Abstract
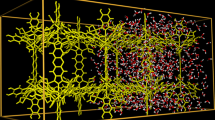
Supercooled water and amorphous ice have a rich metastable phase behaviour. In addition to transitions between high- and low-density amorphous solids1,2, and between high- and low-density liquids3,4,5,6,7,8, a fragile-to-strong liquid transition has recently been proposed9,10, and supported by evidence from the behaviour of deeply supercooled bilayer water confined in hydrophilic slit pores11. Here we report evidence from molecular dynamics simulations for another type of first-order phase transition—a liquid-to-bilayer amorphous transition—above the freezing temperature of bulk water at atmospheric pressure. This transition occurs only when water is confined in a hydrophobic slit pore12,13,14 with a width of less than one nanometre. On cooling, the confined water, which has an imperfect random hydrogen-bonded network, transforms into a bilayer amorphous phase with a perfect network (owing to the formation of various hydrogen-bonded polygons) but no long-range order. The transition shares some characteristics with those observed in tetrahedrally coordinated substances such as liquid silicon15,16, liquid carbon17 and liquid phosphorus18.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Mishima, O., Calvert, L. D. & Whalley, E. ‘Melting’ ice I at 77 K and 10 kbar: a new method of making amorphous solids. Nature 310, 393–394 (1984).
Mishima, O., Calvert, L. D. & Whalley, E. An apparently first-order transition between two amorphous phases of ice induced by pressure. Nature 314, 76–78 (1985).
Poole, P. H., Sciortino, F., Essmann, U. & Stanley, H. E. Phase behaviour of metastable water. Nature 360, 324–328 (1992).
Stanley, H. E. et al. Is there a second critical point in liquid water? Physica A 205, 122–139 (1994).
Tanaka, H. A self-consistent phase diagram for supercooled water. Nature 380, 328–330 (1996).
Tanaka, H. Phase behaviors of supercooled water: Reconciling a critical point of amorphous ices with spinodal instability. J. Chem. Phys. 105, 5099–5111 (1996).
Sciortino, F., Poole, P. H., Essmann, U. & Stanley, H. E. Line of compressibility maxima in the phase diagram of supercooled water. Phys. Rev. E 55, 727–737 (1997).
Mishima, O. & Stanley, H. E. The relationship between liquid, supercooled and glassy water. Nature 396, 329–335 (1998).
Angell, C. A. Water-II is a strong liquid. J. Phys. Chem. 97, 6339–6341 (1993).
Ito, K., Moynihan, C. T. & Angell, C. A. Thermodynamic fragility in liquids and a fragile-to-strong liquid transition in water. Nature 398, 492–495 (1999).
Bergman, R. & Swenson, J. Dynamics of supercooled water in confined geometry. Nature 403, 283–286 (2000).
Bellissent-Funel, M.-C., Chen, S. H. & Zanotti, J. M. X-ray and neutron scattering studies of the structure of water at a hydrophobic surface. J. Chem. Phys. 104, 10023–10029 (1996).
Koga, K., Zeng, X. C. & Tanaka, H. Freezing of confined water: a bilayer ice phase in hydrophobic nanopores. Phys. Rev. Lett. 79, 5262–5265 (1997).
Meyer, M. & Stanley, H. E. Liquid-liquid phase transition in confined water: A Monte Carlo study. J. Phys. Chem. B 103, 9728–9730 (1999).
Thompson, M. O. et al. Melting temperature and explosive crystallization of amorphous silicon during pulsed laser irradiation. Phys. Rev. Lett. 52, 2360–2363 (1984).
Angell, C. A., Borick, S. S. & Grabow, M. H. Glass transitions and first order liquid-metal-to-semiconductor transitions in 4-5-6 covalent systems. J. Non-Cryst. Solids 205, 463–471 (1996).
Glosli, J. N. & Ree, F. H. Liquid-liquid phase transformation in carbon. Phys. Rev. Lett. 82, 4659–4662 (1999).
Katayama, Y. et al. A first-order liquid–liquid phase transition in phosphorus. Nature 403, 170–173 (2000).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Gao, G. T., Zeng, X. C. & Tanaka, H. The melting temperature of proton-disordered hexagonal ice: A computer simulation of TIP4P model of water. J. Chem. Phys. 112, 8534–8538 (2000).
Stillinger, F. H. A topographic view of supercooled liquids and glass formation. Science 267, 1935–1939 (1995).
Gillen, K. T., Douglass, D. C. & Hoch, M. J. R. Self-diffusion in liquid water to -31°. J. Chem. Phys. 57, 5117–5119 (1972).
Goto, K., Hondoh, T. & Higashi, A. Determination of diffusion coefficients of self-interstitials in ice with a new method of observing climb of dislocations by X-ray topography. Jpn J. Appl. Phys. 25, 351–357 (1986).
Smith, R. S. & Kay, B. D. The existence of supercooled water at 150 K. Nature 403, 283–296 (1999).
Zallen, R. in The Physics of Amorphous Solids 63–67 (Wiley, New York, 1998).
Tse, J. S. et al. The mechanisms for pressure-induced amorphization of ice Ih. Nature 400, 647–649 (1999).
Soper, A. K. & Ricci, M. A. Structures of high-density and low-density water. Phys. Rev. Lett. 84, 2881–2884 (2000).
Scala, A., Starr, F. W., La Nave, E., Sciortino, F. & Stanley, H. E. Configurational entropy and diffusivity of supercooled water. Nature 406, 166–169 (2000).
Angell, C. A., Bressel, R. D., Hemmati, M., Sare, E. J. & Tucker, J. C. Water and its anomalies in perspective: tetrahedral liquids with and without liquid-liquid phase transitions. Phys. Chem. Chem. Phys. 2, 1559–1566 (2000).
Tanaka, H. Fluctuation of local order and connectivity of water molecules in two phases of supercooled water. Phys. Rev. Lett. 80, 113–116 (1998).
Acknowledgements
K.K. and H.T. are supported by the Japan Society for the Promotion of Science (JSPS), the Japan Ministry of Education, and the Institute for Molecular Science (IMS). X.C.Z. is supported by the US NSF and Office of Naval Research (ONR), and by a JSPS fellowship.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Koga, K., Tanaka, H. & Zeng, X. First-order transition in confined water between high-density liquid and low-density amorphous phases. Nature 408, 564–567 (2000). https://doi.org/10.1038/35046035
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35046035
This article is cited by
-
Two-dimensional monolayer salt nanostructures can spontaneously aggregate rather than dissolve in dilute aqueous solutions
Nature Communications (2021)
-
Real-space visualization of intercalated water phases at the hydrophobic graphene interface with atomic force microscopy
Frontiers of Physics (2020)
-
Experimental tests for a liquid-liquid critical point in water
Science China Physics, Mechanics & Astronomy (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.