Abstract
Study design:
Cross-sectional, observational, controlled study.
Objective:
High spinal cord injury (SCI) results in disruption of sympathetic vasomotor control. Vasodilatation as a response to local heating is a biphasic mechanism: the first phase (neurogenic) is mediated by the axon-reflex and is modulated by activity of sympathetic nerves. Our objective was to determine whether the response to heat provocation in trunk dermatomes may provide a measure of vasomotor sympathetic function in SCI.
Setting:
National Spinal Injuries Centre, Stoke Mandeville Hospital, Buckinghamshire, UK; Autonomic Unit, The National Hospital for Neurology and Neurosurgery, Queen Square, London, UK; Neurovascular Medicine Unit, Imperial College London at St Mary's Hospital, UK.
Subjects:
A total of 30 subjects were studied; 18 had chronic complete SCI (level C6–T11) and 12 were healthy controls.
Methods:
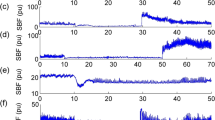
Recordings of skin blood flow (SkBF) were obtained with thermostatic laser Doppler probes placed in the upper trunk (at C4) and lower trunk (T10 or T12) dermatomes.
Results:
SkBF at baseline (SkBFbas) and SkBF at the first peak of vasodilatation (SkBFmax) showed no significant differences between SCI and controls either in upper or lower trunk dermatomes. However, the ratio of SkBFmax/SkBFbas was significantly different in lower trunk dermatomes in SCI at C6–T5 level (7.5±3.5 PU) compared to SCI at T6-T11 level (3.5±1.5 PU) (P<0.01).
Conclusion:
Measurement of SkBF in response to local heating may provide a safe, noninvasive method to assess integrity of sympathetic spinal pathways to the local vasculature. This may aid the classification of the SCI lesions, as the autonomic component currently is not included in the accepted American Spinal Injury Association scoring.
Similar content being viewed by others
Introduction
Autonomic pathways from the brainstem and hypothalamus to the intermediolateral cell column of the spinal cord are interrupted following a spinal cord injury (SCI). In the majority this affects the sympathetic component of the autonomic system, which descends in the thoracic and lumbar segments of the spinal cord, and the parasympathetic innervation to pelvic viscera. As the brain can no longer control the sympathetic outflow below the level of lesion, tonic background excitation of the intermediolateral cells is lost. The result is a loss of modulation of the spinal autonomic reflexes below the lesion with responses to a variety of stimuli rendered ineffective or inappropriate.1, 2 The level and extent of the lesion are important in determining the severity of autonomic dysfunction; thus, low supine basal levels of blood pressure, orthostatic hypotension, and at times autonomic dysreflexia (AD) with substantial hypertension are more likely to occur in patients with complete SCI above the fifth thoracic level.2
Vasodilatation as a response to local heating of nonglabrous skin is a biphasic mechanism.3 The first peak in skin blood flow (SkBF) rise, the neurogenic phase, is mediated by an axon-reflex, initiated the by activity of sensory nerves and modulated by the activity of sympathetic nerves.4 It is a fast responding phase reaching its peak in a few minutes and followed by a brief nadir. The second phase of rise in SkBF is mediated by the local production of endothelial nitric oxide and reaches a plateau in about 30 min.3
Autonomic evaluation to aid classification of SCI using the sympathetic skin response (SSR), which evaluates only one autonomic pathway, involving sympathetic cholinergic fibres to sweat glands has been proposed.5 Evaluation of sympathetic innervation of the vasculature would be of particular value. In a previous study in our units, Kuesgen et al6 investigating axon-reflex vasodilatation in trunk dermatomes above and below the level of lesion in chronic SCI used intradermal injection of histamine, and reported diminished vasodilatation below the level of lesion. We have extended our pilot study7 using a physiological stimulus, which is noninvasive and nonpainful, to induce vasodilatation by controlled local heating of the skin. We hypothesized that as the first peak of the vasodilator response in trunk dermatomes below the level of lesion is dependent on sympathetic activation of blood vessels, this would be impaired in complete SCI where such spinal pathways were disrupted. Determining the degree of cutaneous segmental sympathetic involvement on the trunk was likely to provide a technique to aid the classification of the autonomic component of SCI.
Methods
Subjects
In total, 30 subjects participated in the study. Of these, 18 (17 male and one female, age 38.5±12.6 years (mean±SD)) subjects had complete chronic (time from injury >6 months) SCI, using the American Spinal Injury Association (ASIA) impairment scale,8 which is based on motor and sensory examination. Lesions were localized at the cervical (C6) level (in two subjects) and thoracic level (T1–T11) in the remaining subjects. In order to obtain data from a group as homogeneous as possible, we only included subjects with complete injury caused by a trauma.
