Abstract
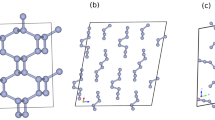
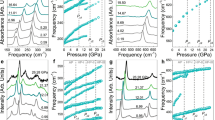
The triple bond of diatomic nitrogen has among the greatest binding energies of any molecule. At low temperatures and pressures, nitrogen forms a molecular crystal in which these strong bonds co-exist with weak van der Waals interactions between molecules, producing an insulator with a large band gap1. As the pressure is raised on molecular crystals, intermolecular interactions increase and the molecules eventually dissociate to form monoatomic metallic solids, as was first predicted for hydrogen2. Theory predicts that, in a pressure range between 50 and 94 GPa, diatomic nitrogen can be transformed into a non-molecular framework or polymeric structure with potential use as a high-energy-density material3,4,5. Here we show that the non-molecular phase of nitrogen is semiconducting up to at least 240 GPa, at which pressure the energy gap has decreased to 0.4 eV. At 300 K, this transition from insulating to semiconducting behaviour starts at a pressure of approximately 140 GPa, but shifts to much higher pressure with decreasing temperature. The transition also exhibits remarkably large hysteresis with an equilibrium transition estimated to be near 100 GPa. Moreover, we have succeeded in recovering the non-molecular phase of nitrogen at ambient pressure (at temperatures below 100 K), which could be of importance for practical use.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Freiman, Yu. A. in Physics of Cryocrystals (eds Manzhelii, V. G. & Freiman, Yu. A.) 538–596 (American Institute of Physics, Woodbury, New York, 1997).
Wigner, E. & Huntington, H. B. On the possibility of a metallic modification of hydrogen. J. Chem. Phys. 3, 764–770 (1935).
McMahan, A. K. & LeSar, R. Pressure dissociation of solid nitrogen under 1 Mbar. Phys. Rev. Lett. 54, 1929–1932 (1985).
Martin, R. M. & Needs, R. Theoretical study of the molecular-to-nonmolecular transformation of nitrogen at high pressures. Phys. Rev. B 34, 5082–5092 (1986).
Mailhiot, C., Yang, L. H. & McMahan, A. K. Polymeric nitrogen. Phys. Rev. B 46, 14419–14435 (1992).
Christe, K. O., Wilson, W. W., Sheehy, J. A. & Boatz, J. A. N5+: A novel homolepic polynitrogen ion as a high energy density material. Angew. Chem. Int. Engl. Edn 38, 2002–2009 (1999).
Helmy, A. A. Calculation of the pressure-induced insulator-metal transition of nitrogen. J. Phys. Condens. Matter 6, 985–988 (1994).
Lewis, S. P. & Cohen, M. L. High-pressure atomic phases of solid nitrogen. Phys. Rev. B 46, 11117–11120 (1992).
Mitas, L. & Martin, R. M. Quantum Monte Carlo of nitrogen: atom, dimer, atomic, and molecular solids. Phys. Rev. Lett. 72, 2438–2441 (1994).
Yakub, E. S. Short-range intermolecular interaction and phase transitions with rearrangement of chemical bonds. J. Low-Temp. Phys. 111, 357–364 (1998).
Nellis, W. J., Holmes, N. C., Mitchell, A. C. & van Thiel, M. Phase transition in fluid nitrogen at high densities and temperatures. Phys. Rev. Lett. 55, 1661–1664 (1984).
Reichlin, R., Schiferl, D., Martin, S., Vanderborgh, C. & Mills, R. L. Optical studies of nitrogen to 130 GPa. Phys. Rev. Lett. 55, 1464–1467 (1985).
Bell, P. M., Mao, H. K. & Hemley, R. J. Observations of solid H2, D2, N2 at pressures around 1.5 megabar at 25 C. Physica B 139–140, 16–20 (1986).
Johnson, K. A. & Ashcroft, N. W. Structure and bandgap closure in dense hydrogen. Nature 403, 632–635 (2000).
Städele, M. & Martin, R. M. Metallization of molecular hydrogen: predictions from exact-exchange calculations. Phys. Rev. Lett. 84, 6070–6073 (2000).
Shimizu, K., Suhara, K., Ikumo, M., Eremets, M. I. & Amaya, K. Superconductivity in oxygen. Nature 393, 767–769 (1998).
Eremets, M. I. et al. Electrical conductivity of Xe at megabar pressures. Phys. Rev. Lett. 83, 2797–2800 (2000).
Goncharov, A. F., Gregoryanz, E., Mao, H. K., Liu, Z. & Hemley, R. J. Optical evidence for nonmolecular phase of nitrogen above 150 GPa. Phys. Rev. Lett. 85, 1262–1265 (2000).
Eremets, M. I. Experimental High-Pressure Techniques 16, 59 (Oxford Univ. Press, Oxford, 1996).
Brovman, E. G., Kagan, Y. & Kholas, A. Structure of metallic hydrogen at zero pressure. Sov. Phys. JETP 34, 1300–1315 (1972).
Hemley, R. J. & Mao, H. K. Phase transition in solid molecular hydrogen at ultrahigh pressures. Phys. Rev. Lett. 61, 857–860 (1988).
Nayarana, C., Luo, H., Orloff, J. & Ruoff, A. L. Solid hydrogen at 342 GPa: no evidence for an alkali metal. Nature 393, 46–49 (1998).
Olijnyk, H. & Jephcoat, A. P. Vibrational dynamics of isotopically dilute nitrogen to 104 GPa. Phys. Rev. Lett. 83, 332–335 (1999).
Mao, H. K., Xu, J. & Bell, P. M. Calibration of the ruby pressure gauge to 800 kbar under quasihydrostatic conditions. J. Geophys. Res. B 91, 4673–4676 (1986).
Acknowledgements
We thank S. Gramsch, V. V. Struzhkin and A. F. Goncharov for useful discussions and comments on the manuscript. This work was supported by the NSF and NASA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eremets, M., Hemley, R., Mao, Hk. et al. Semiconducting non-molecular nitrogen up to 240 GPa and its low-pressure stability. Nature 411, 170–174 (2001). https://doi.org/10.1038/35075531
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35075531
This article is cited by
-
Primitive to conventional geometry projection for efficient phonon transport calculations
npj Computational Materials (2023)
-
Structure determination of ζ-N2 from single-crystal X-ray diffraction and theoretical suggestion for the formation of amorphous nitrogen
Nature Communications (2023)
-
High-energy-density pentazolate salts: CaN10 and BaN10
Science China Physics, Mechanics & Astronomy (2021)
-
Strategy for chemically riveting catenated nitrogen chains
Journal of Molecular Modeling (2019)
-
Metallization and molecular dissociation of dense fluid nitrogen
Nature Communications (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.