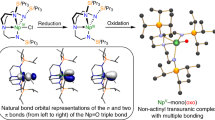
Abstract
The aqueous chemistry of uranium is dominated by the linear uranyl cation [UO2]2+, yet the isoelectronic nitrogen-based analogue of this ubiquitous cation, molecular [UN2], has so far only been observed in an argon matrix. Here, we present three different complexes of [UN2] obtained by the reaction of the uranium pentahalides UCl5 or UBr5 with anhydrous liquid ammonia. The [UN2] moieties are linear, with the U atoms coordinated by five additional ligands (ammonia, chloride or bromide), resulting in a pentagonal bipyramidal coordination sphere that is also commonly adopted by the uranyl cation [UO2(L)5]2+ (L, ligand). In all three cases, the nitrido ligands are further coordinated through their lone pairs by the Lewis-acidic ligands [U(NH3)8]4+ to form almost linear, trinuclear complex cations. Those were characterized by single-crystal X-ray diffraction, Raman and infrared spectroscopy, 14N/15N isotope studies and quantum chemical calculations, which support the presence of two U≡N triple bonds within the [UN2] moieties.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data supporting the findings of this study (powder X-ray patterns, Raman and infrared spectra, and details of the quantum chemical calculations) are available within this Article and its Supplementary Information. Crystallographic data are available free of charge through the Cambridge Crystallographic Data Centre (www.ccdc.cam.ac.uk), with CCDC nos. 1868200 for (1Br8·26NH3), 1984956 (2Br7·10.5NH3) and 1868199 (3Cl6·6NH3). Source data are provided with this paper.
References
Hunt, R. D., Yustein, J. T. & Andrews, L. Matrix infrared spectra of NUN formed by the insertion of uranium atoms into molecular nitrogen. J. Chem. Phys. 98, 6070–6074 (1993).
Wang, X., Andrews, L., Vlaisavljevich, B. & Gagliardi, L. Combined triple and double bonds to uranium: the N≡U═N-H uranimine nitride molecule prepared in solid argon. Inorg. Chem. 50, 3826–3831 (2011).
Andrews, L. et al. Infrared spectra and electronic structure calculations for NN complexes with U, UN and NUN in solid argon, neon and nitrogen. J. Phys. Chem. A 118, 5289–5303 (2014).
Andrews, L., Wang, X., Gong, Y., Vlaisavljevich, B. & Gagliardi, L. Infrared spectra and electronic structure calculations for the NUN(NN)1–5 and NU(NN)1–6 complexes in solid argon. Inorg. Chem. 52, 9989–9993 (2013).
Falcone, M., Chatelain, L., Scopelliti, R., Živković, I. & Mazzanti, M. Nitrogen reduction and functionalization by a multimetallic uranium nitride complex. Nature 547, 332–335 (2017).
Cleaves, P. A. et al. Terminal uranium(v/vi) nitride activation of carbon dioxide and carbon disulfide: factors governing diverse and well-defined cleavage and redox reactions. Chem. Eur. J. 23, 2950–2959 (2017).
Falcone, M., Poon, L. N., Fadaei, T. F. & Mazzanti, M. Reversible dihydrogen activation and hydride transfer by a uranium nitride complex. Angew. Chem. Int. Ed. 57, 3697–3700 (2018).
Fox, A. R., Bart, S. C., Meyer, K. & Cummins, C. C. Towards uranium catalysts. Nature 455, 341–349 (2008).
Fox, A. R., Arnold, P. L. & Cummins, C. C. Uranium–nitrogen multiple bonding: isostructural anionic, neutral and cationic uranium nitride complexes featuring a linear U═N═U core. J. Am. Chem. Soc. 132, 3250–3251 (2010).
Rogozkin, B. D., Stepennova, N. M., Bergman, G. A. & Proshkin, A. A. Thermochemical stability, radiation testing, fabrication and reprocessing of mononitride fuel. At. Energy 95, 835–844 (2003).
