Abstract
We investigated 27 pleomorphic carcinomas of the lung for exon 1 K-ras gene mutations using polymerase chain reaction–single-strand conformation polymophism analysis and direct sequencing. All pleomorphic carcinomas were biphasic, that is, composed of an adeno-, squamous- or large-cell-carcinomatous component associated with a spindle- and/or giant-cell component. Of 27 cases, six (22%) showed K-ras codon 12 mutations, which is a figure higher than that previously reported on in pure sarcoma-like pleomorphic carcinomas. Five tumors displayed the same mutation in both the epithelial and the sarcomatoid components, whereas in one tumor the mutation was restricted to the epithelial component. All mutations occurred in smokers, and were transversions, including GGT (glycine) to TGT (cysteine) change in two cases, to GCT (alanine) in two and to GTT (valine) in two. No significant relationships were found between the occurrence and type of mutations and patients' survival or any other clinicopathological variable, suggesting that K-ras mutations are early events in the development of these tumors. Our results indicate that most, though not all, biphasic pleomorphic carcinomas of the lung are monoclonal in origin, and that cigarette smoking may have a causative role in the development of K-ras alterations in these tumors, as all mutations are transversions.
Similar content being viewed by others
Main
Non-small-cell lung carcinomas are morphologically heterogeneous tumors including three major histological subtypes, namely adenocarcinoma, squamous cell carcinoma and large-cell carcinoma.1, 2 Among most uncommon subtypes, there are poorly differentiated tumors composed of an intimate admixture of carcinoma and sarcoma or sarcoma-like components accounting for 5% or less of all non-small-cell lung carcinomas. These tumors have been variously designated in the past as biphasic and monophasic sarcomatoid carcinoma, pleomorphic carcinoma, spindle cell carcinoma, giant cell carcinoma, pseudosarcoma, pulmonary blastoma and carcinosarcoma.3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 The latest recommendations of the World Health Organization have eliminated most of these terms,1 confirming the designation of pleomorphic carcinomas to either pure neoplasms consisting of sarcomatoid spindle and giant tumor cells or biphasic neoplasms containing sarcomatoid spindle and/or giant cell components (accounting for at least 10% of the tumor mass) and conventional non-small-cell lung carcinoma, including squamous cell carcinoma, adenocarcinoma or large-cell carcinoma. These tumors may be differentiated from carcinosarcomas for the lack of a true malignant heterologous mesenchymal component, such as bone, cartilage, vessels or skeletal muscle,10 and from pulmonary blastomas for the lack of primitive mesenchymal and epithelial tumor cells.1, 20 Pleomorphic carcinomas are basically thought to be epithelial tumors in which neoplastic cells undergo an epithelial–mesenchymal transition21 and express vimentin and other mesenchymal proteins.3, 7, 13, 14, 15, 17, 19, 22
In different anatomical sites, most, but not all, of these tumors composed of carcinoma and sarcoma or sarcoma-like components are considered monoclonal growths (the so-called divergent hypothesis) rather than true collision tumors (convergent hypothesis).20, 23, 24, 25, 26, 27, 28, 29, 30, 31 In turn, these monoclonal growths may originate from a single ancestor undergoing divergent epithelial and mesenchymal differentiation early during the neoplastic transformation (combination theory), or the full-blown carcinoma cells undergo sarcomatoid changes during tumor progression (conversion theory).21, 23, 24, 25
In the lung, a monoclonal origin has been suggested for carcinosarcoma and blastoma using diverse molecular strategies, such as X chromosome inactivation,24 microsatellite analysis,29 and mutational genotyping of p53 gene,28 whereas little is known about clonality of pulmonary pleomorphic carcinomas.28
Using laser-assisted microdissection of formalin-fixed and paraffin-embedded sections, we have investigated the prevalence of K-ras codon 12 mutations in 27 biphasic pleomorphic carcinomas, in both the epithelial and sarcomatoid components. Our results indicate that six of 27 (22%) cases showed K-ras codon 12 mutations, which is a figure higher than that previously reported on in pure sarcoma-like pleomorphic carcinomas.32, 33 Five pleomorphic carcinomas had the same mutation in the epithelial and sarcomatoid components, suggesting a monoclonal origin from a single ancestor.
