Abstract
This paper reviews the role of mast cells in the development and progression of basal cell carcinoma, squamous cell carcinoma and malignant melanoma. Mast cells accumulate around cutaneous malignancies. Current evidence suggests that mast cells contribute to the tumorigenesis of cutaneous malignancies through four mechanisms. (1) Immunosuppression: Ultraviolet-B radiation, the most important initiator of cutaneous malignancies, activates mast cells. Upon irradiation of the skin, trans-urocanic acid in the epidermis isomerizes to cis-urocanic acid, which stimulates neuropeptide release from neural c-fibers. These neuropeptides in turn trigger histamine secretion from mast cells, leading to suppression of the cellular immune system. (2) Angiogenesis: Mast cells are the major source of vascular endothelial growth factor in basal cell carcinoma and malignant melanoma. Vascular endothelial growth factor is one of the most potent angiogenic factors, which also induces leakage of other angiogenic factors across the endothelial cell wall into the matrix. Mast cell proteases reorganize the stroma to facilitate endothelial cell migration. As well, heparin, the dominant mast cell proteoglycan, assists in blood-borne metastasis. (3) Degradation of extracellular matrix: Through its own proteases, and indirectly via interaction with other cells, mast cells participate in degradation of the matrix, which is required for tumor spread. (4) Mitogenesis: Mast cell mediators including fibroblast growth factor-2 and interleukin-8 are mitogenic to melanoma cells. Current evidence supports an accessory role for mast cells in the development and progression of cutaneous malignancies. Emerging data, however, also suggest that mast cells might, in fact, have opposing roles in tumor biology, and the microenvironment could polarize mast cells to possess either promoting or inhibitory effects on tumors.
Similar content being viewed by others
Main
Cutaneous malignancies are the most common cancers. They account for nearly half of all cancers in the United States according to statistics from the American Cancer Society.1 In Australia and New Zealand, where the incidence of skin cancer is the highest in the world, the total number of cutaneous malignancies exceeds that of all other cancers combined by several folds.2, 3 Approximately 77% of cutaneous malignancies are basal cell carcinomas, 20% are squamous cell carcinomas and the remainder consist of malignant melanoma and rarer tumors.4 Basal cell carcinomas arise from mitotic epidermal cells, squamous cell carcinomas from differentiated epithelial keratinocytes and malignant melanomas from epidermal melanocytes.5, 6 Basal cell carcinomas are locally invasive but very rarely (less than 0.1%) metastasize. They tend to spread along the plane of least resistance such as periosteum, perichondrium, fascia and tarsal plate.7 Squamous cell carcinoma has a 2–6% incidence of metastasis.8 Melanoma, however, accounts for most of the mortality from cutaneous malignancies. Many factors affect the prognosis of melanoma, including tumor thickness, ulceration, location and gender. In all, 20% of patients with melanoma will develop metastatic disease and die within 5 years of diagnosis.6
Studies have shown that mast cells accumulate around the margin of these cutaneous malignancies.9, 10, 11, 12, 13, 14 There is compelling evidence that mast cell accumulation among the peritumoral inflammatory infiltrates contributes to a permissive microenvironment for carcinogenesis and metastasis.15, 16, 17
Mast cells originate from the bone marrow and the immature progenitor cells migrate to peripheral tissues and mature in situ.18, 19, 20 Mast cells are not identified in the circulation.21
Mast cells possess many properties that enable them to participate in a diverse range of biological activities. They phagocytoze, process antigens, produce cytokines and release a variety of preformed (eg, histamine, proteoglycans and proteases) and newly formed (eg, leukotrienes and prostaglandins) physiological mediators.20 Mast cells carry an array of adhesion molecules, immune response receptors and other surface molecules which permit them to react to multiple specific and nonspecific stimuli.22 These wide-ranging biological characteristics, their ubiquitous distribution and strategic locations near blood vessels, nerves, inflamed tissues and neoplastic foci enable them to play a central role in a multitude of physiologic, immunologic and pathologic processes.20, 23, 24, 25 We have recently reviewed the biology of mast cells.25
The effects of mast cell mediators on the development and spread of cutaneous malignancies are likely to be mediated through multiple pathways, including immunosuppression, enhancement of angiogenesis, disruption of the extracellular matrix and promotion of tumor cell mitosis (Figure 1).
The accessory roles of mast cells in the progression and spread of cutaneous malignancies. UV-B, ultraviolet-B; SCF, stem cell factor; IL, interleukin; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor; ECs, endothelial cells; TGF-β, transforming growth factor-β; FGF-2, fibroblast growth factor-2.
Mast cell density around cutaneous malignancies
Mast cells have been observed to accumulate around the margin of cutaneous malignancies.9, 10, 11, 12, 13, 14 The increased density of mast cells around many types of tumors is independent of the presence of an inflammatory infiltrate.26 Cohen et al27 find that the degree of peribasal cell carcinoma inflammation does not correlate with the relative density of mast cells. This suggests that mast cells are preferentially recruited to the vicinity of basal cell carcinoma (Figure 2).
Flynn et al,28 in an experiment in which epidermoid carcinoma is induced in the hamster buccal pouches by repeated topical application of dimethylbenzanthracene, demonstrate sequential mast cell migration towards progressive mucosal dysplasia and subsequent development of squamous cell carcinoma. As they migrate from the deep connective tissue to the dysplastic epithelium, mast cell membrane-bound granules fuse to form multichambered sacs. This ultrastructural change takes place when a mast cell is stimulated.29 This observation suggests that as mast cells are attracted to the carcinomatous lesion, they are stimulated to degranulate as well.
