Abstract
Primary cutaneous (PC) CD30-positive large cell lymphoma and lymphomatoid papulosis (LyP) represent the spectrum of PC CD30-positive lymphoproliferative disorders (LPDs) associated with a favorable prognosis. Noncutaneous CD30-positive anaplastic large cell lymphoma (ALCL), although morphologically similar to PC CD30-positive LPDs, seems to be a biologically distinct entity. Cell lines derived from noncutaneous ALCL express CD95 and undergo CD95-induced apoptosis. Little is known about expression or function of CD95/CD95L in cutaneous lesions. We examined a series of PC CD30-positive LPDs and noncutaneous ALCL for expression of CD95/CD95L to investigate possible differences between these histologically similar but biologically distinct entities.
Paraffin-embedded, formalin-fixed tissue sections from 25 cases of CD30-positive LPDs (10 noncutaneous ALCL, 15 PC CD30-positive LPDs) were immunostained for CD3, CD20 (L26), CD43 (Leu22), CD30 (BerH2), anaplastic lymphoma kinase (ALK-1), CD95, and CD95L (C-33). One hundred large atypical cells and 100 small lymphocytes were counted to determine the percentage of CD95/CD95L-positive cells. Statistical analysis using the Mann-Whitney U test was performed.
CD95 expression was slightly higher in the large atypical cells of noncutaneous ALCL compared with PC CD30-positive LPDs (median, 100% versus 94%; P =.003) because of the lower expression of CD95 in LyP. CD95L expression was higher in the surrounding small lymphocytes in PC CD30-positive LPDs (median, 3% versus 13%; P =.002). Expression of CD95 in the small lymphocytes and CD95L in the large atypical cells was not significantly different.
These results support the biologic distinction between cutaneous and noncutaneous CD30-positive LPDs and may have implications in the differing clinical behavior of these entities. Further study of expression and function of apoptosis-related proteins in these entities is warranted.
Similar content being viewed by others
INTRODUCTION
Lymphomatoid papulosis (LyP) and primary cutaneous (PC) CD30-positive anaplastic large cell lymphoma (ALCL) belong to the spectrum of PC lymphoproliferative disorders (LPDs) associated with a favorable prognosis that has been attributed to the expression of CD30 (1, 2). Classically, LyP and PC CD30-positive ALCL differ with respect to clinical presentation. LyP presents as disseminated crops of recurrent, self-healing papules, whereas PC CD30-positive ALCL typically presents as a solitary nodule or multiple nodules confined to the same anatomic region (3, 4). Despite their differing clinical presentations, both of these LPDs are capable of undergoing spontaneous regression. Although this phenomenon is a constant feature of LyP, spontaneous regression has been reported in up to 66% of PC CD30-positive ALCL (range, 25 to 66%) (4, 5, 6, 7). The atypical cells of LyP and cutaneous ALCL can be cytologically identical. Criteria for the histologic distinction between these two entities are imprecise, at times causing a diagnostic dilemma. Ultimately, LyP and PC CD30-positive ALCL have both histologic and clinical overlap, prompting some to consider these entities as ends of a spectrum of PC CD30-positive LPDs (1, 4).
The neoplastic cells of PC CD30-positive LPDs are morphologically similar to those of noncutaneous CD30-positive ALCL. Despite this similarity, these disease processes are biologically distinct and differ with respect to age at onset, prognosis, and immunophenotype. Noncutaneous CD30-positive ALCL have a less favorable prognosis compared with PC lesions (4-year survival rate, 65% versus 92%) (8). Immunophenotypically, these entities differ with respect to the expression of epithelial membrane antigen, the presence of t(2;5), and the expression of anaplastic lymphoma kinase (ALK), all of which have been detected in greater frequency in noncutaneous CD30-positive ALCL (8, 9, 10, 11).