Materials and methods
Patients
A total of 27 consecutive cases of pulmonary biphasic pleomorphic carcinomas were retrieved from the Surgical Pathology files of the City Hospital in Verona and the European Institute of Oncology in Milan, Italy. The clinical characteristics of the patients are summarized in Table 1. There were 25 male and two female cases (M : F ratio 1 : 0.08), aged from 39 to 79 years (mean±s.d.: 62.7±10.2 years; median 64 years). All but one (PLC-26) patient were cigarette smokers, and all but five had local or systemic symptoms at the time of diagnosis, with a Karnowsky's performance status ranging from 70 to 100%. Complete follow-up information was available for all patients, with a mean duration of 37.3±37.8 months (median 18; range 2–114). Recurrent disease at different sites was seen in 15 (56%) patients, 12 (44%) of which died of disease. Two patients (PLC-25 and PLC-29) died of unrelated disease, that is, acute heart failure and adult respiratory distress syndrome, respectively. According to the revised international system for staging lung cancer,1 there were 16 patients with either p-stage IA (six cases) or IB (10 cases), and 11 with either p-stage IIB (eight cases) or IIIA (three cases). Three patients with disease stage IIB and IIIA underwent neoadjuvant or postoperative chemotherapy or radiotherapy, whereas patients with disease stage I did not receive any additional therapy.
For each case, all paraffin blocks were retrieved, and archival hematoxylin and eosin sections reviewed. The diagnosis of pleomorphic carcinoma was rendered according to the current WHO guidelines.1 Percentage and patterns of the sarcomatoid (spindle, giant or mixed, the latter being composed of both spindle and giant cells) and the epithelial (adeno-, squamous- or large-cell carcinoma) components, vascular invasion and tumor necrosis were assessed for every tumor. Necrosis was evaluated semiquantitatively on a scale from absent to 2+ (1+ if ≤50% and 2+ if >50% of the whole tumor area).
Immunohistochemistry
The immunohistochemical experiments were performed using monoclonal antibodies to vimentin (clone V9, Dako, Glostrup, Denmark; dilution 1:50), cytokeratins (clone AE1-AE3, Novocastra Laboratories, Newcastle upon Tyne, UK; dilution 1:50), epithelial membrane antigen (clone E29, Dako; dilution 1:20), and carcinoembryonic antigen (CEA) (clone 11-7, Dako; dilution 1:50), and a commercially available detection kit (EnVision Plus-HRP, Dako), according to either the manufacturer's suggestions or previously refined immunohistochemical methods.34, 35 In each tumor, the sarcomatoid and epithelial components were assessed separately, recording the percentage of neoplastic cells immunoreactive for the different antigens in either components.
Microdissection and K-ras Gene Analysis
For each tumor, different areas corresponding to the epithelial and sarcomatoid component were microdissected from 3 μm-thick hematoxylin- and- eosin-stained sections using a computer-assisted laser micro-dissector (Leica Microsystem, Milan, Italy) according to the manufacturer's instructions. Tumors were entirely scanned if 2 cm or less in size, while two representative tissue blocks were used in larger neoplasms to avoid any bias in the sampling procedure. A total of 16 000–20 000 tumor cells were detached from the epithelial and the sarcomatoid components for genotyping. An equivalent amount of normal cells was microdissected from the surrounding pulmonary parenchyma or bronchial-associated lymphoid tissue of each case. All microdissected fragments were separately placed into microcentrifuge tubes and digested overnight at 55°C in lysis buffer containing 400 μg/ml proteinase K.
DNA was amplified for K-ras exon 1 using specific primers: forward 5′-GACTGAATATAAACTTGTGG-3′, reverse 5′-CTGTATCAAAGAATGGTCCT-3′. Polymerase chain reaction (PCR) was run in 50 μl final volume, containing 1 × PCR Buffer II (Applied Biosystems), 2 mM MgCl2 (Applied Biosystems), 200 μM dNTPs (Amersham Bioscience), 0.5 μM of each primers and 0.025 U/μl AmpliTaq DNA Polymerase (Applied Biosystems). The conditions for PCR were denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s. Two rounds of 30 cycles were used.