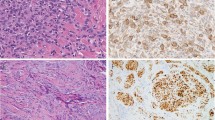
Histopathology studies on human basal cell carcinoma and squamous cell carcinoma have shown that mast cell density is especially high in the more aggressive variants.13, 14, 27, 30, 31 As well, the density of mast cell and microvessels is increased in melanoma compared to benign naevus and melanoma in situ10, 11 (Figure 3).
Adjacent sections of a common naevus (a, b), naevus with architectural disorder and melanocytic atypia (c, d), and metastatic malignant melanoma (e, f) stained with anti-CD31 (a, c, e) for microvessels and antitryptase for mast cells (b, d, f). Note the progressive increase of the number of microvessels (a, c, e) and mast cells located around these microvessels (*) (b, d, f) from common naevus, through naevus with atypia, to metastatic malignant melanoma. (Reproduced with permission from Ribatti et al.68)
Ultrastructural study of mast cells around cutaneous malignancies
Reports on the ultrastructure of mast cells surrounding basal cell carcinoma are contradictory. Janowski et al32 observe that mast cells around basal cell carcinoma have a greater surface area, diameter and perimeter than normal mast cells. Conversely, Tharp et al33 report that mast cells that infiltrate around basal cell carcinoma have no discernible difference in the cell surface area, nuclear size, number and size of cytoplasmic granules. There has been no published report on the ultrastructure of mast cells in squamous cell carcinoma and melanoma.
Ultraviolet-B-induced immunosuppression in photocarcinogenesis
Cutaneous malignancies are highly immunogenic, and a competent immune system should be capable of destroying the cancer cells.34 Therefore, the development of cutaneous malignancies requires firstly, a malignant transformation of skin cells, and secondly, a compromised immunity, especially an impaired cell-mediated immune response.35, 36 There is compelling evidence that ultraviolet-B (280–320 nm) radiation plays a central role in both these mechanisms.36
Exposure of humans and animals to ultraviolet radiation, particularly ultraviolet-B, impairs the function of the immune system, both locally at the site of exposure and systemically. The immunosuppressive effects of ultraviolet radiation have been implicated in the pathogenesis of melanoma and nonmelanoma skin cancers. The association between suppression of immune response by ultraviolet radiation and the development of skin cancers was first demonstrated in the pioneering work of Fisher and Kripke.37, 38 They showed that, unlike most other murine tumors, ultraviolet-induced skin cancers failed to develop when transplanted into syngeneic nonirradiated mice. Interestingly, if the recipient mice were preexposed to ultraviolet radiation, the cancers were able to grow.37, 38
In humans, a suppressed immune function has been demonstrated to be a causative factor for the development of skin cancers. Renal transplant patients, who are therapeutically immunosuppressed, display exceptionally high incidence of squamous cell carcinoma and basal cell carcinoma on sun-exposed sites.39, 40 In the general population, certain individuals seem to be more susceptible than others to the immunosuppressive effects of solar radiation.41 Following exposure of the skin to ultraviolet radiation, 40% of normal subjects exhibit suppressed contact hypersensitivity responses to the topically applied experimental sensitizer, dinitrochlorobenzene, at the site of exposure. However, when skin cancer patients are tested using the same protocol, 92% are susceptible to the inhibitory effects of ultraviolet radiation.41 These results indicate that the ultraviolet-induced suppression of immune function may be a significant risk factor for the development of skin cancers.
It is well established that the skin actively initiates immune responses against foreign antigens. A number of epidermal and dermal cell types participate in skin immune processes. The epidermal Langerhans cells and keratinocytes are largely responsible for initiating immune activation while dermal fibroblasts, dendritic cells, mast cells and endothelial cells maintain and mediate immune responses. Exposure to ultraviolet radiation can directly alter the function of these cell types resulting in the suppression of the immune function.42
Role of mast cells in ultraviolet-B-induced immunosuppression and cutaneous malignancies
Emerging evidence suggests that mast cells may play a critical role in ultraviolet-B-induced immunosuppression43, 44, 45, 46 (Figure 1).
In a study involving South Australian and Danish subjects, patients with a history of basal cell carcinoma and melanoma are found to have a significantly higher, genetically predetermined density of dermal mast cells.47, 48 It is suggested that a higher density of dermal mast cell is a predisposing factor for the development of basal cell carcinoma and melanoma. It has been hypothesized that a higher dermal mast cell density predisposes an individual to ultraviolet-B-induced immunosuppression.47, 49 However, a similar correlation has not been found for patients with squamous cell carcinoma.5 Epidemiological studies have shown that the relative incidence of 1:4 for basal cell carcinoma:squamous cell carcinoma in the general Caucasian population reverses to 3:1 in drug-induced systemic immunosuppression following renal transplant. This indicates a stronger correlation between immunosuppression and the development of squamous cell carcinoma.49 The reason why patients with basal cell carcinoma and melanoma but not those with squamous cell carcinoma are found to have higher dermal mast cell densities is unclear. Grimbaldeston et al5 suggest that the development of squamous cell carcinoma may be supported by other immunomodulatory mechanisms.
