szabo I . 1995 The mitochondrial permeability transition. Biochem.Biophys. Acta 1241: 139–176
Chernyak BV . 1997 Redox regulation of the mitochondrial permeability transition pore. Bioscience Reports 17: 293–302
Schimizu T and Pommier Y . 1997 Camptothecin-induced apoptosis in p53-null HL60 cells and their isolated nuclei: effects of the protease inhibitors Z-VAD-fmk and dichloroisocoumarin suggest an involvement of both caspases and serine proteases. Leukemia 11: 1238–1244
Yoshida A, Pourquier P and Pommier Y . 1998 Purification and characterization of a Mg2+-dependent endonuclease (AN34) from etoposide treated HL60 cells undergoing apoptosis. Cancer Res. 58: 2576–2582
Rothe G and Valet G . 1990 Flow cytometric analysis of respiratory burst activity in phagoxytes with hydroethidine and 2/7′-dichlorofluorescin. J. Leukoc. Biol. 47: 440–448
Carter WO, Narayanan PK and Robinson JP . 1994 Intracellular hydrogen peroxide and superoxide anion detection in endothelial cells. J. Leukoc. Biol. 55: 253–258
Giandomenico AR, Cerniglia GE, Biaglow JE, Stevens CW and Koch CJ . 1997 The importance of sodium pyruvate in assessing damage produced by hydrogen peroxide. Free Radic. Biol. Med. 23: 426–434
Suzuki A, Iwasaki M and Wagai N . 1997 Involvement of cytoplasmic serine protease and CPP32 subfamily in the molecular machinery of caspase 3 activation during Fas-mediated apoptosis. Exp. Cell Res. 233: 48–55
Han Z, Hendrickson EA, Bremner TA and Wyche JH . 1997 A sequential two-step mechanism for the production of the mature p17:p12 form of caspase-3 in vitro. J. Biol. Chem. 272: 13432–13436
Tang D and Kidd VJ . 1998 Cleavage of DFF-45/ICAD by multiple caspases is essential for its function during apoptosis. J. Biol. Chem. 273: 28549–28552
Liu X, Zou H, Slaughter C and Wang X . 1997 DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell 89: 175–184
Crompton M . 1999 The mitochondrial permeability transition pore and its role in cell death. Biochem. J. 341: 233–249
Zamzami N, Marchetti P, Castedo M, Zanin C, Vayssiere J-L, Petit PX and Kroemer G . 1995 Reduction in mitochondrial potential constitutes an early irreversible step of programmed cell death in vivo. J. Exp. Med. 181: 1661–1672
Kroemer G, Petit P, Zamzami N, Vayssiere J-L and Mignotte B . 1995 The biochemistry of programmed cell death. FASEB J. 9: 1277–1287
Marzo I, Brenner C, Zamzami N, Jurgensmeir JM, Susin SA, Vieira HLA, Prevost M-C, Xie Z, Matsuyama S, Reed JC and Kroemer G . 1998 Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science 281: 2027–2031
Kantrow SP and Piantodosi CA . 1997 Release of cytochrome c from liver mitochondria during permeability transition. Biochem. Biophys. Res. Comm. 232: 669–671
Ellerby HM, Martin SJ, Ellerby LM, Naien SS, Rabizadeh S, Salvesen GS, Casiano CA, Cashman NR, Green DR and Bredesen DE . 1997 Establishment of a cell-free system of neuronal apoptosis: comparison of premitochondrial, mitochondrial and postmitochondrial phases. J. Neurosci. 17: 6165–6178
Marzo I, Brenner C, Zamzami N, Susin SA, Beutner G, Brdiczka D, Remy R, Xie Z-H, Reed JC and Kroemer G . 1998 The permeability transition pore complex: A target for apoptosis regulation by caspases and Bcl-2-related proteins. J. Exp. Med. 187: 1261–1271
Zamzami N, Susin SA, Marchetti P, Hirsch T, Gomez-Monterrey I, Castedo M and Kroemer G . 1996 Mitochondrial control of nuclear apoptosis. J. Exp. Med. 183: 1533–1544