K-ras mutations were detected using PCR–single-strand conformation polymorphism (PCR-SSCP) analysis and direct DNA sequencing. For PCR-SSCP, PCR products (5 μl) were mixed with 10 μl of gel loading solution (75% deionized formamide, 12 mM NaOH, 6 mM EDTA and 0.05% xylene cyanol and bromophenol blue), denaturated at 95°C for 2 min and kept on ice until loading on a 15% nondenaturating polyacrylamide gel (30:1 acrylamide:bisacrylamide). To improve band resolution, wells were casted in a 5% polyacrylamide stacking gel. Gels, containing a 1 × TBE buffer were run with a discontinuous buffer system (25 mM tris and 192 mM glycine). Electrophoresis was performed at 90 V for 17–18 h on a PROTEAN II XI cell (BIORAD) at room temperature, and the gels were stained with 0.1% AgNO3. Results of SSCP analysis were confirmed performing two independent experiments, and the DNA samples of all cases exhibiting aberrant bands were sequenced as described.36 Appropriate positive (SW480) and negative (HT29) control cell lines were used in parallel in all experiments for the evaluation of point mutations of exon 1 of the K-ras gene.
Statistical Analysis
Associations of categorical variables were evaluated by Fisher's exact t-test or χ2-test to determine the statistical significance of differences in clinicopathological data according to the presence or absence of K-ras gene mutations. Continuous data were expressed using median values and contrasted employing the Wilcoxon signed-rank test. Overall survival was defined as the time between surgery and the last follow-up or lung cancer-related death. If a patient died without cancer recurrences, the patient's survival time was censored at the time of death. Only lung cancer-related deaths or recurrences were considered as events. Disease-free survival was calculated from the date of surgery to the date of progression or the date of the last follow-up. Survival estimates were calculated with Kaplan–Meier's method and compared by the log rank test. All analyses were carried out using the SAS statistical software (SAS Institute, Inc., Cary, NC, USA). All P-values were based on two-sided testing, and confidence intervals were set at a 95% level.
Results
Pathological Characteristics of Pleomorphic Carcinomas
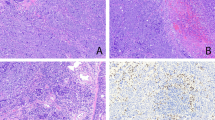
The pathological findings are summarized in Table 2. Grossly, the tumors ranged from 1.9 to 17 cm, with marginal differences in the median values according to tumor stage (4 cm in stage I vs 5.5 cm in stage II–IIIA) (P=0.064). Representative histological features of pleomorphic carcinomas are shown in Figure 1. The epithelial component was poorly differentiated in all but two moderately differentiated cases, and the sarcomatoid component accounted for 15–90% of the whole neoplasms (Figure 1a). The spindle cell sarcomatoid component was either bland-appearing resembling fibrosarcoma or highly atypical resembling malignant fibrous histiocytoma (Figure 1b). Giant cells exhibited multilobated and bizarre nuclei and abundant eosinophilic cytoplasms, sometimes engulfing leukocytes (Figure 1c). The mixed pattern resulted from a variable proportion of highly atypical giant and spindle tumor cells (Figure 1d). Three tumors showed squamous cell carcinoma associated with mixed (two cases) or spindle (one case) sarcomatoid components, 11 tumors showed adenocarcinoma associated with mixed (eight cases), giant (two cases) or spindle (one case) sarcomatoid components, and 12 tumors large-cell carcinoma associated with mixed (six cases), giant (five cases) or spindle (one case) cells. One tumor (PLC-28) included a triple epithelial component (bronchioloalveolar carcinoma, adenocarcinoma and squamous cell carcinoma) associated with a spindle cell component. Tumor necrosis was encountered in all but four cases, ranging from focal (<50% of the whole tumor) in nine cases, to diffuse (>50%) in the remaining 14 tumors. Vascular invasion was recognizable in 16 (59%) cases.
Representative histological features of pulmonary pleopmorphic carcinoma are shown. A typical biphasic appearance is presented, with epithelial cells resembling those of a conventional lung adenocarcinoma (black asterisk), and sarcomatoid spindle tumor cells resembling those of fibrosarcoma (a). The spindle cell component may be highly atypical mimicking a malignant fibrous histiocytoma (b). Giant cells are bizarre with multilobated nuclei and abundant eosinophilic cytoplasm filled by leucocytes (c). The mixed sarcomatoid pattern results from a variable proportion of highly atypical giant (arrows) and spindle tumor cells (d). The epithelial component reacts strongly for cytokeratins, whereas the sarcomatoid cells are more occasionally stained (arrows) (e); vimentin immunostaining decorates especially the sarcomatoid tumor cells, whereas the epithelial cells are less immunoreactive (white asterisks) (f).