Using the W/Wv mice, Hart et al50 demonstrate a direct correlation between mast cell density in the dermis and susceptibility to ultraviolet-B-induced systemic immunosuppression. These mice, which are homozygous for the W (white-spotting) mutation, and therefore severely mast cell deficient, are unresponsive to ultraviolet-induced immunosuppression unless first injected with mast cell precursors derived from the bone marrow at the irradiated site.48, 50 The W locus is on chromosome 5 and encodes the c-kit tyrosine kinase receptor that binds stem cell factor, which is the main growth factor for mast cells and vital for their development, maturation and migration.20
It has been proposed that ultraviolet-B indirectly activates mast cells through two mechanisms. Firstly, the more immediate effect involves isomerization of photo-receptor trans-urocanic acid (UCA) to cis-UCA in the stratum corneum. Cis-UCA stimulates neuropeptide secretion, especially substance P and calcitonin gene-related peptide, from cutaneous sensory nerves.47, 51, 52 These neuropeptides in turn stimulate release of mediators from mast cells. Secondly, irradiated keratinocytes secrete nerve growth factor, which sustains release of neuropeptides from the sensory nerves.47
The critical mast cell products involved in ultraviolet-induced immunosuppression are believed to be tumor necrosis factor-α (TNF-α) and histamine, which are important for the local and systemic effects, respectively34 (Figure 1).
Disruption of the function of Langerhans cell, the principal antigen presenting cell of the skin by ultraviolet radiation has been shown to be an important component of ultraviolet-induced local immunosuppression. Langerhans cells are especially sensitive to the effects of ultraviolet radiation and they undergo morphological and ultrastructural changes upon exposure that may result in altered or diminished antigen presentation. TNF-α has been identified as a key mediator in ultraviolet-induced local immunosuppression. Its level is raised in ultraviolet-exposed skin and it may act by altering Langerhans cell morphology and function.53 Mast cells store large amounts of TNF-α, which are released on activation.50 A murine study suggests that the degree of susceptibility to ultraviolet-B-induced local immunosuppression depends on TNF-α levels within the epidermis after ultraviolet-B irradiation. Intracutaneous administration of TNF-α has been shown to evoke morphological changes in Langerhans cells similar to those observed upon ultraviolet irradiation while antibodies to TNF-α protect Langerhans cells from the effects of ultraviolet radiation.54
Mast cell-derived histamine stimulates prostaglanidin E2 (PGE2) production from keratinocytes. PGE2 alters the cytokine balance in favor of the immunosuppressive interleukin-10 (IL-10) against the immunostimulatory IL-12.55 IL-12 is central to the orchestration of both innate and acquired cell-mediated immune responses.56 IL-12 is able to abort T suppressor cell-induced apoptosis in Langerhans cells. IL-12 has also been shown to cause a remarkable reduction in ultraviolet-specific DNA lesions through the induction of DNA repair.57 Indomethacin, a cyclooxygenase inhibitor, which blocks prostaglandin synthesis, has been shown to cause significant reversal of ultraviolet-B and cis-UCA-induced systemic immunosuppression.50 This finding further affirms the role of prostaglandins in ultraviolet-B-induced systemic immunosuppression.
Histamine also increases suppressor T-cell function by binding to the H2 receptors. These suppressor T cells in turn release higher levels of immune suppressive cytokines including IL-10, and induce apoptosis of antigen-presenting cells.31, 43, 58 Suppression of immune responses to melanoma antigens and functional inactivation of tumor-reactive T cells are important for the development of melanoma in humans. Treatment of mice with cimetidine, an H2 receptor antagonist, has been reported to retard the growth of melanoma.58
Mast cells and angiogenesis
Mast cells stimulate neovascularization at the tumor–host interface12 (Figures 1, 2 and 4). The growth and metastasis of a tumor depends on its ability to elicit new blood supply.59, 60 Acquisition of the angiogenic phenotype, which enables the tumor to establish its independent blood supply, represents an increase in malignancy potential.
(a) Antitryptase monoclonal antibody stain of a melanoma. Note the close relationship between tumor cells, capillaries and mast cells (original magnification × 400). (b) Double immunostaining showing mast cells (stained red with antitryptase rhodamine) accumulation in microvessel-rich (stained green with anti-CD34, FITC) area (original magnification × 200). (Reproduced with permission from Toth et al.12)
Tumor angiogenesis requires a combination of angiogenic factors and stromal remodeling by proteolytic enzymes. Proteolysis of the extracellular matrix not only facilitates endothelial-cell migration, but also releases sequestered latent stores of angiogenic factors.61, 62, 63, 64 The evidence that the intensity of angiogenesis in a human tumor could predict the likelihood of metastasis was first reported in cutaneous melanoma.65 Patients with intermediate thickness (0.76–3.99 mm) melanomas were divided into two groups that were matched for age, sex, Breslow thickness and Clark's level. The group that developed metastasis was found to have a more than two-fold increase in vascular area at the base of their melanoma lesions compared with the metastasis-free group.65 Mast cell accumulation around the margin of tumors has been observed to peak just as the tumors acquire the angiogenic phenotype, preceding growth of new capillaries towards the tumors.26, 66 Peritumoral mast cell count correlates strongly with microvascular density, melanoma progression and prognosis.11, 12
The angiogenic response to implanted melanoma in mast cell-deficient W/Wv mice is delayed and less intense initially. These mice are also less likely to develop lung metastasis. Their angiogenic response and propensity for hematogenous metastasis approach that of their mast cell-proficient counterparts upon mast cell restoration with bone marrow cell injection.67
Mast cells contain various angiogenic factors such as histamine, heparin, transforming growth factor-β (TGF-β), TNF-α, IL-8, fibroblast growth factor-2 (FGF-2) and vascular endothelial growth factor (VEGF).68, 69, 70 Ugurel et al71 show significantly elevated serum levels of FGF-2, VEGF and IL-8 in melanoma patients when compared with healthy subjects. Furthermore, elevated serum levels of these factors correlate positively with the stage of disease and tumor burden, and confers a poor overall survival and high probability of progression 71 (Figure 5).