CD95 ligand (FasL), “the death factor,” is a Type II transmembrane protein that belongs to the tumor necrosis factor family (12, 13, 14). CD95L is expressed by activated T cells, by natural killer cells, within immune-privileged sites such as the eye and testis, and by various tumors (14). The binding of CD95L to its receptor CD95 (Fas) is capable of causing apoptosis, and this system is a major pathway responsible for apoptosis induction (13). CD95 expression is non–lineage specific, and it is constitutively expressed by a variety of epithelial cells and hematopoietic cells (15, 16). The CD95/CD95L system plays an important role within the immune system through T-cell mediated cytotoxicity of virally infected cells, in downregulating immune responses by eliminating activated lymphocytes, and by promoting self-tolerance through the elimination of self-reactive T cells (13, 17). Loss of function of either CD95 or CD95L has been linked to the development of LPDs and autoimmune disorders in mice as well as in humans (18, 19, 20).
Few studies have examined the expression of these apoptosis-related proteins in cutaneous and noncutaneous CD30-positive LPDs (21). Because of the propensity for PC CD30-positive LPDs to undergo regression, examination of factors involved in apoptosis may be relevant in the pathobiology of these processes. Indeed, there is some evidence that apoptosis may play a role in the propensity of some cases of LyP to undergo spontaneous regression (22). We studied the expression of both CD95 and CD95L in PC CD30-positive LPDs and in noncutaneous CD30-positive ALCL by immunohistochemistry to determine whether these proteins are expressed and whether differences in expression exist between these clinically distinct entities.
MATERIALS AND METHODS
We searched the files of the Department of Anatomic Pathology at the Cleveland Clinic Foundation for cases of LyP, PC CD30-positive ALCL, and noncutaneous CD30-positive ALCL. Cases were included in the study only when there was adequate formalin-fixed tissue available for immunohistochemistry and when the cases met the specific inclusion criteria described next.
We searched only for Type A LyP cases because the large, atypical cells in these lesions express CD30 (4). Cases of Type A LyP were included in the study when the patient had clinical history of chronic, recurrent, self-healing skin lesions consistent with the diagnosis of LyP and when the patient had no other concurrent or previous diagnoses of cutaneous lymphoma. Of 31 cases of LyP diagnosed at the Cleveland Clinic Foundation between 1980 and 1998, we identified 8 cases that fulfilled our criteria.
We identified 7 cases of PC CD30-positive ALCL from 107 cases of cutaneous large cell lymphomas diagnosed at the Cleveland Clinic Foundation between 1975 and 1998. Cases were included in the study only when more than 75% of the atypical cells expressed CD30, when the atypical cells demonstrated anaplastic morphology, when there was no extracutaneous involvement at the time of diagnosis, and when there was no history or clinical evidence of concurrent LyP or any other cutaneous lymphoma (4, 7).
We included 9 cases of noncutaneous CD30-positive ALCL in the study from a total of 14 cases diagnosed between 1990 and 1998 at the Cleveland Clinic Foundation. One additional case was included in the study, which was diagnosed in 1983. Classic, monomorphic, and small cell variants of ALCL were included in the study (23, 24).
Immunohistochemistry was performed on formalin-fixed, paraffin-embedded tissue using a sensitive streptavidin-biotin system by means of an automated immunostainer (Ventana Medical Systems, Tucson, AZ). The panel of antibodies performed is listed in Table 1. Microwave antigen retrieval with 10 mm citrate pH 6.0 was used with all antibodies. Staining of germinal center cells and basilar epithelial cells in hyperplastic tonsil was used as a positive control for CD95 (25). Granular cytoplasmic staining for CD95L in germinal center cells of hyperplastic tonsil was used as a positive control. One hundred large atypical cells and 100 small background lymphocytes located among the CD30-positive tumor cells were counted in areas of highest expression under oil immersion to determine the percentage of CD95- and CD95L-positive cells. Multiple sections were examined in cases in which the large atypical cells were relatively sparse. Statistical analysis was performed using the Mann-Whitney U test. Clinical follow-up information was obtained from a variety of sources, including medical records, tumor registries, and correspondence with clinicians.