The immunohistochemical results are summarized in Table 3. The epithelial component, independent of the histological type, size and stage of the tumor was more consistently and more intensely immunoreactive for cytokeratins and epithelial membrane antigen, whereas the opposite occurred for vimentin (Figures 1e and f). CEA immunostaining was observed in only 16 tumors, where it was restricted to the epithelial component.
K-ras Mutations in Pleomorphic Carcinomas
The results are summarized in Table 2 and representative examples shown in Figure 2. K-ras point mutations at codon 12 were found in six (22%) of the 27 cases of pleomorphic carcinomas, whereas no mutation was present in the corresponding normal lung tissue. All K-ras mutations were confined to smoking male patients. The mutated cases were four adenocarcinomas and two large-cell carcinomas with mixed (four cases) or spindle (two cases) sarcomatous components. Four tumors showing a squamous cell carcinoma component, either alone or associated with adenocarcinoma, had no K-ras mutations. In all, but one, pleomorphic carcinoma with mutated K-ras, an identical mutation was detected in both the epithelial and the sarcomatoid components. All point mutations were G:C → T:A and G:C → C:G transversions. In particular, the normal DNA sequence GGT (encoding glycine) at codon 12 was changed to TGT (cysteine) in two cases (PLC-14 and PLC-21), to GCT (alanine) in two cases (PLC-7 and PLC-8) and to GTT (valine) in one case (PLC-25). In the remaining case (PLC-26), the sequence GGT was altered to GTT (valine) in the epithelial component only, whereas the sarcomatoid cells had a germline sequence. No significant relationship was found between the occurrence and type of K-ras mutations and the different clinicopathological variables under evaluation, including tumor type, size and stage, presence of necrosis or vascular invasion, age at diagnosis, gender, and performance status. In particular, no difference in survival was noted between the six patients with tumors carrying K-ras mutations and the 21 patients with tumors lacking K-ras mutations (Figure 3).
A PCR–SSCP analysis of K-ras exon 1 in pleomorphic carcinoma is presented. The cases PLC-14 and PLC-7 show the same abnormal bands in both the epithelial (E) and sarcomatoid (PL) components with respect to the matched normal DNA (N). In case PLC-26, an abnormal band pattern is present in the epithelial component only, while the sarcomatoid component shows the same pattern as the normal DNA. Lack of the lower SSCP bands in the sarcomatoid component of case PLC-14 is due to the loss of the second K-ras allele, as in the SW480 control cell line (C+).
Discussion
The results of the current study on a series of 27 biphasic pleomorphic carcinomas of the lung may be summarized as follows: (i) K-ras mutations occured in 22% of the cases; (ii) the same K-ras mutation was detected in both the epithelial and sarcomatoid components of the same tumor in all, but one case, consistent with a monoclonal origin of the two morphologically distinct components; (iii) all the present and the previously reported K-ras mutations are transversions, consistent with a smoke-related origin of these gene alterations.
The prevalence of K-ras mutations in the current series of biphasic pleomorphic carcinomas is slightly different from that recently reported in two other series of pulmonary pleomorphic carcinomas.28, 32, 33 The different proportion of K-ras mutations among these three series, however, most likely depends on the type of the epithelial component of pleomorphic carcinomas. In fact, in one series, K-ras mutations were absent in 12 biphasic neoplasms predominantly composed of squamous cell carcinoma associated with spindle cells,28 and in the second published series, a 12% prevalence of mutations was reported among 25 pure pleomorphic carcinomas, including exclusively spindle- and giant-cell tumors.32, 33 At variance with these two series, the epithelial component of our biphasic pleomorphic carcinomas was largely represented of adenocarcinoma and large-cell carcinoma. As a matter of fact, among the common lung cancer histotypes, K-ras gene mutations are found in 10–35% of non-small-cell lung carcinomas, especially adenocarcinoma and large-cell carcinomas, and uncommonly in squamous cell carcinomas.37, 38 In the current series, K-ras mutations were found in four (36%) of 11 cases with adenocarcinoma and in two (16%) of 12 with large-cell carcinoma morphology.