Yang X, Chang HY and Baltimore D . 1998 Autoproteolytic activation of procaspases by oligomerization. Mol. Cell 1: 319–325
Hakem R, Hakem A, Duncan GS, Henderson JT, Woo M, Soengas MS, Elia M, de la Pompa JL, Kagi D, Khoo W, Potter J, Yosida R, Kaufman SA, Lowe SW, Penninger JM and Mak TW . 1998 Differential requirement for caspase 9 in apoptotic pathway in vivo. Cell 94: 339–352
Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M, Houtzager VM, Nordstrom PA, Roy S, Vaillancourt JP, Chapman KT and Nicholson DW . 1997 A combinatorial approach defines specificities of members of the caspase family and granzyme B. J. Biol. Chem. 272: 17907–17911
Ramakrishnan N and Catravas GN . 1992 N-(2-mercaptoethyl)-1,3-propanediamine (WR-1065) protects thymocytes from programed cell death. J. Immunol. 148: 1817–1821
Kim JH, Lee EJ, Hyun JW, Kim SH, Mar W and Kim JK . 1998 Reduction of radiation-induced chromosome aberration and apoptosis by dithiothreitol. Arch. Pharm. Res. 21: 683–687
Bustamante J, Slater AFG and Orrenius S . 1995 Antioxidant inhibition of thymocyte apoptosis by dihydrolipoic acid. Free Radic. Biol. Med. 19: 339–347
Talley AK, Dewhurst S, Perry SW, Dollard SC, Gummuluru S, Fine SM, New D, Epstein LG, Gendelman HE and Gelbard HA . 1995 Tumor necrosis factor alpha-induced apoptosis in human neuronal cells: Protection by the antioxidant N-acetylcysteine and the genes bcl-2 and crmA. Mol. Cell. Biol. 15: 2359–2366
Gurr J-R, Bau S-T, Liu F, Lynn S and Jan K-Y . 1999 Dithiothreitol enhances arsenic trioxide-induced apoptosis in NB4 cells. Mol. Pharmacol. 56: 102–109
Held KD, Tuttle SW and Biaglow JE . 1993 Role of the pentose cycle in the oxygen radical-mediated toxicity of the thiol-containing radioprotector dithiothreitol in mammalian cells. Radiat. Res. 134: 383–389
Lennon SV, Martin SJ and Cotter TG . 1991 Dose-dependent induction of apoptosis in human tumour cell lines by widely diverging stimuli. Cell Prolif. 24: 203–214
Deveraux QL and Reed JC . 1999 IAP family proteins –suppressors of apoptosis. Genes Dev. 13: 239–252
Hopcia KL, McCarey YL, Sylvester FC and Held KD . 1996 Radiation-induced apoptosis in HL60 cells: Oxygen effect, relationship between apoptosis and loss of clonogenicity, and dependence of time to apoptosis on radiation dose. Radiat. Res. 145: 315–323
Sellins KS and Cohen JJ . 1987 Gene induction by γ-irradiation leads to DNA fragmentation in lymphocytes. J. Immunol. 139: 3199–3206
Acknowledgements
This work was supported in part by NIH grants CA63997 (to KD Held), CA44982 (to JE Biaglow) and GM30755 (to IE Kochevar) and DOE grant DE-FG07-99ER62874 (to KD Held).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by CJ Thiele
Rights and permissions
About this article
Cite this article
Tartier, L., McCarey, Y., Biaglow, J. et al. Apoptosis induced by dithiothreitol in HL-60 cells shows early activation of caspase 3 and is independent of mitochondria. Cell Death Differ 7, 1002–1010 (2000). https://doi.org/10.1038/sj.cdd.4400726
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4400726
Keywords
This article is cited by
-
Structure optimization of new tumor-selective Passerini α-acyloxy carboxamides as Caspase-3/7 activators
Scientific Reports (2022)
-
Induction of apoptosis in murine leukemia by diarylheptanoids from Curcuma comosa Roxb.
Cell Biology and Toxicology (2011)
-
The role of major apoptotic proteins in Ca2+ dependent cell homeostasis
Pharmaceutical Chemistry Journal (2005)