None of the selected SCI subjects had neurological symptoms or signs suggestive of myelomalacia. A total of 12 (seven male and five female, age 39.2±11.2 years) healthy subjects, who were age-matched with the SCI subjects, participated as controls.
The study was part of the Clinical Initiative supported by the International Spinal Research Trust. Ethics approval was granted by the Aylesbury Vale Local Research Ethics Committee (LREC), the National Hospital for Neurology and Neurosurgery and St Mary's Hospital LREC, London, UK. The study conformed to the principles of the Declaration of Helsinki. All subjects gave their informed consent prior to their participation in the study.
Skin blood flow measurement
Subjects were examined in a quiet room while sitting on a chair or wheelchair; this allowed them to be in the most comfortable position. Throughout the study, they were relaxed but not asleep. Room temperature was maintained at 23±1°C.
SkBF was recorded by laser Doppler flowmetry (Perimed PF4, Stockholm, Sweden) using thermostatic laser Doppler probes (angled small Thermostatic laser Doppler probe, diameter of 10 mm, Perimed probe 457). These probes have both recording and heating elements, thus enabling recording of SkBF while heating the underlying skin surface; SkBF was measured in perfusion units (PU), values that represent the product of the velocity and the concentration of the moving blood cells within the measuring volume.
In SCI, one thermostatic laser Doppler probe was placed on an upper trunk dermatome, C4, which was above the level of lesion in all SCI subjects. The second thermostatic probe was placed on a lower dermatome, T10 or T12, and was below the level of lesion. In healthy controls, one thermostatic laser Doppler probe was placed on an upper trunk dermatome, C4, and the second probe was placed on a lower trunk dermatome, T10. During 10 min of baseline recording the probe temperature was 32°C. Then it was increased to 41°C and was maintained at this level for 30 min. None of the subjects reported the stimulus as painful.
We estimated SkBF at baseline (SkBFbas) and at the initial peak of vasodilatation (SkBFmax). Results are tabulated as mean PU±SD. We also calculated the ratio of initial peak of vasodilatation over baseline SkBF (SkBFmax/SkBFbas), above and below the level of lesion.
Statistical analysis
The statistical software STATISTICA for Windows Release 4.3 (StatSoft Inc., Tulsa, OK, USA) was used to analyse data. The differences in results of SkBF measurements at baseline, at the initial peak of vasodilatation and the SkBFmax/SkBFbas ratio between SCI subjects and controls were analysed with a one-way analysis of variance (ANOVA). The Scheffé test was used to make post hoc comparisons where group effects were found. Differences were considered statistically significant when P-values were less than 0.05.
Results
These are described in the following groups: group A, including 11 (10 male and one female subjects, age 36.5±11.8 years) subjects with complete SCI at cervical and high thoracic level (C6–T5); Group B, including seven (seven male, age 41.5±14.2) subjects with complete SCI at mid to low thoracic level (T6–T11); group C, including 12 (seven male and five female, age 39.2±11.2 years) healthy controls. The necessity of grouping the SCI subjects in two groups according to their level of lesion arises from the fact that vasomotor dysfunction, such as AD, affects high SCI lesions above the fifth thoracic level. However, none of the SCI subjects we studied had AD at the time of investigation.
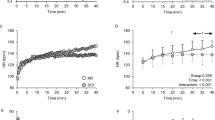
SkBFbas in upper trunk dermatomes in group A was 27.5±18 PU, in group B 18.9±7.9 PU and in group C 26.5±17.9 PU. In lower trunk dermatomes, SkBFbas in group A was 8.6±2.8 PU, in group B 11.6±1.9 PU and in group C 9.4±3.4 PU (Table 1). There was no significant difference among the three groups in upper and lower trunk dermatomes (Figure 1).
SkBFmax in upper trunk dermatomes in group A was 123.6±59.2 PU, in group B 128±92 and in group C 90.7±32.5. In lower trunk dermatomes SkBFmax in group A was 65.3±31.2 PU, in group B 41.5±20.9 and in group C 49±23.9; there was no significant difference (Figure 1).
The ratio of SkBFmax/SkBFbas in upper trunk dermatomes in group A was 6.1±4.3 PU, in group B 7.4±5 PU and in group C 4.3±2.4 PU, with no significant differences among the three groups. However, the SkBFmax/SkBFbas ratio in lower trunk dermatomes in group A was 7.5±3.5 PU, in group B 3.5±1.5 and in group C 5.2±2.1. There was a significant difference (P<0.01) between group A (SCI at C6–T5) and group B (SCI at T6–T11). There was no significant difference between SCI groups A and B and controls (Table 1 and Figure 2).