In the lung, a monoclonal origin has been suggested for carcinosarcoma and blastoma using a variety of molecular approaches in a small number of analyzed tumors, including one carcinosarcoma examined for hypoxanthine-phosphoribosyl transferase gene polymorphism,24 five cases of carcinosarcomas evaluated for 12 polymorphic microsatellite markers,29 and one carcinosarcoma and one blastoma studied for p53 mutations.28 In the present investigation, we found K-ras point mutations in 6/27 (22%) tumors. The occurrence of the same point mutation in both the epithelial and the sarcomatoid component within individual tumors supports a monoclonal origin for most of these biphasic pleomorphic carcinomas, with a statistical odds of only one in 32 for the two distinct neoplastic populations of undergoing the same allele inactivation by random chance alone. Our findings are in keeping with a previous report on a smaller series of biphasic pleomorphic carcinomas (all composed of squamous and spindle cell carcinoma) using p53 gene mutational analysis for the assessment of clonality.28 The latter report suggests a monoclonal histogenesis of pulmonary biphasic pleomorphic carcinomas from a totipotent cell by finding identical p53 mutations in both the epithelial and sarcomatoid components.28 A single tumor in our series (case PLC-26), however, harbored a K-ras mutation in the epithelial but not the sarcomatoid cells, raising the possibility of a biclonal origin for few of these tumors, in keeping with similar findings described also in biphasic carcinomas of the female genital tract.25
Another interesting finding of our investigation is that the sarcomatoid cells in our clonally mutated pleomorphic carcinomas retained variable phenotypic expressions of diverse epithelial markers evaluated according to the percentage of immunoreactive tumor cells, such as cytokeratins, epithelial membrane antigen and/or CEA, whereas showed a strong prevalence of mesenchymal markers, such as vimentin. This differential distribution between the two cell components was independent of tumor stage (Table 3). Therefore, we speculate that biphasic pleomorphic carcinomas may either be metaplastic carcinomas derived from trans-differentiation of malignant epithelial cells into sarcoma-like cells (according to the conversion theory) or arise from a common progenitor that shows an early divergent bi-directional differentiation (according to the combination theory). Our hypothesis of a metaplastic origin of biphasic pleomorphic carcinomas is also supported by three other recent studies, two immunohistochemical dealing with pleomorphic carcinomas19, 22 and another genetic dealing with carcinosarcomas of the lung.28 In the latter, a multistep progression of the sarcomatous component from a common totipotent ancestor through an intermediate stage of carcinoma was prospected, as these tumors showed a greater amount of genetic damages in the mesenchymal than in the epithelial component.28
The association between smoking and pulmonary pleomorphic carcinomas is very strong,7, 14, 15, 17, 32 as re-emphasized also by the current series of patients, in whom all but one were smokers. All K-ras mutations detected in our tumors were transversions, namely G:C → T:A in four cases and G:C → C:G in two cases, exactly paralleling the findings of previous reports showing a higher prevalence of G:C → T:A (67%) than G:C → C:G transversions (33%).32, 33 As transversions resulting from DNA adducts are most often observed in polycyclic aromatic hydrocarbon-exposed smokers, it is likely that a cigarette smoke-related mechanism is involved in the K-ras gene alterations of the subset of mutated pulmonary pleomorphic carcinomas. Interestingly, an Ile–Val polymorphism of the CYP1A1*2 exon 7, encoding an inducible extrahepatic enzyme activating polycyclic aromatic hydrocarbons, has recently been found in 76% of pleomorphic carcinomas (as compared with 12% of the normal population).33 Finally, the lack of any relationship between occurrence of K-ras mutations and any indicator of tumor aggressiveness, such as prevalence of sarcomatoid cells, size, stage, presence of vascular invasion, and patients' survival, could indicate that K-ras mutations are early events in the development of biphasic pleomorphic carcinomas of the lung, as one would expect from a promutagenic factor, such as cigarette smoke. The lack of any relationship with survival and the relatively small number of mutated tumors in our investigation, however, suggest that K-ras gene mutations are not critical events in the development and malignancy progression of pulmonary pleomorphic carcinomas.
In conclusion, we here indicate that most pleomorphic carcinomas of the lung are monoclonal as determined by microdissection-based K-ras gene mutational analysis, and that cigarette smoke may have a causative role in the development of these mutations because all mutations are transversions.
References
Travis WD, Colby TV, Corrin B, et al. Histological Typing of Lung and Pleural Tumours. Springer-Verlag: Berlin/Heidelberg/New York, 1999.
