Abstract
Recent studies of gene expression and immunohistochemistry have shown that protein kinase C-beta II (PKC-beta II) might have prognostic significance in patients with diffuse large B-cell lymphoma (DLBCL). We sought to determine the prognostic significance of the expression of PKC-beta II in patients with nodal DLBCL. Formalin-fixed, paraffin-embedded tissues were stained with a monoclonal antibody to PKC-beta II protein. A total of 125 patients were studied; 83 patients (66%) were in the low-risk International Prognostic Index (IPI) group. Forty-eight patients (38%) were positive for PKC-beta II. Complete remission was obtained in 70%, and was not influenced by the PKC-beta II status (67 vs 71%). The 5-year event-free survival (EFS) was worse in high-risk patients (14 vs 58%, P<0.001) and in those with PKC-beta II positivity (36 vs 49%, P=0.054). In low-risk IPI patients, PKC-beta II expression was related to a worse 5-year overall survival (OS) (60 vs 76%, P=0.033) and a worse 5-year EFS (48 vs 66%, P=0.014). In a Cox regression analysis for EFS, both PKC-beta II expression (hazard ratio=1.68, P=0.037) and the IPI (HR=3.07, P<0.001) were independent poor prognostic factors. PKC-beta II (HR=1.72, P=0.046) and the IPI (HR=5.16, P<0.001) were also independent poor prognostic factors for the OS. PKC-beta II expression, along with the IPI, were associated with a worse EFS and OS in patients with nodal DLBCL specially in low-risk IPI patients.
Similar content being viewed by others
Main
Diffuse large B-cell lymphoma (DLBCL) is the most common type of lymphoma in Western countries, including Brazil.1 It comprises a group of lymphomas characterized by an aggressive clinical course, but which exhibit substantial heterogeneity with respect to morphology, molecular and cytogenetic features, and treatment outcomes. The prognosis of a given patient is currently estimated with a combination of five simple clinical variables that constitute the International Prognostic Index.2
Gene expression profiling, using cDNA or oligonucleotide microarrays, has also been used to identify prognostic subgroups in DLBCL.3, 4 Unfortunately, this technology is expensive and currently impracticable as a clinical tool. Therefore, immunohistochemistry is often used to evaluate the findings of gene expression profiling studies at the protein level.5
Protein kinase C enzymes are a family of serine/threonine kinases that play a major role in signal transduction and contribute to the regulation of cellular differentiation and proliferation.6 Protein kinase C-beta I (PKC-beta I) and beta II are the two major isoforms expressed in B-lymphocytes.7 The RNA expression of both isoforms has been studied in patients with DLBCL, and it seems to be associated with a bad outcome both within the Lymphochip and Affymetrix data sets.3, 4
Two studies have recently analyzed the prognostic impact of PKC-beta II expression by immunohistochemistry in patients with DLBCL, with conflicting results.8, 9 While Hans et al8 verified that the expression of PKC-beta II imparted a bad prognosis to the patients, the study by Sáez et al9 found no association between PKC-beta II expression and clinical outcome in these patients.
The goal of this study was to evaluate the prognostic significance of the expression of PKC-beta II in patients with nodal DLBCL.
Patients and methods
The study population consisted of 125 previously untreated patients with histologically confirmed de novo nodal DLBCL treated with curative intent with standard anthracycline-containing combination chemotherapy at the University Hospital, Federal University of Rio de Janeiro from 1978 to 2001, and at the National Cancer Institute from 1996 to 2001. Patients were selected on the basis of the availability of clinical information and histologic material. Patients with a positive sorology for the HIV virus, and those with primary mediastinal lymphoma, were excluded. Diagnoses were confirmed by histopathologic review, using morphologic and immunologic criteria defined in the WHO classification.10 All cases were CD20 positive.