Matthews, R. B., Chidester, K. M., Hoth, C. W., Mason, R. E. & Petty, R. L. Fabrication and testing of uranium nitride fuel for space power reactors. J. Nucl. Mater. 151, 345–345 (1988).
Diaconescu, P. Actinide chemistry: a tale of two nitrides. Nat. Chem. 2, 705–706 (2010).
Generation IV International Forum. Technology Roadmap Update for Generation IV Nuclear Energy Systems (OECD, 2014).
King, D. M. et al. Isolation and characterization of a uranium(vi)–nitride triple bond. Nat. Chem. 5, 482–488 (2013).
Gardner, B. M. et al. Triamidoamine uranium(iv)–arsenic complexes containing one-, two- and three-fold U–As bonding interactions. Nat. Chem. 7, 582–590 (2015).
Hayton, T. W. et al. Synthesis of imido analogs of the uranyl ion. Science 310, 1941–1943 (2005).
King, D. M. & Liddle, S. T. Progress in molecular uranium-nitride chemistry. Coord. Chem. Rev. 266/267, 2–15 (2014).
King, D. M. et al. Synthesis and structure of a terminal uranium nitride complex. Science 337, 717–720 (2012).
Camp, C., Pécaut, J. & Mazzanti, M. Tuning uranium–nitrogen multiple bond formation with ancillary siloxide ligands. J. Am. Chem. Soc. 135, 12101–12111 (2013).
Evans, W. J., Kozimor, S. A. & Ziller, J. W. Molecular octa-uranium rings with alternating nitride and azide bridges. Science 309, 1835–1838 (2005).
Kushto, G. P., Souter, P. F. & Andrews, L. An infrared spectroscopic and quasirelativistic theoretical study of the coordination and activation of dinitrogen by thorium and uranium atoms. J. Chem. Phys. 108, 7121–7130 (1998).
Andrews, L., Wang, X., Lindh, R., Roos, B. O. & Marsden, C. J. Simple N≡UF3 and P≡UF3 molecules with triple bonds to uranium. Angew. Chem. Int. Ed. 47, 5366–5370 (2008).
Fox, A. R. & Cummins, C. C. Uranium–nitrogen multiple bonding: the case of a four-coordinate uranium(vi) nitridoborate complex. J. Am. Chem. Soc. 131, 5716–5717 (2009).
Evans, W. J., Miller, K. A., Ziller, J. W. & Greaves, J. Analysis of uranium azide and nitride complexes by atmospheric pressure chemical ionization mass spectrometry. Inorg. Chem. 46, 8008–8018 (2007).
Anderson, N. H. et al. Elucidating bonding preferences in tetrakis(imido)uranate(vi) dianions. Nat. Chem. 9, 850–855 (2017).
Schmidt, A.-C., Heinemann, F. W., Maron, L. & Meyer, K. A series of uranium(iv, v, vi) tritylimido complexes, their molecular and electronic structures and reactivity with CO2. Inorg. Chem. 53, 13142–13153 (2014).
Rudel, S. S. et al. Recent advances in the chemistry of uranium halides in anhydrous ammonia. Z. Kristallogr. Cryst. Mater. 233, 817–844 (2018).
Berthet, J.-C., Siddredi, G., Thuery, P. & Ephritikhine, M. Synthesis and crystal structure of pentavalent uranyl complexes. The remarkable stability of UO2X (X = I, SO3CF3) in non-aqueous solutions. Dalton Trans. 14, 3478–3494 (2009).
Berthet, J.-C., Nierlich, M., Miquel, Y., Madic, C. & Ephritikhine, M. Selective complexation of uranium(iii) over lanthanide(iii) triflates by 2,2':6',2''-terpyridine. X-ray crystal structures of [M(OTf)3(terpy)2] and [M(OTf)2(terpy)2(py)][OTf] (M = Nd, Ce, U) and of polynuclear μ-oxo uranium(iv) complexes resulting from hydrolysis. Dalton Trans. 2005, 369–379 (2005).