Corrin B . Pathology of the Lungs. Churchill Livingstone: London/Edinburgh/New York, 2000.
Attanoos RL, Papagiannis A, Suttinont P, et al. Pulmonary giant cell carcinoma: pathological entity or morphological phenotype? Histopathology 1998;32:225–231.
Berho M, Moran CA, Suster S . Malignant mixed epithelial/mesenchymal neoplasms of the lung. Semin Diagn Pathol 1995;12:123–139.
Colby TV, Koss MN, Travis WD . Tumors of the Lower Respiratory Tract. Armed Forces Institute of Pathology: Washington, 1995.
Davis MP, Eagan RT, Weiland LH, et al. Carcinosarcoma of the lung: Mayo Clinic experience and response to chemotherapy. Mayo Clin Proc 1984;59:598–603.
Fishback NF, Travis WD, Moran CA, et al. Pleomorphic (spindle/giant cell) carcinoma of the lung. A clinicopathologic correlation of 78 cases. Cancer 1994;73:2936–2945.
Chang YL, Lee YC, Shih JY, et al. Pulmonary pleomorphic (spindle) cell carcinoma: peculiar clinicopathologic manifestations different from ordinary non-small-cell carcinoma. Lung Cancer 2001;34:91–97.
Humphrey PA, Scroggs MW, Roggli VL, et al. Pulmonary carcinomas with a sarcomatoid element: an immunocytochemical and ultrastructural analysis. Hum Pathol 1988;19:155–165.
Koss MN, Holzer L, Frommelt RA . Carcinosarcomas of the lung. A clinicopathologic study of 66 patients. Am J Surg Pathol 1999;23:1514–1526.
Krefting IP, Nunez LA, Sherer P, et al. Pleomorphic carcinoma (spindle and giant cell) of the lung. MD Med J 1994;43:787–790.
Matsui K, Kitagawa M . Spindle cell carcinoma of the lung. A clinicopathologic study of three cases. Cancer 1991;67:2361–2367.
Nakajima M, Kasai T, Hashimoto H, et al. Sarcomatoid carcinoma of the lung. A clinicopathologic study of 37 cases. Cancer 1999;86:608–616.
Nappi O, Glasner SD, Swanson PE, et al. Biphasic and monophasic sarcomatoid carcinomas of the lung. A reappraisal of ‘carcinosarcomas’ and ‘spindle-cell carcinomas’. Am J Clin Pathol 1994;102:331–340.
Ro JY, Chen JL, Lee JS, et al. Sarcomatoid carcinoma of the lung. Immunohistochemical and ultrastructural studies of 14 cases. Cancer 1992;69:376–386.
Terzi A, Gorla A, Piubello Q, et al. Biphasic sarcomatoid carcinoma of the lung. Eur J Surg Oncol 1997;23:457.
Wick MR, Ritter JH, Humphrey PA . Sarcomatoid carcinomas of the lung. A clinicopathological review. Am J Clin Pathol 1997;108:40–53.
Wick MR, Ritter JH, Nappi O . Inflammatory sarcomatoid carcinoma of the lung: report of three cases and clinicopathologic comparison with inflammatory pseudotumors in adult patients. Hum Pathol 1995;26:1014–1021.
Rossi G, Cavazza A, Sturm N, et al. Pulmonary carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements: a clinicopathologic and immunohistochemical study of 75 cases. Am J Surg Pathol 2003;27:311–324.
Bodner SM, Koss MN . Mutations in the p53 gene in pulmonary blastomas: immunohistochemical and molecular studies. Hum Pathol 1996;27:1117–1123.
Thiery JP . Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer 2002;2:442–454.
Pelosi G, Fraggetta F, Nappi O, et al. Pleomorphic carcinomas of the lung show a selective distribution of gene products involved in cell differentiation, cell cycle control, tumor growth and tumor cell motility: a clinicopathological and immunohistochemical study of 31 cases. Am J Surg Pathol 2003;9:1203–1215.
Thompson L, Wieneke JA, Miettinen M, et al. Spindle cell (sarcomatoid) carcinomas of the larynx. Am J Surg Pathol 2002;26:153–170.
Thompson L, Chang B, Barsky SH . Monoclonal origins of malignant mixed tumors (carcinosarcomas). Am J Surg Pathol 1996;20:277–285.
