Abstract
Study design:
Prospective, randomized, pharmacokinetic study.
Objective:
To determine if cyclosporine-A-mediated inhibition of p-glycoprotein would increase methylprednisolone entry into the central nervous system thereby permitting a reduction in the systemic methylprednisolone dose.
Setting:
Department of Anesthesiology, University of Washington, Seattle, USA.
Methods:
Microdialysis probes were used to obtain cerebrospinal fluid and gluteal muscle extracellular fluid samples for measurement of methylprednisolone concentration in pigs. At time zero, a methylprednisolone bolus was given and an infusion started. At 210 min, after reaching a stable methylprednisolone concentration, a cyclosporine-A bolus was given (either 10 or 30 mg/kg) and microdialysis samples collected until 420 min. Plasma samples were collected at 10, 30 min and then every 30 min until the study's end.
Results:
Cyclosporine-A bolus produced a dose-dependant increase in methylprednisolone concentration in plasma, muscle and cerebrospinal fluid. Importantly, the magnitude of the increase in cerebrospinal fluid was significantly greater than the increase in plasma and muscle.
Conclusions:
The relatively greater increase in cerebrospinal fluid concentrations of methylprednisolone is consistent with increased penetration of the blood–brain barrier secondary to cyclosporine-mediated p-glycoprotein inhibition. Theoretically, increased methylprednisolone entry into the central nervous system should allow a reduction in the systemic methylprednisolone dose and a consequent decrease in glucocorticoid-mediated side effects.
Similar content being viewed by others
Introduction
High-dose methylprednisolone begun within 8 h of spinal cord injury has been shown to improve neurological function.1, 2, 3, 4 However, its use is also associated with a significant increase in complications related to systemic immunosuppression. Specifically, the incidence of wound infection, sepsis, pneumonia, and the duration of intensive care unit stay are all increased in patients receiving high-dose methylprednisolone.5, 6, 7, 8 Importantly, some methylprednisolone-mediated morbidities/side effects put patients at increased risk of complications known to contribute to secondary neurological injury (eg, sepsis-induced hypotension,9, 10, 11 glucocorticoid-medicated hyperglycemia12). Consequently, methylprednisolone-related side effects may actually work at crosspurposes to any salutary neurological benefits of methylprednisolone therapy.
We have previously shown that methylprednisolone entry into the central nervous system is actively opposed by p-glycoprotein,13 an active transporter present on the surface of capillary endothelial cells within the central nervous system and an important component of the blood–brain barrier.14, 15 Thus, when targeting the central nervous system, very large systemic doses of methylprednisolone must be administered to overwhelm p-glycoprotein's transport capacity and achieve therapeutic concentrations in the spinal cord.
With this in mind, one approach to improving the therapeutic index for methylprednisolone therapy would be to improve its entry into the central nervous system by blocking p-glycoprotein. Improved entry into the central nervous system would allow a reduction in the systemic methylprednisolone dose necessary to achieve therapeutic concentrations in the spinal cord and would decrease the risk of immunosuppressive side effects.
The goal of this study was to demonstrate just such a pharmacologic approach to improving the therapeutic index for systemic methylprednisolone therapy. To achieve this goal, we examined the effect of cyclosporine-A, a prototypical competitive inhibitor of p-glycoprotein, on methylprednisolone concentrations in plasma, cerebrospinal fluid and muscle.
Materials and methods
All studies were approved by the University of Washington Institutional Animal Care and Use Committee. Guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care were followed throughout.
Farm-bred, mixed-breed, pigs of both genders weighing 14.0±2.6 kg were used. Animals were housed in rooms with 12 h light–dark cycles and given twice daily feedings of pig chow (Purina, St Louis, MO, USA) and ad libitum access to water.
Surgical preparation
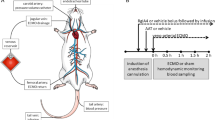
On the study day, anesthesia was induced by mask inhalation of isoflurane (4–5%) in oxygen and the animals were orotracheally intubated after muscle relaxation by intramuscular succinylcholine (100 mg). Anesthesia was maintained with isoflurane (1.5–2%) in oxygen and the animals were mechanically ventilated to maintain end-tidal CO2 at 36–38 mmHg (Datex Airway Gas Analyzer Type GAO, Datex, Helsinki, Finland). Femoral arterial and venous cannulae were placed via cutdown for arterial pressure monitoring, blood sampling, maintenance fluid administration (normal saline at 4 ml/kg/h) and study drug administration. Muscle relaxation was maintained by adding pancuronium bromide to the maintenance fluid infusion (1 mg/ml).