Fortier, S., Brown, J. L., Kaltsoyannis, N., Wu, G. & Hayton, T. W. Synthesis, molecular and electronic structure of UV(O)[N(SiMe3)2]3. Inorg. Chem. 51, 1625–1633 (2012).
Gardner, B. M. et al. Homologation and functionalization of carbon monoxide by a recyclable uranium complex. Proc. Natl Acad. Sci. USA 109, 9265–9270 (2012).
Olovsson, I. The crystal structures of the triammines of the ammonium halides. Acta Chem. Scand. 14, 1453–1465 (1960).
Olovsson, I. The crystal structure of tetrammineammonium iodide. Acta Chem. Scand. 14, 1466–1474 (1960).
Roßmeier, T. Supramolekulare Chemie mit Ammoniak—Strukturchemie neuer Ammoniak-Proton-Komplexe (Regensburg, 2005).
Roßmeier, T., Reil, M. & Korber, N. First characterization of the ammine–ammonium complex [{NH4(NH3)4}2(μ-NH3)2]2+ in the crystal structure of [NH4(NH3)4][B(C6H5)4]·NH3 and the [NH4(NH3)4]+ complex in [NH4(NH3)4][Ca(NH3)7]As3S6·2NH3 and [NH4(NH3)4][Ba(NH3)8]As3S6·NH3. Inorg. Chem. 43, 2206–2212 (2004).
Roßmeier, T. & Korber, N. First characterization of an extended ammonium–ammonia complex [NH4(NH3)4+(μ-NH3)2] in the crystal structure of [NH4(NH3)4][Co(C2B9H11)2]·2NH3. Z. Anorg. Allg. Chem. 630, 2665–2668 (2004).
Schmidt, K. H. & Müller, A. Vibrational spectra and force constants of pure ammine complexes. Coord. Chem. Rev. 19, 41–97 (1976).
Wagner, W. D. & Nakamoto, K. Resonance Raman spectra of nitridoiron(v) porphyrin intermediates produced by laser photolysis. J. Am. Chem. Soc. 111, 1590–1598 (1989).
Bullock, J. I. Raman and infrared spectroscopic studies of the uranyl ion: the symmetric stretching frequency, force constants and bond lengths. J. Chem. Soc. A 1969, 781–784 (1969).
Nocton, G. et al. Synthesis, structure and bonding of stable complexes of pentavalent uranyl. J. Am. Chem. Soc. 132, 495–508 (2010).
Juza, R. & Meyer, W. Über uran-nitrid-chlorid, -bromid und -jodid. Z. Anorg. Allg. Chem. 366, 43–50 (1969).
Murasik, A., Furrer, A. & Szczepaniak, W. Crystal-field levels in UBr3 determined by neutron spectroscopy. Solid State Commun. 33, 1217–1219 (1980).
Knizia, G. Intrinsic atomic orbitals: an unbiased bridge between quantum theory and chemical concepts. J. Chem. Theory Comput. 9, 4834–4843 (2013).
Kaltsoyannis, N. Computational study of analogues of the uranyl ion containing the −NUN− unit: density functional theory calculations on UO22+, UON+, UN2, UO(NPH3)3+, U(NPH3)24+, [UCl4{NPR3}2] (R = H, Me), and [UOCl4{NP(C6H5)3}]−. Inorg. Chem. 39, 6009–6017 (2000).
Wei, F., Wu, G., Schwarz, W. H. E. & Li, J. Geometries, electronic structures and excited states of UN2, NUO+ and UO22+: a combined CCSD(T), RAS/CASPT2 and TDDFT study. Theor. Chem. Acc. 129, 467–481 (2011).
Scheibe, B., Rudel, S. S., Buchner, M. R., Karttunen, A. J. & Kraus, F. A 1D coordination polymer of UF5 with HCN as a ligand. Chem. Eur. J. 23, 291–295 (2017).
