Abstract
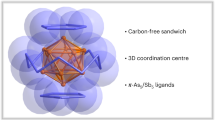
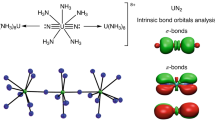
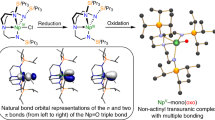
RECENT work1–5 has shown that gold-based ligands in compounds of carbon and nitrogen can induce novel molecular structures with coordination numbers at C and N as high as 5 and 6. These phenomena must be ascribed to metal–metal interactions (Au…Au), which can overrule bonding in classical configurations. Here we describe a study of the molecular structures of tetra(auro)ammonium ((LAu)4N+) and tetra(auro)arsonium ((LAu)4As+) cations (where L is a ligand). We find that the classical tetrahedral structure in these four-coordinate compounds is abandoned in favour of a square-pyramidal geometry once the radius of the central element is too large to allow for metal–metal bonding in a tetrahedral geometry. Thus, whereas the nitrogen compounds adopt a tetrahedral structure, for the larger arsenic atom an arsenic-capped square of gold atoms represents a more favourable core geometry. We have not yet been able to prepare the intermediate phosphorus compound, but we expect it also to have the square-pyramidal structure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Grohmann, A., Riede, J. & Schmidbaur, H. Nature 345, 140–142 (1990).
Schmidbaur, H. Gold. Bull. 23, 11–20 (1990).
Scherbaum, F., Grohmann, A., Huber, B., Krüger, C. & Schmidbaur, H. Angew. Chem. Int. Edn Engl. 27, 1544–1546 (1988).
Scherbaum, F., Grohmann, A., Müller, G. & Schmidbaur, H. Angew. Chem. Int. Edn Engl. 28, 463–465 (1989).
Scherbaum, F., Huber, B., Müller, G. & Schmidbaur, H. Angew Chem. Int. Edn Engl. 27, 1542–1544 (1988).
Greenwood, N. N. & Earnshaw, A. N. Chemistry of the Elements (Pergamon, Oxford, 1986).
Hoffmann, R. Angew. Chem. Int. Edn Engl. 21, 711–723 (1982).
Stone, F. G. A. Angew. Chem. Int. Edn Engl. 23, 85–95 (1984).
Hall, K. P. & Mingos, D. M. P. Prog, inorg. Chem. 32, 237–254 (1984).
Slovokhotov, Yu. L. & Struchkov, Yu. T. J. organometall. Chem. 277, 143–146 (1984).
Perevalova, E. G., Smyslova, E. I., Dyadchenko, V. P., Grandberg, K. I. & Nesmeyanov, A. N. Izv. Akad. Nauk SSSR Ser. Khim. 1455 (1980) (in Russian).
Brodbeck, A. thesis, Univ. of Tübingen (1990).
Brodbeck, A. & Strähle, J. Acta Crystallogr. A46, C-232 (1990).
Kolb, A. & Bissinger, P. thesis, Techn. Univ. München (1990).
Grohmann, A., Riede, J. & Schmidbaur, H. J. chem. Soc. Dalton Trans. 783–788 (1991).
Ramamoorthy, V. & Sharp, P. R. Inorg. Chem. 29, 3336–3340 (1990).
Weidenhiller, G., Steigelmann, O., Riede, J. & Schmidbaur, H. Angew. Chem. Int. Edn Engl. 29, 433–435 (1991).
Becker, G., Gutekunst, G. & Wessely, H. J. Z. anorg. allg. Chem. 462, 113–129 (1980).
Nesmeyanov, A. N. et al. J. organometall. Chem. 201, 343–349 (1980).
Schmidbaur, H., Aly, A. A. M. & Schubert, U. Angew. Chem. Int. Edn Engl. 17, 846–847 (1978).
Jones, P. G. Gold Bull. 19, 46–57 (1986).
Rösch, N., Görling, A., Ellis, D. E. & Schmidbaur, H. Angew. Chem. Int. Edn Engl. 28, 1357–1359 (1989).
Rösch, N., Görling, A., Ellis, D. E. & Schmidbaur, H. Inorg. Chem. (in the press).
Mingos, D. M. P. J. Chem. Soc. Dalton Trans. 1163–1170 (1976).
Evans, D. G., Mingos, D. M. P. J. organometall. Chem. 232, 171–174 (1982).
Mingos, D. M. P. Nature 345, 113–114 (1990).
Mingos, D. M. P. & Kanters, R. P. F. J. organometall. Chem. 384, 405–415 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zeller, E., Beruda, H., Kolb, A. et al. Change of coordination from tetrahedral gold–ammonium to square-pyramidal gold–arsonium cations. Nature 352, 141–143 (1991). https://doi.org/10.1038/352141a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/352141a0
This article is cited by
-
Design of a gold clustering site in an engineered apo-ferritin cage
Communications Chemistry (2022)
-
Density functional study of closed-shell attraction on X(ML)3 +(X = O, S, Se; M = Au, Ag, Cu) systems
Journal of Molecular Modeling (2006)
-
Some recent highlights in gold chemistry
Gold Bulletin (2003)
-
The aurophilicity phenomenon: A decade of experimental findings, theoretical concepts and emerging applications
Gold Bulletin (2000)
-
Gold chemistry with ferrocene derivatives as Ligands
Gold Bulletin (1999)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.