To permit placement of custom made microdialysis probes into the cerebrospinal fluid, the L5, L1, and T9 lamina were exposed and a small (2–3 mm) laminotomy was made to reveal the dura mater. A 1–2 mm hole was incised in the dura and arachnoid maters and a microdialysis probe was inserted into the cerebrospinal fluid. The dialysis probes were fixed in place by cyanoacrylate glue and dental acrylic was used to seal the laminotomies. Mock cerebrospinal fluid (NaCl 140 mEq, NaHCO3 25 mEq, KCl 2.9 mEq, MgCl2 0.4 mEq, urea 3.5 mEq, glucose 4.0 mEq, and CaCl2 2.0 mEq; pH 7.38–7.42; 295 mOsm) was pumped through the dialysis probes at 10 μl/min.
In addition to the dialysis probes placed in the cerebrospinal fluid, a probe was also placed percutaneously in a gluteal muscle. Since muscle capillaries do not express p-glycoprotein, this sample site served as a control to distinguish p-glycoprotein-related effects of cyclosporine-A from non-p-glycoprotein-related effects.
Microdialysis probe manufacture
Custom loop microdialysis probes were made as previously described.13, 16, 17, 18 Briefly, the probes were made from cellulose dialysis fibers (Spectrum Medical Industries Inc., Houston, TX, USA) with a 215 μm inside diameter, a 235 μm outside diameter, and a molecular weight cutoff of 6000 Da. Epoxy cement was used to coat all but the center 20 mm of the dialysis fiber, thus creating a 20 mm ‘dialysis window’. The epoxy was spread evenly by running a 2 cm length of PE-10 tubing over the fiber while the epoxy was still wet. After the epoxy had cured, a 90 μm diameter tungsten wire (Hamilton Company, Reno, NV, USA) was placed in the lumen of the dialysis probe and the probe bent in half. The wire allowed the probe to be bent without occluding the lumen. A silicone elastomer was spread in the shape of a cone at the base of the probe; this served to plug the hole in the meninges through which the probe was inserted. The probes were allowed to ‘cure’ for 24 h before use and all probes were used within 72 h of manufacture.
Study paradigm
Following surgical preparation, the animals received a 2.7 mg/kg methylprednisolone bolus followed immediately by a 2.7 mg/kg/h continuous infusion of methylprednisolone. This infusion continued throughout the 420 min of the study. At the 210 min point, in a randomized fashion, the animals received either a 10 or a 30 mg/kg bolus of cyclosporine-A.
Dialysate was collected continuously as 10 min samples (ie, 100 μl) from all four microdialysis probes. Arterial blood samples (3 ml) were collected at time 0, 10, and 30 min and then every 30 min for the remainder of the study.
Drug assays
Dialysate (40 μl) was mixed with 20 μl of internal standard (fluoxymesterone 1 ng/μl) and placed in an autosampler vial. A 50 μl portion of this sample was injected on the HPLC.
Plasma samples (0.5 ml) were placed in a silanized 13 × 100 screw-cap culture tube and 20 μl of internal standard (fluoxymesterone 35 ng/μl) and 4 ml of methylene chloride were added. The samples were vortexed, the aqueous layer discarded, and 1.5 ml of 0.1 N NaOH and 1.5 ml of deionized water was added. The samples were vortexed, the tubes centrifuged and the aqueous layer again discarded. The methylene chloride phase was decanted into a clean silanized culture tube and evaporated under a stream of air at 40°C. The residue was dissolved in 100 μl of HPLC mobile phase and filtered using a 0.2 μm nylon centrifuge filter. A 50 μl portion was injected on the HPLC.
The HPLC consisted of a Hewlett Packard 1050 series with autosampler, pump, and UV detector. The column was a Supelco LC-18-DB 150 mm × 4.6 mm × 5 μm. The mobile phase consisted of 65% 10 mM potassium phosphate mono-basic and 35% acetonitrile pumped at 1 ml/min. The peaks are detected at 242 nm. Quantification is based upon peak height ratios between methylprednisolone and the internal standard, plotted as a linear regression.
The interday coefficient of variation was 5–7% and the limits of quantitation were 5 ng/ml for plasma and 10 ng/ml for dialysate.