Brown, D., Berry, J. A. & Holloway, J. H. Halogen exchange reactions involving uranium-(v) and -(vi) halides. J. Chem. Soc. Dalton Trans. 1982, 1385–1388 (1982).
Deubner, H. L. et al. A revised structure model for the UCl6 structure type, novel modifications of UCl6 and UBr5, and a comment on the modifications of protactinium pentabromides. Chem. Eur. J. 25, 6402–6411 (2019).
STOE WinXPOW (STOE & Cie, 2015); https://www.stoe.com/
X-Area (STOE & Cie, 2018); https://www.stoe.com/
X-RED32 (STOE & Cie, 2012); https://www.stoe.com/
X-SHAPE (STOE & Cie, 2013); https://www.stoe.com/
LANA—Laue Analyzer (STOE & Cie, 2019); https://www.stoe.com/
Sheldrick, G. M. SHELXS-97 (1997); http://shelx.uni-ac.gwdg.de/SHELX/index.php
Sheldrick, G. M. SHELXT—integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 71, 3–8 (2015).
Sheldrick, G. M. SHELXL-2016/6 (2016); http://shelx.uni-ac.gwdg.de/SHELX/index.php
Hübschle, C. B., Sheldrick, G. M. & Dittrich, B. ShelXle: a Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 44, 1281–1284 (2011).
OPUS (Bruker Optik, 2009); https://www.bruker.com/
Ahlrichs, R., Bär, M., Häser, M., Horn, H. & Kölmel, C. Electronic structure calculations on workstation computers: the program system turbomole. Chem. Phys. Lett. 162, 165–169 (1989).
TURBOMOLE versions 7.2 and 7.3 (TURBOMOLE, 2017); http://www.turbomole.com
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Adamo, C. & Barone, V. Toward reliable density functional methods without adjustable parameters: the PBE0 model. J. Chem. Phys. 110, 6158–6170 (1999).
Schäfer, A., Huber, C. & Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 100, 5829–5835 (1994).
Cao, X. & Dolg, M. Segmented contraction scheme for small-core actinide pseudopotential basis sets. J. Mol. Struct. THEOCHEM 673, 203–209 (2004).
Küchle, W., Dolg, M., Stoll, H. & Preuss, H. Energy-adjusted pseudopotentials for the actinides. Parameter sets and test calculations for thorium and thorium monoxide. J. Chem. Phys. 100, 7535–7542 (1994).
Eichkorn, K., Treutler, O., Öhm, H., Häser, M. & Ahlrichs, R. Auxiliary basis sets to approximate Coulomb potentials. Chem. Phys. Lett. 240, 283–290 (1995).
Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 8, 1057–1065 (2006).
Sierka, M., Hogekamp, A. & Ahlrichs, R. Fast evaluation of the Coulomb potential for electron densities using multipole accelerated resolution of identity approximation. J. Chem. Phys. 118, 9136–9148 (2003).
Klamt, A. & Schürmann, G. COSMO: a new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 2, 799–805 (1993).
Rappoport, D. & Furche, F. Lagrangian approach to molecular vibrational Raman intensities using time-dependent hybrid density functional theory. J. Chem. Phys. 126, 201104 (2007).
Knizia, G. & Klein, J. E. M. N. Electron flow in reaction mechanisms—revealed from first principles. Angew. Chem. Int. Ed. 54, 5518–5522 (2015).
Acknowledgements
S.S.R. and F.K. thank the Deutsche Forschungsgemeinschaft for generous funding. A.J.K. thanks CSC, the Finnish IT Center for Science, for computational resources. We thank B. Roling for use of his Raman spectrometer. We thank U. Müller for bringing [UN2] to our attention many years ago.