Stage-dependent increase of serum levels of angiogenic factors in 125 malignant melanoma patients. The bars represent the percentage of patients with a serum level above a threshold (bFGF >3.19 pg/ml, VEGF >363.8 pg/ml, IL-8 >226.8 pg/ml), calculated using the receiver operating characteristic curve analysis. Stage I, primary melanoma; stage II, regional lymph node and/or intransit metastasis; stage III, distant metastasis. (Adapted with permission from Ugurel et al.71)
Mast cells around cutaneous melanoma (50–90% of mast cells) and basal cell carcinoma (90.2% of mast cells) are the major source of VEGF (Figures 6 and 7a and b).12, 14 VEGF is one of the most potent angiogenic factors which contributes to neovascularization by promoting mitosis of endothelial cells and inducing hyperpermeability in microvessels, leading to extravasation of other proangiogenic factors into the extracellular matrix.72, 73 Tumor cells and stromal cells in turn secrete cytokines such as TGF-α and platelet-derived growth factor, which perpetuate mast cell expression of VEGF.72
Double immunofluorescence stain of a malignant melanoma showing peritumoral zone rich in mast cells close to tumour. Most of the MCs show immunoreaction for both antitryptase (red) and anti-VEGF (yellow). Note the absence of VEGF expression in the MCs, microvessels, inflammatory cells and tumor cells within the tumor. Far from the tumor margin, MC react mostly with antitryptase antibody but not VEGF (original magnification × 200). (Reproduced with permission from Toth et al.12)
Frozen sections of a basal cell carcinoma with positive staining for anti-tryptase (highlighting mast cells) (a), VEGF (b), IL-8 (c), and RANTES (d) monoclonal antibodies. (Reproduced with permission from Aoki et al.14)
In an elegant study, Coussens et al74 show that mast cell-deficient human papillomavirus-infected transgenic mice have severely attenuated tumor angiogenesis and growth when compared with their wild-type littermates. This study shows that tryptase and chymase, the two mast cell-specific proteases, contribute to neovascularization during squamous cell carcinogenesis.74 Tryptase is a fibroblast mitogen and chemoattractant, and it stimulates type α1(I) procollagen mRNA synthesis in human fibroblasts in vitro.75, 76 Preceding malignant transformation in angiogenic dysplasia, high levels of tryptase has been observed, with increased dermal fibroblasts and type α1(I) procollagen mRNA widely expressed in the stroma. This observation supports the role for tryptase in matrix reorganization associated with neovascularization.74 Blair et al77 demonstrate that addition of tryptase to microvascular endothelial cell cultures causes a pronounced increase of capillary growth by more than 20-folds, and this can be suppressed by specific tryptase inhibitors. Chymase can directly and indirectly, through its ability to activate progelatinase B, trigger proteolysis of the extracellular matrix thereby releasing sequestered angiogenic factors including VEGF and FGF.74
Heparin, the dominant proteoglycan in mast cells, has many properties including being mitogenic for endothelial cells.78, 79 It also stimulates migration of cultured capillary endothelial cells.66 Its anticoagulant effect prevents microthrombi in the new vessels, which helps propagation of metastases.
Mast cell also produces IL-8, which exhibits potent angiogenic activities both in vitro and in vivo.80 In all, 50% of melanoma in the vertical growth phase, 100% of melanoma metastatic lesions, and none of the radial growth phase melanoma express IL-8.81 It is believed that IL-8 exerts its angiogenic activity through the induction of matrix metalloproteinase 2, thereby facilitating endothelial cell migration through the stroma and consequently, assisting tumor metastasis.80 IL-8 has also been implicated in tumor angiogenesis in basal-cell carcinoma14 (Figure 7c).
Other mast cell mediators known for their roles in tumor angiogenesis include histamine, FGF and TNF-α.62 However, their angiogenic role has not been studied specifically for cutaneous malignancies.
Mast cells and the degradation of extracellular matrix
Mast cell plays an accessory role in the degradation of extracellular matrix, the first of a series of linked sequential steps for a tumor to establish successful metastasis74, 82, 83 (Figure 1).
Dabbous et al84 show that mast cell degranulation is commonly associated with disruption and lysis of the connective tissue matrix occurring in tumor infiltration. Mast cells have been implicated in this process either directly through the action of their enzymes, or indirectly through modulation of the collagenolytic activity of fibroblasts, macrophages and tumor cells.85, 86 Tryptase activates latent metalloproteinases and plasminogan activator, which degrade the extracellular matrix.86 Heparin enhances both the activity and production of collagenase in vitro.87 Heparin also releases plasminogen activator from endothelial cells.88 Other mast cell mediators such as FGF-2, TGF-β, IL-3 and IL-4 can stimulate collagenase and β-hexosaminidase production by fibroblasts, and IL-1 by macrophages. These factors work in concert to loosen up the stromal milieu to facilitate tumor invasion.68, 86, 89, 90
Mitogenic effect of mast cells
There is increasing evidence that mast cells support tumor progression by providing direct mitogenic stimulation of cancer cells89 (Figure 1). Among the mediators released by mast cells, FGF-2 and IL-8 are directly mitogenic to melanocytes and melanoma cells.91, 92
Several studies have shown a close correlation between melanoma progression and the degree to which the tumor cells react to FGF-2.11, 89 The most important gene of the FGF receptor family, fgfr-1 gene is expressed throughout the human melanocytic system, albeit at higher levels in melanoma than in naevi and normal melanocytes. Antisense targeting of fgfr-1 in melanoma cells completely blocks tumor growth, inhibits intratumoral angiogenesis causing extensive tumor necrosis, and can even lead to regression in melanoma in vivo.93, 94
Tumor–host paracrine loop
As mast cells' recruitment to the vicinity of cutaneous malignancies is independent of an inflammatory infiltrate, these tumors must secrete mast cell chemoattractants, thus forming a paracrine loop (Figure 1).