RESULTS
Clinical
Of the 15 cases of PC CD30-positive LPDs, there were 8 cases of LyP and 7 cases of PC CD30-positive ALCL. The majority of patients were male (80%). The mean age at diagnosis was 47 years (range, 9 to 71 years). All eight patients with LyP presented with multicentric skin lesions. Five of the seven patients with PC CD30-positive ALCL (71%) presented with solitary lesions, and the remaining two patients had multiple skin lesions localized to one anatomic site. The extremities were the most common site involved (12 cases), followed by the trunk (7 cases) and face (5 cases). The majority of skin lesions had either a papular or nodular component (10 cases).
Of the 10 cases of primary noncutaneous CD30-positive ALCL, the majority of patients were male (70%). The mean age at diagnosis was 47 years (range, 11 to 66 years). The majority of cases involved lymph nodes (six cases); other sites of primary involvement included the chest wall, colorectum, liver, and thyroid gland.
The clinical follow-up is summarized in Table 2. The mean follow-up periods for LyP, PC CD30-positive ALCL, and noncutaneous CD30-positive ALCL were 61 mo (range, 12 to 119 mo), 68 mo (range, 7 to 177 mo), and 55 mo (range, 9 to 102 mo), respectively.
Morphology
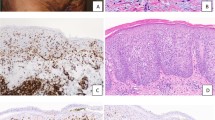
The majority of LyP cases consisted of a bandlike and perivascular infiltrate of large atypical cells intermixed with inflammatory cells. Several cases (50%) had ulceration of the overlying epidermis. There was minimal epidermotropism of the large atypical cells.
The majority of PC CD30-positive ALCL cases consisted of a diffuse dermal proliferation of cells with anaplastic morphology. The atypical cells consisted of irregular, pleomorphic nuclei with prominent nucleoli and abundant cytoplasm. The associated inflammatory infiltrate was considerably less than that seen in the LyP cases.
The noncutaneous CD30-positive cases consisted predominantly of the common type with a sheetlike or sinusoidal proliferation of cells with anaplastic morphology. Three cases consisted of a monomorphic population of CD30-positive cells, and one case was a small cell variant of ALCL in which the large CD30-positive cells were few in number and predominantly perivascular in location.
Immunohistochemistry
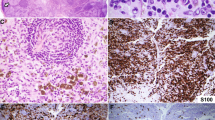
Of the 15 cases of PC CD30-positive LPD, 13 cases had T-cell phenotype with CD3 and/or CD43 expression (87%). Two cases consisted of null cells, which stained negatively for CD3, CD43, and CD20 (13%). Four of the noncutaneous CD30-positive ALCL cases (40%) demonstrated T-cell origin, and six cases (60%) had a null phenotype. The immunophenotypic profile of each case is summarized in Table 2. The morphologic and phenotypic features of LyP, PC CD30-positive ALCL, and noncutaneous CD30-positive ALCL are illustrated in Figures 1, 2, and 3, respectively.
In the eight LyP cases, the expression of CD30 by the atypical cells ranged from 25% to more than 90%. By definition, all seven of the PC CD30-positive ALCL cases had CD30 expression in more than 75% of the atypical cells. All 10 cases of noncutaneous CD30-positive ALCL demonstrated CD30 expression in more than 80% of the atypical cells. In each case, the CD30-positive cells demonstrated membranous and/or Golgi zone immunoreactivity.
ALK-1 staining showed that 2 of the 10 noncutaneous CD30-positive ALCL expressed ALK. In contrast, none of the cutaneous cases demonstrated ALK-1 immunoreactivity (Table 2).
The results of CD95 and CD95L expression are summarized in Table 3. In general, CD95 expression was present in a cytoplasmic and membranous pattern. CD95L demonstrated predominantly a focal granular cytoplasmic pattern. The expression of CD95 was slightly higher in the large atypical cells of noncutaneous CD30-positive ALCL compared with PC CD30-positive LPDs (median, 100% versus 94%; P =.003); there was no detectable difference in CD95 expression in the background small lymphocytes. However, CD95L expression was higher in the background small lymphocytes in PC CD30-positive LPDs as compared with noncutaneous CD30-positive ALCL (median, 13% versus 3%; P =.002). The expression of CD95L in the large atypical cells was not significantly different.