Author information
Authors and Affiliations
Contributions
S.S.R. conceived and designed experiments and interpreted crystal structures, powder patterns and spectra for 18+ and 36+. S.S.R. and M.M. performed experiments and analytics on 18+ and 36+. M.M. contributed to the manuscript. H.L.D. conceived, designed and performed experiments on 27+. H.L.D. and M.M. interpreted the single-crystal structure of 27+. A.J.K. conceived and designed the quantum chemical calculations, interpreted results and wrote the theoretical parts of the manuscript. F.K. designed and guided research and interpreted the single-crystal structure determinations, powder patterns and spectra. S.S.R. and F.K. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Raman spectra of 1Br8·26NH3 and 3Cl6·6NH3 measured in fused-silica tubes at room temperature.
a, 1Br8·26NH3 in NH3 crystallized at −40 °C. b, Decomposition product of 1Br8·26NH3 in NH3 obtained at room temperature. c, Solution over 1Br8·26NH3 in NH3. d) 3Cl6·6NH3 in NH3 obtained at room temperature. Bands at 850–950 cm−1 are assigned to the U≡N stretching vibration. The abbreviation rt means room temperature, the laser wavelength was λ = 532 nm.
Extended Data Fig. 3 Diamond ATR-FTIR spectra of the decomposition products of 1Br8·26NH3 and 3Cl6·6NH3.
a, b, Decomposition products of 1Br8·26NH3 (a) and 3Cl6·6NH3 (b) both after removal of liquid ammonia at room temperature. Uranyl impurities would result in a band at ~911–960 cm−1. The abbreviation rt stands for room temperature.
Extended Data Fig. 4 Powder X-ray diffraction pattern of the residue of the reaction of UBr5 with ammonia after heating to 600 °C in a sealed steel ampoule under argon.
Reflection positions of UNBr are marked with red strokes, those of UBr3 with blue strokes. Reflections marked with an asterisk belong to a yet unidentified compound. Measured using CuKα1 radiation in a 0.3 mm capillary.
Extended Data Fig. 5 Valence IBOs for cation 18+. Valence IBOs for cation 18+.
a, Uter−µ-N interaction. b, U−NH3 interaction. c) N−H bond. The red and green orbitals are the IBOs; atom colour code: U, cyan; N, blue; H, white. The valence IBOs shown in Fig. 3 (main text) and the four singly-occupied IBOs with four unpaired f-electrons of the Uter atom are omitted. For further details, see the caption of Fig. 3 (main text). The Isovalue for IBO isosurface plots is 0.08 a.u.
Supplementary information
Supplementary Information
Synthesis of starting materials, Supplementary Figs. 1–4, Tables 1–4 and references.
Supplementary Data 1
Crystallographic data for compound 1Br8·26NH3 (CCDC 1868200).
Supplementary Data 2
Crystallographic data for compound 2Br7·10.5NH3 (CCDC 1984956).
Supplementary Data 3
Crystallographic data for compound 3Cl6·6NH3 (CCDC 1868199).
Source data
Source Data 1
Reference orbitals for intrinsic bond orbital (IBO) analysis.
Source Data 2
Cartesian coordinates of the studied systems in XYZ format.
Rights and permissions
About this article
Cite this article
Rudel, S.S., Deubner, H.L., Müller, M. et al. Complexes featuring a linear [N≡U≡N] core isoelectronic to the uranyl cation. Nat. Chem. 12, 962–967 (2020). https://doi.org/10.1038/s41557-020-0505-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-0505-5
This article is cited by
-
Thorium(iv)–antimony complexes exhibiting single, double, and triple polar covalent metal–metal bonds
Nature Chemistry (2024)
-
A charged diatomic triple-bonded U≡N species trapped in C82 fullerene cages
Nature Communications (2022)
-
Photochemical Synthesis of Transition Metal-Stabilized Uranium(VI) Nitride Complexes
Nature Communications (2022)
-
Filling the equatorial garland of uranyl ion: its content and limitations
Journal of Inclusion Phenomena and Macrocyclic Chemistry (2021)