Reed et al89 demonstrate that melanoma cells recruit mast cells in vivo by producing mast cell chemotactic and mitogenic factors such as IL-3, and the level of IL-3 in the stroma correlates with melanoma progression. This result, however, contradicts another report that shows the absence of IL-3 receptor on human mast cells.95 Poole et al66, using agarose migration assay, demonstrate that mast cells migrate towards undefined factors released from a variety of tumors including melanoma.
Basal cell carcinoma cells secrete stem cell factor and IL-1, which can stimulate mast cell proliferation and degranulation, thus forming a paracrine loop.10, 28, 31
Opposing roles for mast cells?
The body of evidence presented thus far supports a tumorigenic role for mast cells in the development and progression of cutaneous malignancies. Could this be an incomplete portrayal of the mast cells? Could mast cells in fact fulfil opposing roles depending on the microenvironment in which they reside, playing the Jekyll and Hyde of tumor growth?17
This dual role for mast cells certainly seems probable. Firstly, mast cells have a vast array of mediators, some of which have promoting, and others, inhibitory effects on malignancies.17 Secondly, the phenotypic expression of mast cell is not static and its secretory pattern alters according to the microenvironment. Mast cells have the ability to secrete individual granules (in contrast to indiscriminate degranulation in an anaphylactic reaction) or distinct mediators selectively.96, 97 For instance, acidity inhibits allergic degranulation but promotes IL-4 production. IL-6 can be secreted without histamine in vitro; and murine mast cells can secrete VEGF without parallel release of serotonin.98, 99, 100
Several studies have shown a tumor cytotoxic role for mast cells in cutaneous malignancies.14, 101, 102, 103
Schittek et al demonstrate that mast cell-deficient W/Wv mice develop melanoma metastasis more readily than their +/+ littermates. It has been hypothesized that histamine increases prostacyclin synthesis by endothelial cells, and that prostacyclin has a potent antimetastatic action.101
Certain mast cell mediators including TNF-α, IL-1, IL-6 and interferon-γ have been reported to suppress melanoma cell growth.102, 103
Aoki et al84 show that mast cells in basal cell carcinoma express mRNA of RANTES, which is a potent chemoattractant for many types of inflammatory cells including neutrophils, eosinophils and monocytes (Figure 7d). RANTES is also known to have the ability to selectively attract T cells of the CD4+/CD45RO+ phenotype, which may contribute to antitumor immunity.
Conclusion
Mast cell attracts as much interest as controversy today in a variety of physiological and pathological processes, including cutaneous malignancies. The divergence in opinion on the functional role of mast cell in these tumors is not surprising given its versatility and the plethora of mediators it secretes, which have wide-ranging and sometimes opposing effects. However, the great majority of studies to date support an accessory role for mast cells in the development and progression of cutaneous malignancies.
References
American Cancer Society. Cancer Facts and Figures 2005. American Cancer Society: Atlanta, 2005, pp 1–5.
Australian Institute of Health and Welfare and Australasian Association of Cancer Registries. Cancer in Australia 2001. Canberra, 2004, pp 6–9.
Health Sponsorship Council [Report]. Sunsmart in New Zealand: facts, information and articles 2005. Wellington, New Zealand.
Fleming I, Amonette R, Monaghan T . Principles of management of basal and squamous cell carcinoma of the skin. Cancer 1995;75:699–704.
Grimbaldeston M, Skov L, Finlay-Jones J, et al. Squamous cell carcinoma is not associated with high dermal mast cell prevalence in humans. J Invest Dermatol 2002;119:1204–1206.
Wagner J, Gordon M, Chuang T, et al. Current therapy of cutaneous melanoma. Plast Reconstr Surg 2000;105:1774–1799.
Netscher D, Spira M . Basal cell carcinoma: an overview of tumor biology and treatment. Plast Reconstr Surg 2004;113:74e–94e.
Rudolph R, Zelac D . Squamous cell carcinoma of the skin. Plast Reconstr Surg 2004;114:82e–94e.
Cawley E, Hoch-Ligeti C . Association of tissue mast cells and skin tumors. Arch Dermatol 1961;83:146–150.
Duncan L, Richards L, Mihm Jr M . Increased mast cell density in invasive melanoma. J Cutan Pathol 1998;25:11–15.
Ribatti D, Ennas M, Vacca A, et al. Tumor vascularity and tryptase-positive mast cells correlate with a poor prognosis in melanoma. Eur J Clin Invest 2003;33:420–425.
Toth T, Toth-Jakatics R, Jimi S, et al. Cutaneous malignant melanoma: correlation between neovascularization and peritumor accumulation of mast cells overexpressing vascular endothelial growth factor. Hum Pathol 2000;31:955–960.
Humphreys T, Monteiro M, Murphy G . Mast cells and dendritic cells in basal cell carcinoma stroma. Dermatol Surg 2000;26:200–204.
Aoki M, Pawankar R, Niimi Y, et al. Mast cells in basal cell carcinoma express VEGF, IL-8 and RANTES. Int Arch Allergy Immunol 2003;130:216–223.