McCluggage WG . Malignant biphasic uterine tumours: carcinosarcomas or metaplastic carcinomas. J Clin Pathol 2002;55:321–325.
Fujii H, Yoshida M, Gong ZX, et al. Frequent genetic heterogeneity in the clonal evolution of gynecological carcinosarcoma and its influence on phenotypic diversity. Cancer Res 2000;60:114–120.
Ansari-Lari MA, Hoque MO, Califano J, et al. Immunohistochemical p53 expression patterns in sarcomatoid carcinomas of the upper respiratory tract. Am J Surg Pathol 2002;26:1024–1031.
Holst VA, Finkelstein S, Colby TV, et al. p53 an K-ras mutational genotyping in pulmonary carcinosarcoma, spindle cell carcinoma, and pulmonary blastoma: implications for histogenesis. Am J Surg Pathol 1997;21:801–811.
Dacic S, Finkelstein SD, Sasatomi E, et al. Molecular pathogenesis of pulmonary carcinosarcoma as determined by microdissection-based allelotyping. Am J Surg Pathol 2002;26:510–516.
Sreenan JJ, Hart WR . Carcinosarcomas of the female genital tract. A pathological study of 29 metastatic tumors: further evidence for the dominant role of the epithelial component and the conversion theory of histogenesis. Am J Surg Pathol 1995;19:666–674.
Gronau S, Menz CK, Melzner I, et al. Immunohistomorphologic and molecular cytogenetic analysis of a carcinosarcoma of the urinary bladder. Virchows Arch 2002;440:436–440.
Przygodzki RM, Koss MN, Moran CA, et al. Pleomorphic (giant and spindle cell) carcinoma is genetically distinct from adenocarcinoma and squamous cell carcinoma by K-ras-2 and p53 analysis. Am J Clin Pathol 1996;106:487–492.
Przygodzki RM, Koss MN, O'Leary TJ . Pleomorphic (giant and/or spindle cell) carcinoma of lung shows a high percentage of variant CYP1A1*2. Mol Diagn 2001;6:109–115.
Pelosi G, Pastorino U, Pasini F, et al. Independent prognostic value of fascin-1 immunoreactivity in stage I non-small cell lung cancer. Br J Cancer 2003;88:537–547.
Pelosi G, Pasini F, Olsen Stenholm C, et al. p63 immunoreactivity in lung cancer. Yet another player in the development of squamous cell carcinomas? J Pathol 2002;198:100–109.
Moore PS, Zamboni G, Brighenti A, et al. Molecular characterization of pancreatic serous mycrocystic adenomas. Evidence for a tumor suppressor gene on chromosome 10q. Am J Pathol 2001;158:317–321.
Fong KM, Sekido Y, Minna JD . Molecular pathogenesis of lung cancer. J Thorac Cardiovasc Surg 1999;118:1136–1152.
Sekido Y, Fong KM, Minna JD . Progress in understanding the molecular pathogenesis of human lung cancer. Biochim Biophys Acta 1998;1378:F21–F59.
Acknowledgements
This investigation was generously supported by AIRC (Associazione Italiana per la Ricerca sul Cancro), Fondazione Cassa di Risparmio di Verona (Bando 2001) and CNR-MIUR ‘Diagnostica molecolare in oncologia’.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pelosi, G., Scarpa, A., Manzotti, M. et al. K-ras gene mutational analysis supports a monoclonal origin of biphasic pleomorphic carcinoma of the lung. Mod Pathol 17, 538–546 (2004). https://doi.org/10.1038/modpathol.3800058
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800058
Keywords
This article is cited by
-
Integrated molecular characterization reveals potential therapeutic strategies for pulmonary sarcomatoid carcinoma
Nature Communications (2020)
-
Molecular profiles of EGFR, K-ras, c-met, and FGFR in pulmonary pleomorphic carcinoma, a rare lung malignancy
Journal of Cancer Research and Clinical Oncology (2011)
-
Combined small-cell carcinoma of the lung with quadripartite differentiation of epithelial, neuroendocrine, skeletal muscle, and myofibroblastic type
Virchows Archiv (2011)
-
Lung carcinoma with spindle and/or giant cell: a clinicopathological analysis of 17 cases
The Chinese-German Journal of Clinical Oncology (2009)
-
Sarcomatoid carcinomas of the lung—are these histogenetically heterogeneous tumors?
Virchows Archiv (2006)