DISCUSSION
Although LyP and PC CD30-positive ALCL often can be distinguished by clinical and morphologic features, there is overlap prompting some to consider them part of a continuum of PC CD30-positive LPDs. The relationship between these entities is supported by the conversion of LyP to CD30-positive ALCL in a minority of cases (1, 5). In contrast, there is mounting evidence that noncutaneous CD30-positive ALCL, although morphologically similar to its cutaneous counterpart, is biologically distinct. Multiple studies have shown that the t(2;5) and expression of ALK is absent in PC CD30-positive LPDs but present in the majority of noncutaneous CD30-positive ALCL (9, 10, 11, 24, 26). Our experience with cutaneous CD30-positive LPDs is similar in that none of our cases expressed ALK. Two of 10 cases of noncutaneous CD30-positive ALCL expressed ALK, a number lower than often reported (9). However, our results are consistent with previous reports demonstrating ALK positivity in younger patients and lack of expression in older patients (27). The two ALK-positive noncutaneous CD30-positive ALCL cases were from the youngest patients (11 and 27 years), and the mean age of the remaining eight patients was much older (54.4 years). Few studies have examined the differences, if any, in expression of apoptosis-related proteins in these entities. They may be of interest, particularly when one considers the propensity for some PC CD30-positive LPDs to undergo spontaneous regression.
Kikuchi and Nishikawa (22) examined the apoptotic index in 50 patients with cutaneous LPDs, including 10 cases of LyP, by detecting internucleosomal breaks in apoptotic cells. LyP had a significantly higher rate of apoptosis compared with all other cutaneous LPDs (P <.001). Possibly related to this, we noted higher CD95L expression by the background small lymphocytes in PC CD30-positive LPDs as compared with noncutaneous CD30-positive ALCL. This may have biologic significance because expression of functional CD95 has been demonstrated in CD30-positive lymphoma cells in vitro. Dirks et al. (16) demonstrated apoptosis of a CD95-expressing noncutaneous CD30-positive ALCL cell line after treatment with anti-CD95 antibodies.
We recognize the technical limitations of this study. The differences in expression of CD95L in the background small lymphocytes admittedly are small. Furthermore, clear surface expression of CD95L was not present. However, Müllauer et al. (28) also described a granular staining pattern for CD95L in a survey of non-Hodgkin’s lymphomas. In addition, surface expression of CD95L is sometimes difficult to demonstrate even by flow cytometry (personal observation). It is possible that surface CD95L may be decreased as a result of proteolytic cleavage of the antigen into a soluble form (29).
CD95 expression was seen in the majority of tumor cells in both entities with slightly higher expression in the noncutaneous lesions. Because CD95 is expressed in most lymphomas (30, 31) and relatively high levels were seen in our cases (median >90%), the significance of this finding is uncertain. Our results are similar to those reported recently. Paulli et al. (21) studied the expression of CD95 in 25 patients with cutaneous CD30-positive LPDs by immunohistochemistry. They reported similar results with CD95 expression in more than 80% of the atypical cells in 100% of LyP cases (8 of 8) and 100% of cutaneous ACLC (5 of 5) examined. Xerri and colleagues (30) demonstrated CD95 expression in more than 50% of cells in 8 of 8 ALCL.
In conclusion, we found a significantly higher expression of CD95L in background small lymphocytes in PC CD30-positive LPDs compared with noncutaneous CD30-positive ALCL. In addition, we demonstrated CD95 expression in the great majority of atypical cells of both cutaneous CD30-positive LPDs and noncutaneous CD30-positive ALCL. These results may have implications in their differing biologic and clinical features. Because the expression of CD95 and CD95L may not always correlate with function as a result of mutation or other downstream alterations, further study of the expression and function of apoptosis-related proteins in these entities is warranted.