Coussens L, Werb Z . Inflammatory cells and cancer: think different!. J Exp Med 2001;193:F23–F26.
Nakayama T, Yao L, Tosato G . Mast cell-derived angiopoietin-1 plays a critical role in the growth of plasma cell tumors. J Clin Invest 2004;114:1317–1325.
Theoharides T, Conti P . Mast cells: the Jekyll and Hyde of tumor growth. Trends Immunol 2004;25:235–241.
Cotran R, Kumar V, Collins T, et al. Robbins Pathologic Basis of Disease, 6th edn. WB Saunders: Philadelphia, 1999, p 196.
Abbas A, Lichtman A, Pober J . Cellular and Molecular Immunology, 3rd edn. WB Saunders: Philadelphia, 1997, pp 303–306.
Church M, Levi-Schaffer F . The human mast cell. J Allergy Clin Immunol 1997;99:155–160.
Roitt I, Brostoff J, Male D . Immunology, 6th edn. Mosby: London, 2001, p 330.
Metcalfe D, Baram D, Merkori Y . Mast cells. Physiol Rev 1997;77:1033.
Gurish M, Austen K . The diverse roles of mast cells. J Exp Med 2001;194:F1–F5.
Castells M . Mast cells: molecular and cell biology. Int J Asthma, Allergy Immunol 1999;1:1–20.
Tan ST, Wallis R, He Y, et al. Mast cells and hemangioma. Plast Reconst Surg 2004;113:999–1011.
Kessler D, Langer R, Pless N, et al. Mast cells and tumor angiogenesis. Int J Cancer 1976;18:703–709.
Cohen M, Rogers G . The significance of mast cells in basal cell carcinoma. J Am Acad Dermatol 1995;33:514–517.
Flynn E, Schwartz J, Shklar G . Sequential mast cell infiltration and degranulation during experimental carcinogenesis. J Cancer Res Clin Oncol 1991;117:115–122.
Dvorak A, Galli S, Schulman E, et al. Basophil and mast cell degranulation: ultrastructural analysis of mechanisms of mediator release. Fed Proc 1983;42:2510–2515.
Claudatus Jr J, d'Ovidio R, Lospalluti M . Skin tumors and reactive cellular infiltrate: further studies. Acta Derm Venereol 1986;66:29–34.
Erkilic S, Erbagci Z . The significance of mast cells associated with basal cell carcinoma. J Dermatol 2001;28:312–315.
Janowski P, Strzelecki M, Brzezinska-Blaszczyk E, et al. Computer analysis of normal and basal cell carcinoma mast cells. Med Sci Monit 2001;7:260–265.
Tharp M, Glass M, Seelig Jr L . Ultrastructural morphometric analysis of human mast cells in normal skin and pathological cutaneous lesions. J Cutan Pathol 1988;15:78–83.
Hart P, Grimbaldeston M, Finlay-Jones J . Sunlight, immunosuppression and skin cancer: role of histamine and mast cells. Clin Exp Pharmacol Physiol 2001;28:1–8.
Streilein J, Taylor J, Vincek V, et al. Immune surveillance and sunlight-induced skin cancer. Immunol Today 1994;15:174–179.
Matsumura Y, Ananthaswamy H . Molecular mechanisms of photocarcinogenesis. Front Biosci 2002;7:d765–d783.
Kripke M . Immunological unresponsiveness induced by ultraviolet radiation. Immunol Rev 1984;10280:87–102.
Fisher M, Kripke M . Systemic alteration induced in mice by ultraviolet light irradiation and its relationship to ultraviolet carcinogenesis. Proc Natl Acad Sci USA 1977;74:1688–1692.
Penn I . Post-transplant malignancy: the role of immunosuppression. Drug Saf 2000;23:101–113.
Bavinck J, De Boer A, Vermeer B, et al. Sunlight, keratotic skin lesions and skin cancer in renal transplant recipients. Br J Dermatol 1993;129:242–249.
Yoshikawa T, Rae V, Bruins-Slot W, et al. Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in humans. J Invest Dermatol 1990;95:530–536.
Wallis R . Mast cell involvement in ultraviolet radiation induced immunosuppression. In: Science. University of Otago: Wellington, 2004, pp 1–4.
Aubin F . Mechanisms involved in ultraviolet light-induced immunosuppression. Eur J Dermatol 2003;13:515–523.
Elmets C, Bergstresser P, Tigelaar R, et al. Analysis of the mechanism of unresponsiveness produced by haptens painted on skin exposed to low dose ultraviolet radiation. J Exp Med 1983;158:781–794.
Fisher M, Kripke M . Systemic alteration induced in mice by ultraviolet light irradiation and its relationship to ultraviolet carcinogenesis. Proc Natl Acad Sci USA 1977;74:1688–1692.
Daynes R, Spellman C . Evidence for the generation of suppressor cells by ultraviolet radiation. Cell Immunol 1977;31:182–187.
Grimbaldeston M, Skov L, Finlay-Jones J, et al. Increased dermal mast cell prevalence and susceptibility to development of basal cell carcinomas in humans. Methods 2002;28:90–96.
Grimbaldeston M, Pearce A, Robertson B, et al. Association between melanoma and dermal mast cell prevalence in sun-unexposed skin. Br J Dermatol 2004;150:895–903.
Rowe D, Carroll R, Day C . Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. J Am Acad Dermatol 1992;26:976.
Hart P, Townley S, Grimbaldeston M, et al. Mast cells, neuropeptides, histamine, and prostaglandins in UV-induced systemic immunosuppression. Methods 2002;28:79–89.
