Abstract
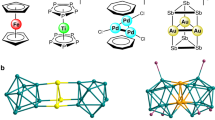
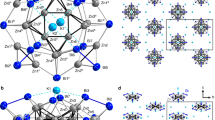
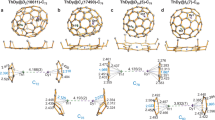
Traditionally, a metallocene complex comprises a metal centre sandwiched between two aromatic organic ligands and such complexes have been extensively investigated. Carbon-free analogues of metallocene with homoleptic As5 or Sb5 ligands, however, have remained experimentally elusive, especially analogues of higher nuclearity. Here we report the synthesis and characterization of sandwich-type clusters [Pn@M12Pn10(Pn5)2]4–/5– (Pn = As, Sb; M = Co, Fe), where the endohedral icosahedral cluster [Pn@M12] can be regarded as a spherical-like three-dimensional coordination centre surrounded by a Pn10 ring and two Pn5 pentagonal caps. Quantum chemical calculations reveal that the [Pn@M12] has orbitals that mimic 1S, 1P and 1D electronic shells, which can interact with the outer (Pn5)2 ring layer. These interactions occur because the frontier orbitals are dominated by the (n − 1)d block orbitals of the metal atoms. The π1-As5 orbitals interact with 1S and part of the 1P shell of the central core, while the π2- and π3-As5 orbitals interact with the symmetry-allowed part of the 1P and 1D shells. The characteristics of this orbital interaction are analogous to those of mononuclear metallocenes.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2204629 (1′), 2204630 (2′), 2204596 (3′). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. All other data supporting the findings of this study are available within the Article and its Supplementary Information.
References
Kealy, T. J. & Pauson, P. L. A new type of organo-iron compound. Nature 168, 1039–1040 (1951).
Fischer, E. O. & Pfab, W. Cyclopentadien-Metallkomplexe, ein neuer Typ metallorganischer Verbindungen. Z. Naturforsch. B 7, 377–379 (1952).
Wilkinson, G., Rosenblum, M., Whiting, M. C. & Woodward, R. B. The structure of iron bis-cyclopentadienyl. J. Am. Chem. Soc. 74, 2125–2126 (1952).
Togni, A. & Halterman, R. L. Metallocenes: Synthesis Reactivity Applications (Wiley, 1998).
Gómez Arrayás, R., Adrio, J. & Carretero, J. C. Recent applications of chiral ferrocene ligands in asymmetric catalysis. Angew. Chem. Int. Ed. 45, 7674–7715 (2006).
Jaouen, G., Vessières, A. & Top, S. Ferrocifen type anti cancer drugs. Chem. Soc. Rev. 44, 8802–8817 (2015).
Luo, J., Hu, B., Hu, M., Wu, W. & Liu, T. L. An energy-dense, powerful, robust bipolar zinc–ferrocene redox-flow battery. Angew. Chem. Int. Ed. 61, e202204030 (2022).
Nakahata, M., Takashima, Y., Yamaguchi, H. & Harada, A. Redox-responsive self-healing materials formed from host–guest polymers. Nat. Commun. 2, 511 (2011).
Liu, J. et al. An organometallic super-gelator with multiple-stimulus responsive properties. Adv. Mater. 20, 2508–2511 (2008).
Mathey, F. Les phosphacymantrenes, premiers heterocycles phosphores dotes d’une veritable chimie ‘aromatique’. Tetrahedron Lett. 17, 4155–4158 (1976).
Maigrot, N., Avarvari, N., Charrier, C. & Mathey, F. Synthesis of 1,2-diphospholide Ions. Angew. Chem. Int. Ed. 34, 590–592 (1995).
Bartsch, R., Hitchcock, P. B. & Nixon, J. F. First structural characterisation of penta- and hexa-phosphorus analogues of ferrocene. Synthesis, crystal and molecular structure of the air-stable, sublimable iron sandwich compounds [Fe(η5-C2R2P3)2], and [Fe(η5-C3R3P2)(η5-C2R2P3)] (R = But). J. Chem. Soc., Chem. Commun. 1146–1148 (1987).
Scherer, O. J., Hilt, T. & Wolmershäuser, G. [{CpR(OC)2Fe}2(μ-η1:η1-P4)]: starting material for the synthesis of iron sandwich compounds with a 1,2,3-triphospholylligand and of a trinuclear iron complex with a P11 ligand. Angew. Chem. Int. Ed. 39, 1425–1427 (2000).
Scherer, O. J. & Brück, T. [(η5-P5)Fe(η5-C5Me5)], a pentaphosphaferrocene derivative. Angew. Chem. Int. Ed. 26, 59 (1987).