Lymphomas with lymph node involvement clinically dominant, as well as those presenting at the spleen, were considered as primary nodal. Those lymphomas with extensive disease involving both nodal and extranodal sites were considered nodal. Lymphomas presenting in extranodal organs with no or only minor lymph node involvement were considered primary extranodal.11
Immunohistochemistry
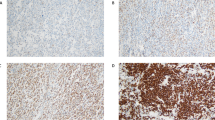
Four-micrometer paraffin sections from formalin-fixed material were dehydrated and deparaffinized according to standard procedures. Heat-induced antigen retrieval was then performed. The slides were incubated overnight with a monoclonal antibody to PKC-beta II (P-3203, Sigma, Saint Louis, MO, USA) in a 1:80 dilution. Cases were considered positive if the antibody expression was detected in >10% of the lymphoma cells.9 Reactive tonsils were used as external positive controls. The PKC-beta II antibody stained normal plasma cells and mantle zone lymphocytes strongly.
The results of immunohistochemistry were based on 500 cell counts. The staining was even in most cases. When staining was uneven, the areas where the positivity was higher were analyzed.
Statistical Analysis
Fisher's exact test (two-sided) was used to compare categorical variables, while numeric data were compared with the Mann–Whitney test. Survival curves were determined according to the Kaplan–Meier method, and compared using the log-rank test. Overall survival was calculated as the time from diagnosis to the date of death or last contact. Event-free survival (EFS) was calculated as the time from diagnosis to the date of disease progression, relapse, death from any cause or last contact. Multivariate survival analysis was performed with Cox's stepwise proportional hazards model. The SPSS version 11.0 software (Chicago, IL, USA) was used for data analysis.
Results
The patients consisted of 71 men and 54 women, with a median age of 60 years (range, 19–83 years). The Ann Arbor stage was I or II in 68 patients (54%) and 42 patients (34%) presented with B symptoms. The serum LDH was elevated in 60 patients (48%). Extranodal disease was present in 47 patients (38%). Data were available for IPI classification in 118 patients, and 83 patients (70%) were in the low-risk group. The median follow-up of the surviving patients was 5.3 years (0.19–11.5). The 5-year overall survival (OS) and the 5-year EFS for all patients were 54 and 44%, respectively.
Forty-eight patients (38%) were positive for PKC-beta II. The staining was cytoplasmic in all cases, but the membrane was also stained in 10 cases (Figure 1). There were no differences in LDH, age, B symptoms or Ann Arbor stage according to the expression of PKC-beta II (Table 1). More females than males were PKC-beta II positive (Table 1). Although there were more patients with involvement of two or more extranodal sites in the PKC-beta II negative group, the IPI risk-groups did not differ according to PKC-beta II expression (Table 1). Complete remission was obtained in 70%, and was not influenced by PKC-beta II status (Table 2).
The 5-year overall survival was significantly better in the low-risk IPI group than in the high-risk group (70 vs 18%, P<0.001). The 5-year event free survival was also better in the low-risk IPI group (58 vs 14%, P<0.001). PKC-beta II positive patients had a worse 5-year EFS but a similar 5-year overall survival (Table 2). However, when only the patients in the low-risk IPI group were analyzed, those with PKC-beta II expression had a worse 5-year overall survival and a worse 5-year EFS (Table 2 and Figure 2). Membrane staining had no prognostic significance.
A Cox regression analysis was performed to assess the independent role of the expression of PKC-beta II and the IPI. In this model, both PKC-beta II expression and the IPI remained as independent poor prognostic factors for EFS (HR=1.68, P=0.037 and HR=3.07, P<0.001, respectively) and for OS (HR=1.72, P=0.046 and HR=5.16, P<0.001, respectively).
Discussion
In this study, we have examined the prognostic value of the expression of PKC-beta II in a group of patients with nodal DLBCL. The positivity for PKC-beta II was associated with a shortened EFS. The results of the multivariate analysis confirmed the independent predictive value of both the International Prognostic Index and the expression of PKC-beta II for the EFS and the overall survival. However, PKC-beta II only added statistically significant prognostic information in low-risk IPI patients.
Sáez et al9 have recently studied the expression of 52 selected molecules in a series of 235 DLBCL. Cases were considered positive for PKC-beta II when more than 10% of the cells were stained. Among the 186 cases tested, 30% were positive, but PKC beta II expression had no impact in the failure-free survival.9 However, primary extranodal lymphomas were included in their analysis.