Discussion
The main finding of this study is that the axon-reflex vasodilatation is preserved below the level of lesion in chronic complete SCI. However, our results showed a significant difference in the skin vasodilator response to local skin heating below the level of lesion in complete SCI with high lesions (C6–T5) as compared to SCI with low lesions (T6–T11). Our technique evaluated the integrity of the axon-reflex vasodilatation where the sensory axon initiates the response, which is then modulated by the sympathetic system. Studies in animals9 and humans4 indicate that sympathetic vasoconstriction can oppose the effect of antidromic vasodilatation. Whether in our study the reduced SkBF below the level of injury in response to local heating in low lesions is due to increased vasoconstriction or reduced vasodilatation is debatable. However, our results support impairment in vasomotor control below the level of lesion in subjects with SCI at C6–T5 when compared with lesions at T6–T11.
This study focused on vasomotor function using a novel approach, applying a physiological stimulus and recording the response from the dermatomal skin segment from where the reflex was initiated. In AD, in subjects with high lesions above T5 but not below, a sensory stimulus below the level of lesion triggers an exaggerated response that results in hypertension, which is due to vasoconstriction mediated by increased sympathoneural activity.10 Paroxysmal hypertension may arise from stimuli from skin, skeletal muscle, abdominal or pelvic viscera activation.2 In our study, in subjects with high SCI lesions, a sensory stimulus locally applied below the level of lesion increased vasodilation at the same site of stimulation, in contrast to low SCI lesions. Vasomotor function in high SCI is impaired because of the inability of higher autonomic centres to increase sympathetic activity, as is observed with head-up postural change causing orthostatic hypotension.2 The inability to oppose heat-provoked vasodilatation by descending sympathetic pathways may be one reason for the greater vasodilatation in high lesions, where a larger proportion of sympathetic pathways is separated from central control. Also, high lesions are known to be supersensitive to the effects of vasodilator agents or stimuli. This has been described in response to hypoglycaemia, which causes a fall in blood pressure,11 and to vasodilator drugs.12 This has been attributed to impaired sympathetically mediated vasoconstriction, to oppose vasodilatation. This may be an additional factor accounting for the greater heat-provoked vasodilatation in high lesions.
There are numerous studies evaluating the sympathetic cholinergic component of sweat function. These studies have used the SSR, a noninvasive technique that assesses sympathetic cholinergic sudomotor pathways. Magnifico et al13 demonstrated that the SSR is absent in pure autonomic failure, where sympathetic cholinergic function is impaired, but not when there was a selective adrenergic deficit, as in dopamine-beta-hydroxylase deficiency, where sympathetic cholinergic function is preserved. The SSR can be elicited above but not below the level of lesion in SCI, and is a reflection of the integrity of sympathetic cholinergic pathways to sweat glands.5, 14, 15 In our present study, we used the axon-reflex vasodilatation to evaluate the sympathetic vasomotor component, as this has not been fully investigated as a method to aid classification of SCI.
Previous work in humans16, 17 demonstrated that heating one limb caused an increase in blood flow through the controlateral limb. The response was abolished when these nerves were blocked or cut, indicating that sympathetic nerves mediated this response to ‘indirect heating’. The response may have been mediated by the activity of vasodilator nerves and/or release of vasoconstrictor tone.18 This pattern of thermoregulatory response in the hand and forearm also occurs in other extremities like the feet, ears, nose and lips,19 where blood vessels are normally subjected to high levels of vasoconstrictor tone because of their richer adrenergic sympathetic innervation.18 However, skin in more proximal parts of the body, like the trunk (chest, abdomen and back), upper arms and thighs, has less vasoconstrictor tone;18 whether increases in SkBF in these areas are more likely to be mediated by increased vasodilator nerve activity remained a possibility.
In a previous pilot study using a similar approach,7 heat-provoked vasodilatation in chronic SCI at T4–T9 was diminished in the foot when compared to the hand. The sympathetic innervation of the hand and foot differs considerably from that of trunk dermatomes, being denser in the former. In this study with evaluations in trunk dermatomes, there were differences. Whether this relates to differences between the areas studied and their degree of adrenergic innervation remain to be investigated further.
Our present study focused on segmental SkBF in the trunk; the vasodilator activity studied was local and unilateral and evaluated the integrity of axon-reflex activity in a temperature-controlled environment. The changes in local SkBF therefore are likely to be due to changes in segmental vasomotor control. We demonstrated a difference in vasomotor control in SCI subjects below the level of lesion that depends upon lesion level, being greater in those in whom there was substantial sympathetic denervation. Our method utilizes commercially available apparatus that is also used in routine clinical setting and uses a physiological stimulus that is safe and not painful. Whether this measurement of SkBF in response to local heating can be exploited to aid classification of the sympathetic component of a SCI needs further study in complete and incomplete lesions at different segmental levels.