Scheer, M., Deng, S., Scherer, O. J. & Sierka, M. Tetraphosphacyclopentadienyl and triphosphaallyl ligands in iron complexes. Angew. Chem. Int. Ed. 44, 3755–3758 (2005).
Heinl, C. et al. The missing parent compound [(C5H5)Fe(η5-P5)]: synthesis, characterization, coordination behavior and encapsulation. Chemistry 27, 7542–7548 (2021).
Peresypkina, E., Virovets, A. & Scheer, M. Organometallic polyphosphorus complexes as diversified building blocks in coordination chemistry. Coord. Chem. Rev. 446, 213995 (2021).
Giusti, L. et al. Coordination chemistry of elemental phosphorus. Coord. Chem. Rev. 441, 213927 (2021).
Urne, E., Brennessel, W. W., Cramer, C. J., Ellis, J. E. & von Ragué Schleyer, P. A carbon-free sandwich complex [(P5)2Ti]2−. Science 295, 832–834 (2002).
Wang, Z. C., Qiao, L., Sun, Z. M. & Scheer, M. Inorganic ferrocene analogue [Fe(P4)2]2–. J. Am. Chem. Soc. 144, 6698–6702 (2022).
Scherer, O., Blath, C. & Wolmershäuser, G. Ferrocene mit einem Pentaarsacyclopentadienyl-Liganden. J. Organomet. Chem. 387, C21–C24 (1990).
Breunig, H. J., Burford, N. & Rösler, R. Stabilization of a pentastibacyclopentadienyl ligand in the triple-decker sandwich complexes [{(η5-1,2,4-tBu3C5H2)Mo}2(μ,η5-Sb5)] and [(η5-1,2,4-tBu3C5H2)Mo(μ,η5-Sb5)Mo(η5-1,4-tBu2-2-MeC5H2)]. Angew. Chem. Int. Ed. 39, 4148–4150 (2000).
Scherer, O. J., Wiedemann, W. & Wolmershäuser, G. [(η5-C5H4R)(CO)3Cr]2Cr(μ,η5-As5)Cr(η5-C5H4Me)], ein Tripeldecker-Sandwichkomplex mit unverzerttem cyclo-As5-Mitteldeck. J. Organomet. Chem. 361, C11–C14 (1989).
Lein, M., Frunzke, J. & Frenking, G. Structures and bonding of the sandwich complexes Ti(η5-E5)22– (E = CH, N, P, As, Sb): a theoretical study. Inorg. Chem. 42, 2504–2511 (2003).
Frunzke, J., Lein, M. & Frenking, G. Structures, metal–ligand bond strength, and bonding analysis of ferrocene derivatives with group-15 heteroligands Fe(η5-E5)2 and FeCp(η5-E5) (E = N, P, As, Sb). A theoretical study. Organometallics 21, 3351–3359 (2002).
Murahashi, T. et al. Discrete sandwich compounds of monolayer palladium sheets. Science 313, 1104–1107 (2006).
Teramoto, M. et al. Three-dimensional sandwich nanocubes composed of 13-atom palladium core and hexakis-carbocycle shell. J. Am. Chem. Soc. 140, 12682–12686 (2018).
Pan, F. X. et al. An all-metal aromatic sandwich complex [Sb3Au3Sb3]3‒. J. Am. Chem. Soc. 137, 10954–10957 (2015).
Xu, H. L. et al. A sandwich-type cluster containing Ge@Pd3 planar fragment flanked by aromatic nonagermanide caps. Nat. Commun. 11, 5286 (2020).
Spiekermann, A., Hoffmann, S. D., Kraus, F. & Fässler, T. F. [Au3Ge18]5‒—a gold–germanium cluster with remarkable Au–Au interactions. Angew. Chem. Int. Ed. 46, 1638–1640 (2007).
Moses, M. J., Fettinger, J. C. & Eichhorn, B. W. Interpenetrating As20 fullerene and Ni12 icosahedra in the onion-skin [As@Ni12@As20]3− ion. Science 300, 778–780 (2003).
Wang, Y. et al. Sb@Ni12@Sb20–/+ and Sb@Pd12@Sb20n cluster anions, where n = +1, −1, −3, −4: multi-oxidation-state clusters of interpenetrating platonic solids. J. Am. Chem. Soc. 139, 619–622 (2017).
Li, Z. Y. et al. Counterion-induced crystallization of intermetalloid matryoshka clusters [Sb@Pd12@Sb20]3−,4−. Dalton Trans. 46, 3453–3456 (2017).