Statistical analysis
Steady-state methylprednisolone concentrations in blood, cerebrospinal fluid dialysate and gluteal muscle dialysate were determined by averaging the concentration present in samples collected over the hour before the cyclosporine bolus (ie, between 150 and 210 min). Steady-state methylprednisolone concentration after the cyclosporine-A bolus was determined by averaging the concentration present in dialysate and blood samples collected over the final hour of the study (ie, between 360 and 420 min). These steady-state concentrations were used for all comparisons of methylprednisolone concentration within and between sampling sites.
The statistical significance of the change in steady-state methylprednisolone concentration within each sample site after cyclosporine-A administration was determined by paired t-test. Differences between sampling sites in the magnitude of the change in methylprednisolone concentration was determined by analysis of variance. A P-value <0.05 was considered statistically significant. Data are reported as mean±SD.
Results
Figure 1a and b shows the raw concentration versus time data for both the 10 and 30 mg/kg cyclosporine groups. Differences in methylprednisolone concentrations among plasma, muscle, and cerebrospinal fluid sampling sites were all statistically significant with the highest concentrations in plasma and the lowest in cerebrospinal fluid. Figure 2a and b shows the same data normalized for the methylprednisolone concentration at the time that the cyclosporine-A bolus was administered.
(a) Methylprednisolone concentration in cerebrospinal fluid dialysate, muscle dialysate, and plasma before and after 10 mg/kg cyclosporine bolus administered at time=210 min. Plasma concentrations were significantly greater than muscle concentrations, which were significantly greater than CSF concentrations. (b) Methylprednisolone concentration in cerebrospinal fluid dialysate, muscle dialysate, and plasma before and after 30 mg/kg cyclosporine bolus administered at time=210 min. Plasma concentrations were significantly greater than muscle concentrations which were significantly greater than CSF concentrations
(a) Relative methylprednisolone concentration in cerebrospinal fluid dialysate (L4 level), muscle dialysate, and plasma before and after 10 mg/kg cyclosporine bolus administered at time=210 min. Methylprednisolone concentrations are expressed as a fraction of the concentration present in the sample collected at each site at the time of the cyclosporine bolus. (b) Relative methylprednisolone concentration in cerebrospinal fluid dialysate (L4 level), muscle dialysate, and plasma before and after 30 mg/kg cyclosporine bolus administered at time=210 min. Methylprednisolone concentrations are expressed as a fraction of the concentration present in the sample collected at each site at the time of the cyclosporine bolus
The coefficient of variation of the eight microdialysis samples used to calculate steady-state cerebrospinal fluid methylprednisolone concentrations for each animal between 150–210 and 360–420 min averaged 8.5±5.0%. The eight microdialysis samples used to calculate steady-state muscle methylprednisolone concentrations for each animal during the same time periods averaged 10.9±5.6%. The three plasma samples used to calculate steady-state methylprednisolone concentrations for each animal during the same time periods averaged 7.9±7.0%. These small coefficients of variation are comparable to the interday variability in the reproducibility of the methylprednisolone assay and indicate that drug concentrations were steady over the time periods analyzed.
Both cyclosporine doses produced statistically significant increases in methylprednisolone concentration in muscle, plasma and all cerebrospinal fluid sampling sites. At all sampling sites, the magnitude of the increase in methylprednisolone concentration was significantly greater for the 30 mg/kg cyclosporine-A dose than for the 10 mg/kg dose (Table 1).
Importantly, the percentage increase in methylprednisolone concentration in cerebrospinal fluid compartments following cyclosporine-A was significantly greater than the simultaneous percentage increase in the plasma concentration of methylprednisolone (Figure 3; Table 1). This was true for both cyclosporine-A doses. In contrast, the percentage increase in methylprednisolone concentration in muscle was not different than the simultaneous percentage increase in the plasma concentration of methylprednisolone at either cyclosporine-A dose (Figure 3; Table 1).
Discussion
We have previously shown that methylprednisolone entry into the CNS is opposed by p-glycoprotein expressed by capillary endothelial cells of the blood–brain/spinal cord barrier.13 Our finding in this study that, prior to cyclosporine administration, methylprednisolone concentrations in muscle (which does not express p-glycoprotein) exceed simultaneous concentrations in CSF is consistent with this earlier observation. In addition, our finding that cyclosporine-A produced a greater percentage increase in cerebrospinal fluid concentrations of methylprednisolone than occurred in plasma or muscle suggests that p-glycoprotein inhibition is an effective method to increase methylprednisolone delivery to the CNS.