References
Paulli M, Berti E, Rosso R, Boveri E, Kindl S, Klersy C, et al. CD30/Ki-1-positive lymphoproliferative disorders of the skin—clinicopathologic correlation and statistical analysis of 86 cases: a multicentric study from the European Organization for Research and Treatment of Cancer Cutaneous Lymphoma Project Group. J Clin Oncol 1995; 13: 1343–1354.
Beljaards RC, Meijer CJ, Scheffer E, Toonstra J, van Vloten WA, vander Putte SC, et al. Prognostic significance of CD30 (Ki-1/Ber-H2) expression in primary cutaneous large-cell lymphomas of T-cell origin: a clinicopathologic and immunohistochemical study in 20 patients. Am J Pathol 1989; 135: 11169–11178.
Tomaszewski MM, Lupton GP, Krishnan J, May DL . A comparison of clinical, morphological and immunohistochemical features of lymphomatoid papulosis and primary cutaneous CD30 (Ki-1)-positive anaplastic large cell lymphoma. J Cutan Pathol 1995; 22: 310–318.
Willemze R, Beljaards RC . Spectrum of primary cutaneous CD30 (Ki-1)-positive lymphoproliferative disorders: a proposal for classification and guidelines for management and treatment. J Am Acad Dermatol 1993; 28: 973–980.
Willemze R, Kerl H, Sterry W, Berti E, Cerroni L, Chimenti S, et al. EORTC classification for primary cutaneous lymphomas: a proposal from the Cutaneous Lymphoma Study Group of the European Organization for Research and Treatment of Cancer. Blood 1997; 90: 354–371.
Bernier M, Bagot M, Broyer M, Farcet JP, Gaulard P, Wechsler J . Distinctive clinicopathologic features associated with regressive primary CD30 positive cutaneous lymphomas: analysis of 6 cases. J Cutan Pathol 1997; 24: 157–163.
Beljaards RC, Kaudewitz P, Berti E, Gianotti R, Neumann C, Rosso R, et al. Primary cutaneous CD30-positive large cell lymphoma: definition of a new type of cutaneous lymphoma with a favorable prognosis. A European Multicenter Study of 47 patients. Cancer 1993; 71: 2097–2104.
de Bruin PC, Beljaards RC, van Heerde P, Van Der Valk P, Noorduyn LA, Van Krieken JH, et al. Differences in clinical behaviour and immunophenotype between primary cutaneous and primary nodal anaplastic large cell lymphoma of T-cell or null cell phenotype. Histopathology 1993; 23: 127–135.
Nakamura S, Shiota M, Nakagawa A, Yatabe Y, Kojima M, Motoori T, et al. Anaplastic large cell lymphoma: a distinct molecular pathologic entity—a reappraisal with special reference to p80(NPM/ALK) expression. Am J Surg Pathol 1997; 21: 1420–1432.
Beylot-Barry M, Lamant L, Vergier B, de Muret A, Fraitag S, Delord B, et al. Detection of t(2;5)(p23;q35) translocation by reverse transcriptase polymerase chain reaction and in situ hybridization in CD30-positive primary cutaneous lymphoma and lymphomatoid papulosis. Am J Pathol 1996; 149: 483–492.
DeCoteau JF, Butmarc JR, Kinney MC, Kadin ME . The t(2;5) chromosomal translocation is not a common feature of primary cutaneous CD30+ lymphoproliferative disorders: comparison with anaplastic large-cell lymphoma of nodal origin. Blood 1996; 87: 3437–3441.
Suda T, Takahashi T, Golstein P, Nagata S . Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell 1993; 75: 1169–1178.
Nagata S, Golstein P . The Fas death factor. Science 1995; 267: 1449–1456.
Nagata S . Fas ligand and immune evasion. Nature Med 1996; 2: 1306–1307.