Garssen J, Buckley T, van Loveren H . A role for neuropeptides in UVB-induced systemic immunosuppression. Photochem Photobiol 1998;68:205–210.
Wille J, Kydonieus A, Murphy G . Cis-urocanic acid induces mast cell degranulation and release of preformed TNF-alpha: a possible mechanism linking UVB and cis-urocanic acid to immunosuppression of contact hypersensitivity. Skin Pharmacol Physiol 1999;12:18–27.
Walsh L . Ultraviolet B irradiation of skin induces mast cell degranulation and release of tumor necrosis factor-alpha. Immunol Cell Biol 1995;73:226–233.
Kurimoto I, Streilein J . Deleterious effects of cis-urocanic acid and UVB radiation on langerhans cell and on induction of contact hypersensitivity are mediated by tumor necrosis factor-alpha. J Invest Dermatol 1992;99:69S–70S.
Harizi H, Juzan M, Pitard V, et al. Cyclooxygenase-2-issued prostaglandin E2 enhances the production of endogenous IL-10, which down-regulates dendritic cell functions. J Immunol 2002;168:2255–2263.
Kalinski P, Hilkens C, Snijders A, et al. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2 promote type 2 cytokine production in maturing human naive Th cells. J Immunol 1997;159:28.
Schwarz A, Stander S, Berneburg M, et al. Interleukin 12 suppresses ultraviolet radiation-induced apoptosis by inducing DNA repair. Nat Cell Biol 2002;4:26–31.
Nordlund J, Askenase P . The effect of histamine, antihistamine, and mast cell stabilizer on the growth of Cloudman melanoma cells in DBA/2 mice. J Invest Dermatol 1983;81:28–31.
Folkman J . What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst 1990;82:4–6.
Algire G, Chalkley H, Legallais F, et al. Vascular reactions of normal and malignant tumors in vivo. I. Vascular reactions of mice to wounds and to normal and neoplastic transplants. J Natl Cancer Inst 1945;6:73–85.
Coussens L, Werb Z . Matrix metalloproteinases and the development of cancer. Chem Biol 1996;3:895–904.
Meninger C . Mast cells and tumor-associated angiogenesis. In: Marone G (ed). Human Basophils and Mast Cells: Clinical Aspects. Karger: Basel, 1995, pp 238–256.
Brown L, Detmar M, Claffey K, et al. Vascular permeability factor/vascular endothelial growth factor. A multifunctional angiogenic cytokine. In: Goldberg I, Rosen E (eds). Regulation of Angiogenesis. Birkhauser: Basel, 1997, pp 233–269.
Friedl A, Chang Z, Tierney A, et al. Differential binding of fibroblast growth factor-2 and -7 to basement membrane heparin sulfate. Am J Pathol 1997;150:1443–1455.
Srivastava A, Laidler P, Davies R, et al. The prognostic significance of tumor vascularity in intermediate-thickness (0.76–4.0 mm thick) skin melanoma. Am J Pathol 1988;133:419–423.
Poole T, Zetter B . Stimulation of rat peritoneal mast cell migration by tumor-derived peptides. Cancer Res 1983;43:5857–5861.
Starkey J, Crowle P, Taubenberger S . Mast-cell deficient W/Wv mice exhibit a decreased rate of tumor angiogenesis. Int J Cancer 1988;42:48–52.
Ribatti D, Vacca A, Ria R, et al. Neovascularization, expression of fibroblast growth factor-2, and mast cells with tryptase activity increase simultaneously with pathological progression in human malignant melanoma. Eur J Cancer 2003;39:666–674.
Gordon J, Burd P, Galli S . Mast cells as a source of multifunctional cytokines. Immunol Today 1990;11:458–464.
Reed J, Albino A, McNutt N . Human cutaneous mast cells express b-FGF. Lab Invest 1995;72:215–222.
Ugurel S, Rappl G, Tilgen W, et al. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol 2001;19:577–583.
Sawatsubashi M, Yamada T, Fukushima N, et al. Association of vascular endothelial growth factor and mast cells with angiogenesis in laryngeal squamous cell carcinoma. Virchows Arch 2000;436:243–248.
Dvorak H, Brown L, Detmar M, et al. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 1995;146:1029–1039.
Coussens L, Raymond W, Bergers G, et al. Inflammatory mast cells upregulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev 1999;13:1382–1397.
Cairns J, Walls A . Mast cell tryptase stimulates the synthesis of type I collagen in human lung fibroblasts. J Clin Invest 1997;99:1313–1321.
Gruber B, Kew R, Jelaska A, et al. Human mast cells activate fibroblasts. J Immunol 1997;158:2310–2317.
Blair R, Meng H, Marchese M, et al. Human mast cells stimulate vascular tube formation. Tryptase is a novel, potent angiogenic factor. J Clin Invest 1997;99:2691–2700.
Roche W . Mast cells and tumor angiogenesis: the tumor-mediated release of an endothelial growth factor mast cells. Int J Cancer 1985;36:721–728.
Duncan J, Brown F, McKinnon A, et al. Patterns of angiogenic response to mast cell granule constituents. Int J Microcirc Clin Exp 1992;11:21–33.
Bar-Eli M . Role on interleukin-8 in tumor growth and metastasis of human melanoma. Pathobiology 1999;67:12–18.
Singh R, Varney M, Bucana C, et al. Expression of interleukin-8 in primary and metastatic malignant melanoma of the skin. Melanoma Res 1999;9:383–387.
Stetler-Stevenson W, Aznavoorian S, Liotta L . Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Dev Biol 1993;9:541–573.