However, the increase in cerebrospinal fluid methylprednisolone concentration that resulted from cyclosporine-A was not solely the result of p-glycoprotein inhibition. Cyclosporine-A also increased plasma concentrations of methylprednisolone. The increase in methylprednisolone plasma concentration likely resulted from inhibition of hepatic metabolism of methylprednisolone by cytochrome P450-3A4.19 Most p-glycoprotein inhibitors, including cyclosporine-A, are also competitive cytochrome P450-3A4 inhibitors. In fact, some investigators consider the two proteins to act in a coordinated and synergistic fashion within some tissues (eg, gastrointestinal epithelium, hepatocytes) to limit absorption or promote elimination of a large number of molecules.20, 21, 22
The cyclosporine-mediated increase in methylprednisolone plasma concentration produced a comparable increase in methylprednisolone concentration in muscle (Figure 3). Since muscle does not express p-glycoprotein, and therefore does not actively exclude methylprednisolone, one would expect methylprednisolone concentrations in muscle to parallel those in plasma, which is what was found. In contrast, within the cerebrospinal fluid the increase in methylprednisolone concentration following cyclosporine-A bolus was significantly greater than can be explained by the simultaneous plasma methylprednisolone increase (Figure 2).
The relatively greater increase in cerebrospinal fluid methylprednisolone concentration compared to the increase in plasma concentration can best be explained by a reduction in the barrier to methylprednisolone entry into the CNS. The most likely mechanism for a reduction in the efficacy of the barrier to methylprednisolone entry is cyclosporine-A-mediated inhibition of p-glycoprotein. Thus, these data indicate that p-glycoprotein inhibition represents a potential method to enhance methylprednisolone entry into the CNS.
Since spinal cord injury increases permeability of the blood–spinal cord barrier,23, 24 it is reasonable to consider whether p-glycoprotein inhibition is necessary to increase methylprednisolone concentrations in the injured spinal cord. However, Braughler and Hall demonstrated that methylprednisolone entry into injured spinal tissue was greater than in uninjured tissue only if methylprednisolone was administered within 1 h of injury. If administered more than 1 h after injury, methylprednisolone concentration in injured spinal tissue was no different than in uninjured tissue. These findings would suggest that methylprednisolone permeability across the blood–spinal cord barrier is only very transiently increased and that methylprednisolone entry into the spinal cord is likely to be increased even in the injured spinal cord by p-glycoprotein inhibition.
The potential clinical advantage of improving methylprednisolone entry into the CNS from the plasma is that the methylprednisolone dose could be decreased with the potential for a reduction in glucocorticoid-mediated systemic side effects. In particular, reduced immune suppression would be expected to decrease the risk of wound infection, sepsis, and pneumonia that has been noted in multiple studies in which high-dose methylprednisolone was used in the treatment of acute spinal cord injury. Importantly, a reduction in the incidence or severity of these infectious complications may well have a positive impact on neurologic recovery. For example, hypotension, which frequently complicates sepsis, has been identified as an important contributor to secondary neurologic injury.11
Similarly, methylprednisolone-induced hyperglycemia may contribute to secondary neurologic injury. Animal models have demonstrated that hyperglycemia is an important contributor to secondary neurologic injury,12, 25 and hyperglycemia has been implicated as a contributor to poor neurologic outcome in humans.26, 27 For example, Lam et al27 observed that head-injured patients with a postoperative blood glucose concentration greater than 200 mg/dl had a significantly worse neurologic outcome than did patients with a lower blood glucose concentration. Rovlias and Kotsou26 have reported very similar results. Consequently, use of a p-glycoprotein inhibitor that increases methylprednisolone entry into the CNS and thereby permits a reduction in the systemic methylprednisolone dose may improve neurological outcome.