Stegmaier, S. & Fässler, T. F. A bronze matryoshka: the discrete intermetalloid cluster [Sn@Cu12@Sn20]12– in the ternary phases A12Cu12Sn21 (A = Na, K). J. Am. Chem. Soc. 133, 19758–19768 (2011).
Jinlong, Y., Chuanyun, X., Shangda, X. & Kelin, W. Stability, electronic and magnetic properties, and reactivity of icosahedral MCo12 clusters. Phys. Rev. B 48, 12155–12163 (1993).
Zhang, H., Cui, C. & Luo, Z. The doping effect of 13-atom iron clusters on water adsorption and O–H bond dissociation. J. Phys. Chem. A 123, 4891–4899 (2019).
Moses, M. J., Fettinger, J. C. & Eichhorn, B. W. [Ni5Sb17]4– transition-metal Zintl ion complex: crossing the Zintl border in molecular intermetalloid clusters. Inorg. Chem. 46, 1036–1038 (2007).
Ruiz-Martínez, A., Casanova, D. & Alvarez, S. Polyhedral structures with an odd number of vertices: nine-coordinate metal compounds. Chemistry 14, 1291–1303 (2008).
Walter, M. et al. A unified view of ligand-protected gold clusters as superatom complexes. Proc. Natl Acad. Sci. USA 105, 9157–9162 (2008).
Wei, J. et al. Looking at platinum carbonyl nanoclusters as superatoms. Nanoscale 14, 3946–3957 (2022).
Gascoin, F. & Sevov, S. C. Synthesis and characterization of the ‘metallic salts’ A5Pn4 (A = K, Rb, Cs and Pn = As, Sb, Bi) with isolated zigzag tetramers of Pn44− and an extra delocalized electron. Inorg. Chem. 40, 5177–5181 (2001).
Wakatsuki, Y., Yamazaki, H., Lindner, E. & Bosamle, A. 1,3-Butadiene-1,4-diyl)(η5-cyclopentadienyl)-(triphenylphosphine)cobalt with various substituents. Inorg. Synth. 26, 189–200 (1989).
Sheldrick, G. M. SHELXT—integrated space-group and crystal-structure determination. Acta Crystallogr. A 71, 3–8 (2015).
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42, 339–341 (2009).
Spek, A. L. Structure validation in chemical crystallography. Acta Crystallogr. D 65, 148–155 (2009).
te Velde, G. et al. Chemistry with ADF. J. Comp. Chem. 22, 931–967 (2001).
Ernzerhof, M. & Scuseria, G. Assessment of the Perdew–Burke–Ernzerhof exchange-correlation functional. J. Chem. Phys. 110, 5029 (1999).
Adamo, C. & Barone, V. Physically motivated density functionals with improved performances: the modified Perdew–Burke–Ernzerhof model. J. Chem. Phys. 116, 5933 (2002).
Ziegler, T. & Rauk, A. On the calculation of bonding energies by the Hartree Fock Slater method. Theor. Chim. Acta 46, 1–10 (1977).
Mitoraj, M. & Michalak, A. Applications of natural orbitals for chemical valence in a description of bonding in conjugated molecules. J. Mol. Model. 14, 681–687 (2008).
Mitoraj, M. P., Michalak, A. & Ziegler, T. A combined charge and energy decomposition scheme for bond analysis. J. Chem. Theory Comput. 5, 962–975 (2009).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (92161102, 21971118 and 22003048) and the Natural Science Foundation of Tianjin City (numbers 21JCZXJC00140 and 20JCYBJC01560) to Z.-M.S. Z.-M.S. thanks the 111 project (B18030) from China (MOE). A.M.C. thanks ANID for regular grant 1221676.
Author information
Authors and Affiliations
Contributions
Z.-M.S. conceived and directed the research. X.-H.Y. synthesized compounds 1′ and 2′. W.-X.C. synthesized compound 3′. A.M.-C. and T.Y. performed the computational studies. X.-H.Y. and W.-X.C. performed the single-crystal X-ray diffraction, EDX and ESI-MS, and analysed the data. All authors co-wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Giovanni Maestri and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary handling editor: Alison Stoddart, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–19, Discussion and Tables 1–7.
Supplementary Data 1
Crystallographic data for complex 1′ (CCDC 2204629).
Supplementary Data 2
Crystallographic Data for complex 2′ (CCDC 2204630).
Supplementary Data 3
Crystallographic data for complex 3′ (CCDC 2204596).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yue, XH., Chen, WX., Yang, T. et al. Carbon-free sandwich compounds based on arsenic and antimony with icosahedral metal cores. Nat. Synth 2, 423–429 (2023). https://doi.org/10.1038/s44160-023-00247-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-023-00247-0