Woolley D, Whitehead R, Walker R, et al. Mast cell-tumor cell interactions: matrix degradation and the demonstration of histamine H2 receptors on human melanoma. Adv Exp Med Biol 1988;233:81–88.
Dabbous M, Walker R, Haney L, et al. Mast cells and matrix degradation at sites of tumor invasion in rat mammary adenocarcinoma. Br J Cancer 1986;54:459–465.
Baram D, Vaday G, Salamon P, et al. Human mast cells release metalloproteinase-9 on contact with activated T cells: juxtacrine regulation by TNF-alpha. J Immunol 2001;167:4009–4016.
Stack M, Johnson D . Human mast cell tryptase activates single-chain urinary-type plasminogen activator. J Biol Chem 1994;269:9416–9419.
Sakamoto S, Goldhaber P, Glimcher M . The effect of heparin on the amount of enzyme released in tissue culture and on the activity of the enzyme. Calcif Tissue Int 1973;12:247–258.
Markwardt F, Klocking H . Heparin-induced release of plasminogen activator. Haemostasis 1977;6:370–374.
Reed J, McNutt N, Bogdany J, et al. Expression of the mast cell growth factor interleukin-3 in melanocytic lesions correlates with an increased number of mast cells in the perilesional stroma: implications for melanoma progression. J Cutan Pathol 1996;23:495–505.
Yamamoto T, Katayama I, Nishioka K . Expression of stem cell factor in basal cell carcinoma. Br J Dermatol 1997;137:709–713.
Halaban R, Kwon B, Ghosh S, et al. bFGF as an autocrine growth factor for human melanoma. Oncogene Res 1988;3:177.
Schadendorf D, Moller A, Algermissen B, et al. IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor. J Immunol 1993;151:2667.
Wang Y, Becker D . Antisense targeting of basic fibroblast growth factor and fibroblast growth factor-1 in human melanomas blocks intratumoral angiogenesis and tumor growth. Nat Med 1997;3:887–893.
Becker D, Lee P, Rodeck U, et al. Inhibition of the fibroblast growth factor receptor 1 (FGFR-1) gene in human melanocytes and malignant melanomas leads to inhibition of proliferation and signs indicative of differentiation. Oncogene 1992;7:2302–2313.
Nilsson G, Butterfield JH, Nilsson K, et al. Stem cell factor is a chemotactic factor for human mast cells. J Immunol 1994;153:3717–3723.
Theoharides T, Bondy P, Tsakalos N, et al. Differential release of serotonin and histamine from mast cells. Nature 1982;297:229–231.
Sieghart W, Theoharides T, Alper S, et al. Calcium-dependent protein phosphorylation during secretion by exocytosis in the mast cell. Nature 1978;275:329–331.
Frossi B, De Carli M, Daniel J, et al. Oxidative stress stimulates IL-4 and IL-6 production in mast cells by an APE/Ref-1-dependent pathway. Eur J Immunol 2003;33:2168–2177.
Gagari E, Tsai M, Lantz CS, et al. Differential release of mast cell interleukin-6 via c-kit. Blood 1997;89:2654–2663.
Boesiger J, Tsai M, Maurer M, et al. Mast cells can secrete vascular permeability factor/vascular endothelial cell growth factor and exhibit enhanced release after immunoglobulin E-dependent upregulation of Fce receptor I expression. J Exp Med 1998;188:1135–1145.
Schittek A, Issa H, Stafford J, et al. Growth of pulmonary metastases of B16 melanoma in mast cell-free mice. J Surg Res 1985;38:24–28.
Lu C, Kerbel R . Interleukin-6 undergoes transition from a paracrine growth inhibitor to autocrine stimulator during human melanoma progression. J Cell Biol 1993;120:1281.
Swope V, Abdel-Malek Z, Kassem L, et al. Interleukins 1a and 6 and necrosis factor a are paracrine inhibitors of human melanocyte proliferation and melanogenesis. J Invest Dermatol 1991;96:180–185.
Acknowledgements
Dr S Ch'ng is the recipient of the Max Lovie Research Fellowship awarded by the Reconstructive Plastic Surgery Research Foundation. Her work is also supported by grants from the Pub Charity and the Trusts Charitable Foundation. We thank Mr Brennan Thomas for his assistance in the preparation of the illustrations.
Author information
Authors and Affiliations
Corresponding author
Additional information
Parts of this work were presented at the Royal Australasian College of Surgeons' Annual Scientific Congress, Perth, Australia, May 9–13, 2005.
Rights and permissions
About this article
Cite this article
Ch'ng, S., Wallis, R., Yuan, L. et al. Mast cells and cutaneous malignancies. Mod Pathol 19, 149–159 (2006). https://doi.org/10.1038/modpathol.3800474
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800474
Keywords
This article is cited by
-
BMP6-induced modulation of the tumor micro-milieu
Oncogene (2019)
-
Mast Cells in Renal Cancer: Clinical Morphological Correlations and Prognosis
Bulletin of Experimental Biology and Medicine (2017)
-
Fibrosis and Mast Cells in Colorectal Lesions: Significance in Adenoma-Colorectal Cancer Sequence and Association with Diet
Journal of Gastrointestinal Cancer (2016)
-
Age-associated inflammation connects RAS-induced senescence to stem cell dysfunction and epidermal malignancy
Cell Death & Differentiation (2015)
-
Pharmacologically Antagonizing the CXCR4-CXCL12 Chemokine Pathway with AMD3100 Inhibits Sunlight-Induced Skin Cancer
Journal of Investigative Dermatology (2014)