The ideal p-glycoprotein inhibitor would be a drug that has little or no toxicity and which does not simultaneously inhibit the related cytochromes involved in drug metabolism. While pharmaceutical companies are working on just such a drug, it is not yet clinically available. We could have chosen other clinically available drugs (eg, digoxin, verapamil, vinca alkaloids, daunamycin, etc) as competitive p-glycoprotein inhibitors, but these drugs are acutely toxic or fatal in the doses needed to produce demonstrable p-glycoprotein inhibition in vivo. Thus, cyclosporine-A was an appropriate choice for a ‘proof of principle’. However, these data should not be construed as advocating simultaneous administration of cyclosporine-A and methylprednisolone in humans with spinal cord injury. Although cyclosporine-A has been shown to improve neurological function when it is administered alone in animal models of acute neurological injury,28, 29, 30, 31, 32, 33, 34, 35, 36 its coadministration with high-dose methylprednisolone is untested in humans and the potential for significant morbidity secondary to profound immunosuppression is a concern.
In summary, this study demonstrates that coadministering the p-glycoprotein inhibitor, cyclosporine-A, increases entry of methylprednisolone into the CNS, although it also increases plasma methylprednisolone concentration by inhibiting methylprednisolone metabolism. The findings suggest that current efforts by the pharmaceutical industry to develop more potent and less toxic p-glycoprotein inhibitors that do not simultaneously inhibit cytochrome p-450 may improve the efficacy of methylprednisolone in treating acute spinal cord trauma.
References
Young W, Bracken MB . The second national acute spinal cord injury study. J Neurotrauma 1992; 9 (Suppl 1): S397–S405.
Bracken MB, Holford TR . Neurological and functional status 1 year after acute spinal cord injury: estimates of functional recovery in National Acute Spinal Cord Injury Study II from results modeled in National Acute Spinal Cord Injury Study III. J Neurosurg 2002; 96: 259–266.
Bracken MB et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med 1990; 322: 1405–1411.
Bracken MB et al. Methylprednisolone and neurological function 1 year after spinal cord injury. Results of the National Acute Spinal Cord Injury Study. J Neurosurg 1985; 63: 704–713.
Gerndt SJ et al. Consequences of high-dose steroid therapy for acute spinal cord injury. J Trauma 1997; 42: 279–284.
Galandiuk S, Raque G, Appel S, Polk Jr HC . The two-edged sword of large-dose steroids for spinal cord trauma. Ann Surg 1993; 218: 419–425; discussion 425–427.
Bracken MB et al. Efficacy of methylprednisolone in acute spinal cord injury. JAMA 1984; 251: 45–52.
Bracken MB et al. Administration of methylprednisolone for 24 or 48 h or tirilazad mesylate for 48 h in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA 1997; 277: 1597–1604.
Pietropaoli JA, Rogers FB, Shackford SR, Wald SL, Schmoker JD, Zhuang J . The deleterious effects of intraoperative hypotension on outcome in patients with severe head injuries. J Trauma 1992; 33: 403–407.
Winchell RJ, Simons RK, Hoyt DB . Transient systolic hypotension. A serious problem in the management of head injury. Arch Surg 1996; 131: 533–539; discussion 539.
Brain Trauma Foundation, American Association of Neurological Surgeons: The Brain Trauma Foundation. The American Association of Neurological Surgeons. The Joint Section on Neurotrauma and Critical Care. Hypotension J Neurotrauma 2000; 17: 591–595.
Cherian L, Hannay HJ, Vagner G, Goodman JC, Contant CF, Robertson CS . Hyperglycemia increases neurological damage and behavioral deficits from post-traumatic secondary ischemic insults. J Neurotrauma 1998; 15: 307–321.
Koszdin KL, Shen DD, Bernards CM . Spinal cord bioavailability of methylprednisolone after intravenous and intrathecal administration: the role of P-glycoprotein. Anesthesiology 2000; 92: 156–163.
Potschka H, Fedrowitz M, Loscher W . Multidrug resistance protein MRP2 contributes to blood–brain barrier function and restricts antiepileptic drug activity. J Pharmacol Exp Ther 2003; 306: 124–131.
Zong J, Pollack GM . Modulation of P-glycoprotein transport activity in the mouse blood–brain barrier by rifampin. J Pharmacol Exp Ther 2003; 306: 556–562.
Kopacz DJ, Bernards CM . Effect of clonidine on lidocaine clearance in vivo: a microdialysis study in humans. Anesthesiology 2001; 95: 1371–1376.
Bernards CM, Kopacz DJ . Effect of epinephrine on lidocaine clearance in vivo: a microdialysis study in humans. Anesthesiology 1999; 91: 962–968.
Bethune CR, Bernards CM, Bui-Nguyen T, Shen DD, Ho RJ . The role of drug–lipid interactions on the disposition of liposome-formulated opioid analgesics in vitro and in vivo. Anesth Analg 2001; 93: 928–933.