On the other hand, Hans et al8 tested the prognostic value of PKC-beta II and cyclin D2 in a series of 200 patients with DLBCL. A cutoff of 50% for PKC-beta II and 30% for cyclin D2 was chosen, based on a survival tree method. Tumor expression of both proteins was associated with a worse overall survival. PKC-beta II was positive in 22% of the patients. Only patients in the low-risk IPI group who expressed either of the two proteins had a significantly worse overall survival than patients lacking both markers. Patients in the high-risk group presented a similar trend, but the difference in survival did not reach statistical significance.8 Although extranodal lymphomas were included, these findings are very similar to the results of the present study. In both cases, however, the lack of statistical significance might have been due to the smaller number of patients available for analysis in the high-risk IPI group.
The different cutoff points used in these two studies appear to be overly dependent on each data set. When our cases were reassessed using the 50% cutoff,8 PKC-beta II lost its prognostic meaning for the 5-year overall survival (55 vs 50% for negative and positive cases, P=0.53) and the 5-year EFS (45 vs 43% for negative and positive cases, P=0.82). As in all studies involving immnophenotyping of lymphomas, a better standardization of the methodology is clearly needed if these methods are ever to influence clinical decisions.12
It is also noteworthy that, in the present study, PKC-beta II did not influence the complete remission rate. Its adverse impact was derived solely from a higher risk of relapse in PKC-beta II positive patients (44 vs 24%). If this finding is confirmed in further studies, it might suggest that PKC-beta II positive cells are able to remain viable in low counts after standard treatment of the lymphoma.
It has been recently suggested that cases with a membrane pattern of PKC-beta II expression have a poorer survival.13 In the present study, however, membrane positivity was found in only 8% of the cases, was always accompanied by cytoplasmic positivity, and had no impact on survival.
To our knowledge, this is the first study to address the prognostic impact of PKC-beta II expression in a sample of DLBCL of nodal origin. Substantial clinical and prognostic differences between nodal and extranodal DLBCL have been recently pointed out in an analysis of 1575 patients.14 Owing to the peculiarities of the various extranodal lymphomas, it seems more appropriate, when studying prognosis, to analyze separately the nodal lymphomas.
The pathophysiologic role of PKC-beta II is only now being elucidated. The protein kinase C family of enzymes is involved in several cell responses, including proliferation. An increase in protein kinase C-beta II has been reported in colon tumors, when compared to normal colonic epithelium.15 An increased expression of PKC-beta on B cells, when compared to T cells, plasma cells and myeloid cells, has also been observed.7 Recently, PKC-beta II has been linked to the NF-kappa B activation pathway. In a cell model without PKC-beta II expression, there was no phosphorilation of a key kinase related with NF-kappa B function.16 The most compelling evidence for a role of PKC-beta II in lymphomagenesis came from gene expression studies. PKC-beta I and II were among the genes differentially expressed in patients with diffuse large B cell lymphoma patients who were not cured with standard treatment.3
PKC-beta II expression might also be relevant from a therapeutic standpoint, since PKC-beta inhibitors are currently undergoing evaluation.17 Wu et al studied four DLBCL cell lines with detectable to abundant PKC-beta transcripts. After incubation with the inhibitor LY436881, the PKC enzymatic activity was reduced to undetectable levels, with a decrease in proliferation and an increase in apoptosis at clinically achievable doses.18 This finding led to a phase II study of enzastaurin (LY317615) in the treatment of relapsed DLBCL.19 Preliminary results indicate that the drug is well tolerated and, in heavily treated patients, a 22% freedom from progression was obtained.
In conclusion, the expression of PKC-beta II seems to be associated with a worse prognosis, especially within the low-risk International Prognostic Index group. This information might contribute to the design of a better prognostic tool that could be used to identify patients for improved risk-adapted therapies.
References
Milito CB, Morais JC, Nucci M, et al. Classification of non-Hodgkin's lymphoma: morphological and immunological study of 145 cases. J Bras Patol Med Lab 2002;38:315–324. Available from World Wide Web: 〈http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1676–244420020000400011&lng=en&nrm=iso〉. ISSN 1676–2444.
The International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med 1993;329:987–994.
Shipp MA, Ross KN, Tamayo P, et al. Diffuse large B-cell lymphoma outcome prediction by gene-expression profiling and supervised machine learning. Nat Med 2002;8:68–74.
Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000;403:503–511.
Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004;103:275–282.
Nishizuka Y . Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science 1992;258:607–614.
Mischak H, Kolch W, Goodnight J, et al. Expression of protein kinase C genes in hemopoietic cells is cell-type and B cell-differentiation stage specific. J Immunol 1991;147:3981–3987.
Hans CP, Weisenburger DD, Greiner TC, et al. Expression of PKC-beta or cyclin D2 predicts for inferior survival in diffuse large B-cell lymphoma. Mod Pathol 2005;18:1377–1384.
Sáez A-I, Sáez AJ, Artiga M-J, et al. Building an outcome predictor model for diffuse large B-cell lymphoma. Am J Pathol 2004;164:613–622.
Harris NL, Jaffe ES, Diebold J, et al. The World Health Organization classification of hematological malignancies report of the Clinical Advisory Committee Meeting, Airlie House, Virginia, November 1997. Mod Pathol 2000;13:193–207.
López-Guillermo A, Colomo L, Jiménez M, et al. Diffuse Large B-Cell Lymphoma: Clinical and Biological Characterization and Outcome According to the Nodal or Extranodal Primary Origin. J Clin Oncol 2005;23:2797–2804.
Spector N, Milito CB, Biasoli I, et al. P53 expression as a prognostic indicator in hodgkin's lymphoma. J Clin Oncol 2005;23:3158–3159.
Briones J, Espinosa I, Bordes R, et al. Membrane expression of PKC-beta 2 protein identifies a sugroup of patients with diffuse large B-cell lymphoma with poor survival [abstract]. In: 9th International Conference on Malignant Lymphoma; 2005 Jun 8–11. Lugano (Switzerland). Ann Oncol (Suppl 5). Oxford Journals: Oxford (England), 2005, p. v.58. Abstract 078.
Moller MB, Pedersen NT, Christensen BE . Diffuse large B-cell lymphoma: clinical implications of extranodal versus nodal presentation-a population-based study of 1575 cases. Br J Haematol 2004;124:151–159.
Dempsey EC, Newton AC, Mochly-Rosen D, et al. Protein kinase isoforms and the regulation of diverse cell responses. Am J Physiol Lung Cell Mol Physiol 2000;279:L429–L438.
Saijo K, Mecklenbrauker I, Santana A, et al. Protein kinase C beta controls nuclear factor kappaB activation in B cells through selective regulation of the IkappaB kinase alpha. J Exp Med 2002;195:1647–1652.
Teicher BA, Alvarez E, Mendelsohn LG, et al. Enzymatic rationale and preclinical support for a potent protein kinase Cβ inhibitor in cancer therapy. Adv Enzyme Regul 1999;39:313–327.
Wu E, Aguiar RCT, Savage KJ, et al. PKCβ: a rational therapeutic target in diffuse large B-cell lymphoma [abstract]. In: 44th Annual Meeting of the American Society of Hematology; 2002 Dec 6–10. Philadelphia (PA). Blood. The American Society of Hematology: Washington DC, 2002, p. 202a. Abstract 757.
Robertson M, Kahl B, Vose J, et al. A phase II study of enzastaurin, a protein kinase C-β (PKCβ) inhibitor, in the treatment of relapsed diffuse large B-cell lymphoma (DLBCL) [abstract]. In: 47th Annual Meeting of The American society of Hematology; 2005 Dec 10–13. Atlanta (GA). Blood. The American Society of Hematology: Washington DC, 2005, p. 275a. Abstract 934.
Acknowledgements
We thank Cesonia de Assis Martinusso and Alyson Rosario Jr for helping with the immunohistochemistry. This work was supported by grants from CNPq, CAPES and FAPERJ.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schaffel, R., Morais, J., Biasoli, I. et al. PKC-beta II expression has prognostic impact in nodal diffuse large B-cell lymphoma. Mod Pathol 20, 326–330 (2007). https://doi.org/10.1038/modpathol.3800738
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800738
Keywords
This article is cited by
-
Prognostic impact of protein kinase C β II expression in R-CHOP-treated diffuse large B-cell lymphoma patients
Modern Pathology (2010)
-
Expression of Protein Kinase C Family in Human Hepatocellular Carcinoma
Pathology & Oncology Research (2010)