Leithauser F, Dhein J, Mechtersheimer G, Koretz K, Bruderlein S, Henne C, et al. Constitutive and induced expression of APO-1, a new member of the nerve growth factor/tumor necrosis factor receptor superfamily, in normal and neoplastic cells. Lab Invest 1993; 69: 415–429.
Dirks W, Schone S, Uphoff C, Quentmeier H, Pradella S, Drexler HG . Expression and function of CD95 (FAS/APO-1) in leukaemia-lymphoma tumour lines. Br J Haematol 1997; 96: 584–593.
Lynch DH, Ramsdell F, Alderson MR . Fas and FasL in the homeostatic regulation of immune responses. Immunol Today 1995; 16: 569–574.
Nagata S, Suda T . Fas and Fas ligand: lpr and gld mutations. Immunol Today 1995; 16: 39–43.
Rieux-Laucat F, Le Deist F, Hivroz C, Roberts IA, Debatin KM, Fischer A, et al. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science 1995; 268: 1347–1349.
Fisher GH, Rosenberg FJ, Straus SE, Dale JK, Middleton LA, Lin AY, et al. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell 1995; 81: 935–946.
Paulli M, Berti E, Boveri E, Kindl S, Bonoldi E, Gambini C, et al. Cutaneous CD30+ lymphoproliferative disorders: expression of bcl-2 and proteins of the tumor necrosis factor receptor superfamily. Hum Pathol 1998; 29: 1223–1230.
Kikuchi A, Nishikawa T . Apoptotic and proliferating cells in cutaneous lymphoproliferative diseases. Arch Dermatol 1997; 133: 829–833.
Chott A, Kaserer K, Augustin I, Vesely M, Heinz R, Oehlinger W, et al. Ki-1-positive large cell lymphoma: a clinicopathologic study of 41 cases. Am J Surg Pathol 1990; 14: 439–448.
Kinney MC, Collins RD, Greer JP, Whitlock JA, Sioutos N, Kadin ME . A small-cell-predominant variant of primary Ki-1 (CD30)+ T-cell lymphoma. Am J Surg Pathol 1993; 17: 859–868.
Nguyen PL, Harris NL, Ritz J, Robertson MJ . Expression of CD95 antigen and bcl-2 protein in non-Hodgkin’s lymphomas and Hodgkin’s disease. Am J Pathol 1996; 148: 847–853.
Wood GS, Hardman DL, Boni R, Dummer R, Kim YH, Smoller BR, et al. Lack of the t(2;5) or other mutations resulting in expression of anaplastic lymphoma kinase catalytic domain in CD30+ primary cutaneous lymphoproliferative disorders and Hodgkin’s disease. Blood 1996; 88: 1765–1770.
Shiota M, Nakamura S, Ichinohasama R, Abe M, Tadaatsu A, Takeshita M, et al. Anaplastic large cell lymphomas expressing the novel chimeric protein p80NPM/ALK: a distinct clinicopathologic entity. Blood 1995; 86: 1954–1960.
Müllauer L, Mosberger I, Chott A . Fas ligand expression in nodal non-Hodgkin’s lymphoma. Mod Pathol 1998; 11: 369–375.
Tanaka M, Itai T, Adachi M, Nagata S . Downregulation of Fas ligand by shedding. Nature Med 1998; 4: 31–36.
Xerri L, Carbuccia N, Parc P, Hassoun J, Birg F . Frequent expression of FAS/APO-1 in Hodgkin’s disease and anaplastic large cell lymphomas. Histopathology 1995; 27: 235–241.
Plumas J, Jacob MC, Chaperot L, Chaperot L, Molens JP, Sotto JJ, et al. Tumor B cells from non-Hodgkin’s lymphoma are resistant to CD95 (Fas/Apo-1)-mediated apoptosis. Blood 1998; 91: 2875–2885.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sigel, J., Hsi, E. Immunohistochemical Analysis of CD30-Positive Lymphoproliferative Disorders for Expression of CD95 and CD95L. Mod Pathol 13, 446–451 (2000). https://doi.org/10.1038/modpathol.3880076
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3880076