Varis T, Kaukonen KM, Kivisto KT, Neuvonen PJ . Plasma concentrations and effects of oral methylprednisolone are considerably increased by itraconazole. Clin Pharmacol Ther 1998; 64: 363–368.
Zhang Y, Benet LZ . The gut as a barrier to drug absorption: combined role of cytochrome P450 3A and P-glycoprotein. Clin Pharmacokinet 2001; 40: 159–168.
Patel J, Mitra AK . Strategies to overcome simultaneous P-glycoprotein mediated efflux and CYP3A4 mediated metabolism of drugs. Pharmacogenomics 2001; 2: 401–415.
Wacher VJ, Wu CY, Benet LZ . Overlapping substrate specificities and tissue distribution of cytochrome P450 3A and P-glycoprotein: implications for drug delivery and activity in cancer chemotherapy. Mol Carcinog 1995; 13: 129–134.
Sharma HS . Neurotrophic factors attenuate microvascular permeability disturbances and axonal injury following trauma to the rat spinal cord. Acta Neurochir Suppl 2003; 86: 383–388.
Sharma HS . Pathophysiology of blood–spinal cord barrier in traumatic injury and repair. Curr Pharm Des 2005; 11: 1353–1389.
Wass CT, Scheithauer BW, Bronk JT, Wilson RM, Lanier WL . Insulin treatment of corticosteroid-associated hyperglycemia and its effect on outcome after forebrain ischemia in rats. Anesthesiology 1996; 84: 644–651.
Rovlias A, Kotsou S . The influence of hyperglycemia on neurological outcome in patients with severe head injury. Neurosurgery 2000; 46: 335–342; discussion 342–343.
Lam AM, Winn HR, Cullen BF, Sundling N . Hyperglycemia and neurological outcome in patients with head injury. J Neurosurg 1991; 75: 545–551.
Diaz-Ruiz A et al. Lipid peroxidation inhibition in spinal cord injury: cyclosporin-A vs methylprednisolone. Neuroreport 2000; 11: 1765–1767.
Diaz-Ruiz A et al. Cyclosporin-A inhibits lipid peroxidation after spinal cord injury in rats. Neurosci Lett 1999; 266: 61–64.
Uchino H et al. Differential neuroprotection by cyclosporin A and FK506 following ischemia corresponds with differing abilities to inhibit calcineurin and the mitochondrial permeability transition. Neurobiol Dis 2002; 10: 219–233.
Miyata K, Omori N, Uchino H, Yamaguchi T, Isshiki A, Shibasaki F . Involvement of the brain-derived neurotrophic factor/TrkB pathway in neuroprotecive effect of cyclosporin A in forebrain ischemia. Neuroscience 2001; 105: 571–578.
Yoshimoto T, Uchino H, He QP, Li PA, Siesjo BK . Cyclosporin A, but not FK506, prevents the downregulation of phosphorylated Akt after transient focal ischemia in the rat. Brain Res 2001; 899: 148–158.
Uchino H et al. Amelioration by cyclosporin A of brain damage in transient forebrain ischemia in the rat. Brain Res 1998; 812: 216–226.
Li PA, Uchino H, Elmer E, Siesjo BK . Amelioration by cyclosporin A of brain damage following 5 or 10 min of ischemia in rats subjected to preischemic hyperglycemia. Brain Res 1997; 753: 133–140.
Uchino H, Elmer E, Uchino K, Lindvall O, Siesjo BK . Cyclosporin A dramatically ameliorates CA1 hippocampal damage following transient forebrain ischaemia in the rat. Acta Physiol Scand 1995; 155: 469–471.
Sullivan PG, Thompson M, Scheff SW . Continuous infusion of cyclosporin A postinjury significantly ameliorates cortical damage following traumatic brain injury. Exp Neurol 2000; 161: 631–637.
Acknowledgements
This work was funded by the National Institutes of Health (NS38911), Bethesda, MD, USA.
Author information
Authors and Affiliations
Additional information
This work was performed at the Department of Anesthesiology, University of Washington.
Rights and permissions
About this article
Cite this article
Bernards, C. Cyclosporine-A-mediated inhibition of p-glycoprotein increases methylprednisolone entry into the central nervous system. Spinal Cord 44, 414–420 (2006). https://doi.org/10.1038/sj.sc.3101863
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101863