References
Harati Y . Autonomic disorders associated with spinal cord injury. In: Low PA (ed). Clinical Autonomic Disorders. 2nd edn. Lippincott-Raven Publishers: New York, USA 1997, pp 455–461.
Mathias CJ, Frankel HL . Autonomic disturbances in spinal cord lesions. In: Mathias CJ, Bannister R (eds). Autonomic Failure. A Textbook of Clinical Disorders of the Autonomic Nervous System. 4th edn. Oxford University Press: New York, USA 2002. pp 494–513.
Minson CT, Berry LT, Joyner MJ . Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol 2001; 91: 1619–1626.
Hornyak ME, Naver HK, Rydenhag B, Wallin G . Sympathetic activity influences the vascular axon reflex in the skin. Acta Physiol Scand 1990; 139: 77–84.
Cariga P, Catley M, Mathias CJ, Savic G, Frankel HL, Ellaway PH . Organisation of the sympathetic skin response in spinal cord injury. J Neurol Neurosurg Psychiatry 2002; 72: 356–360.
Kuesgen B, Frankel HL, Anand P . Decreased cutaneous sensory axon-reflex vasodilatation below the lesion in patients with complete spinal cord injury. Somatosens Mot Res 2002; 19: 149–152.
Nicotra A, Asahina M, Mathias CJ . Skin vasodilator response to local heating in human chronic spinal cord injury. Eur J Neurol 2004; 11: 835–837.
Maynard Jr FM et al. International standards for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Spinal Cord 1997; 35: 266–274.
Häbler HJ, Wasner G, Jänig W . Interaction of sympathetic vasoconstriction and antidromic vasodilatation in the control of skin blood flow. Exp Brain Res 1997; 113: 402–410.
Mathias CJ, Christensen NJ, Corbett JL, Frankel HL, Spalding JM . Plasma catecholamines during paroxysmal neurogenic hypertension in quadriplegic man. Circ Res 1976; 39: 204–208.
Mathias CJ, Frankel HL, Turner RC, Christensen NL . Physiological responses to insulin hypoglycaemia in spinal man. Paraplegia 1979; 17: 319–326.
Mathias CJ . Neurological disturbances of the cardiovascular system. Doctor of Philosophy Thesis, University of Oxford, 1976.
Magnifico F, Misra VP, Murray NMF, Mathias CJ . The sympathetic skin response in peripheral autonomic failure – evaluation in pure autonomic failure, pure cholinergic dysautonomia and dopamine-beta-hydroxylase deficiency. Clin Auton Res 1998; 8: 133–138.
Curt A, Weinhardt C, Diets V . Significance of sympathetic skin response in the assessment of autonomic failure in patients with spinal cord injury. J Auton Nerv Syst 1996; 61: 175–180.
Curt A, Nitsche B, Rodic B, Scurch B, Dietz V . Assessment of autonomic dysreflexia in patients with spinal cord injury. J Neurol Neurosurg Psychiatry 1997; 62: 473–477.
Lewis T, Pickering GW . Vasodilatation in the limbs in response to warming the body; with evidence for sympathetic vasodilator nerves in man. Heart 1931; 16: 33–51.
Roddie IC, Shepherd JT, Whelan RF . The contribution of constrictor and dilator nerves to the skin vasodilatation during body heating. J Physiol 1957; 136: 489–497.
Roddie IC . Sympathetic vasodilatation in human skin. J Physiol 2003; 548: 336–337.
Roddie IC . In: Shepherd JT, Abboud FM (eds). Handbook of Physiology. Section 2. The Cardiovascular System, Vol III, Part 1. Am Physiol Society: Bethesda 1983. pp 285–317.
Acknowledgements
We gratefully acknowledge the support of the International Spinal Research Trust for this study, which formed part of the Clinical Initiative Programme of the Trust.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nicotra, A., Asahina, M., Young, T. et al. Heat-provoked skin vasodilatation in innervated and denervated trunk dermatomes in human spinal cord injury. Spinal Cord 44, 222–226 (2006). https://doi.org/10.1038/sj.sc.3101837
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101837
Keywords
This article is cited by
-
Peripheral vascular function in spinal cord injury: a systematic review
Spinal Cord (2013)
-
Enhanced phase synchronization of blood flow oscillations between heated and adjacent non-heated sacral skin
Medical & Biological Engineering & Computing (2012)
-
Autonomic assessment of animals with spinal cord injury: tools, techniques and translation
Spinal Cord (2009)
-
Assessment of the sympathetic level of lesion in patients with spinal cord injury
Spinal Cord (2009)