Abstract
Study design: Retrospective review of patient data.
Objectives:(i) To determine the incidence and time of deep vein thrombosis (DVT) under low molecular weight heparin (LMWH) prophylaxis in spinal cord injury (SCI), (ii) to determine the incidence and time of heterotopic ossification (HO) and (iii) to assess a possible aetiologic relationship in the pathogenesis of DVT and HO.
Setting: Swiss Paraplegic Centre, Nottwil.
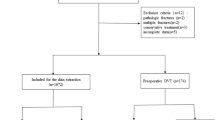
Methods: We analyzed the incidence of DVT and HO in 1209 SCI patients (275 first rehabilitations) at the Swiss Paraplegic Centre Nottwil from 1998 to 2000. Clinical files and laboratory data were scrutinised for particularities preceding DVT and HO.
Results: The incidence of DVT was 6.55% for first rehabilitation compared to only 1.59% in all patients hospitalised. DVT was complicated by pulmonary embolism (PE) in 1.45% and 0.47% respectively. Incidence of HO was 8% for first rehabilitation and 1.82% for all patients hospitalised. In first rehabilitation patients the peak for DVT occurred around day 30 contrary to HO with a peak around day 120. In single patients HO was identified by MRI as a rapidly progressing process. Laboratory profiles were inflammatory in both HO and DVT. Increased physical activity preceding HO was observed in four patients. In two patients acute HO was complicated by ipsilateral DVT.
Conclusion: Prophylaxis with LMWH and elastic stockings significantly reduces the frequency of DVT during first rehabilitation in SCI. DVT and HO are both associated with laboratory parameters of non-infectious inflammation. The later onset of HO coinciding with ongoing mobilisation, argues for a different pathogenetic mechanism. Acute HO of the hip region appears to favour ipsilateral DVT by well known thrombogenic mechanisms.
Similar content being viewed by others
Introduction
Deep vein thrombosis (DVT) is a frequent complication of spinal cord injury (SCI).1,2,3 According to the internationally accepted criteria of Bounameaux et al.4 acute paraplegics have a high risk of developing DVT because stabilisation of the spine is a major orthopaedic procedure, and because of a well-known hypercoagulability associated with SCI.5,6
The real incidence of DVT in SCI is not known. Previous studies are difficult to compare because of different methods and non-homogeneous population.7 According to several studies DVT most frequently occurred between day 5 and 12 after the injury.8,9 In the era before prophylaxis with unfractionated heparin the incidence of DVT was up to 70% (reviewed by Merli et al.8). Even with low molecular weight heparin (LMWH) prophylaxis the incidence in acute SCI is still about 10%.10,11
Heterotopic ossification (HO) is a frequent complication in acute SCI.12,13,14 HO is characterised by an inflammatory process involving the muscles and resulting in formation of new bone preferentially around joints. This results in reduced mobility and a delay in recovery. Despite many efforts the pathogenesis of HO is still not known (reviewed by Wang et al.15). Individual cases point to an inflammatory component16,17 and other studies suggest a comorbidity of HO and DVT.18,19,20 A related aetiology was proposed but never proved.18,19 Perkash et al.19 demonstrated that an elevated level of d-dimer correlates with the activity of HO. Recent experimental studies suggest a central role of muscle-localised pluripotential mesenchymal cells and genes specific for enchondral ossification in the formation of the new ectopic bone.21,22
The goal of this work is to assess (i) the incidences of DVT and HO in SCI patients at the Swiss Paraplegic Centre (SPC) Nottwil, (ii) the interval between the event of SCI and the diagnosis of these complications and (iii) to examine mechanisms of interaction between DVT and HO.
Methods
Subjects
We investigated retrospectively 1209 patients between 1st January 1998 and 31st December 2000 at the SPC Nottwil (Table 1). The mean age of the patients was 46.6 years (5–90). Most of the patients had traumatic SCI and were male. Paralysis due to surgery was classified as trauma. We excluded patients without neurological deficits, with cerebral pathology, myelopathies without paralysis, congenital paralysis, herniation of intervertebral discs and psychiatric disorders. A patient with a newly diagnosed paralysis was considered as a first rehabilitation. Rehabilitation occurring at least two months after the event of the SCI was considered as re-rehabilitation. The 275 subjects with a mean age of 49.7 years (7–85) were first-rehabilitation patients and 934 with a mean age of 45.6 years (5–90) were re-rehabilitation patients.
DVT prophylaxis
All first rehabilitation patients (except the children) had DVT prophylaxis with LMWH (5000 IE Fragmin® once daily subcutaneously during bed rest, and additionally elastic stockings strength II during the first six weeks of mobilisation. DVT prophylaxis was started the day of hospitalisation. Duration of prophylaxis was thus dependent on the time elapsed until full mobilisation, ie for paraplegics at least 10 weeks and for quadriplegics at least 15 weeks. For re-rehabilitation the same prophylactic scheme was used but some individual patients refused to wear elastic stockings.
Diagnosis of DVT and HO
DVT was suspected when a staff member (physician, physiotherapist, nurse) on the daily ward rounding noted a physical change as localised swelling (mainly of the lower leg), temperature differences or redness. DVT was diagnosed by Doppler ultrasound and, if necessary, confirmed by contrast phlebography. HO was suspected by staff members (primarily physiotherapists but also physicians and nurses) when a progressive loss of motion of a particular joint, (mainly the hip), was observed. HO was diagnosed with magnetic resonance tomography (MRT) and/or computer tomography (CT). When DVT or HO was suspected laboratory investigations including peripheral blood counts c reactive protein (CRP) and alkaline phosphatase (aP) were performed on a routine basis. D-dimer levels, however, were only determined after special request of the responsible physician.
Statistics
The statistical evaluation was performed with the χ-square t-test.
Results
Altogether we found 27 DVT with an incidence of 1.59%. Eighteen of them occurred during first rehabilitation and nine during re-rehabilitation (Table 2) the incidence was 6.55% and 0.63% respectively. The risk of DVT during first rehabilitation was significantly higher than during a later hospitalisation (P<0.001). At first rehabilitations the mean interval between the beginning of the SCI and the DVT was 80.9 days (13–307) (Figure 1). If we exclude the latest two DVT, the mean interval is 55 days (13–160). Thirteen patients had an adequate prophylaxis when the DVT occurred. Nine of them had LMWH, one heparin, two oral anticoagulation together with LMWH, and one patient had oral anticoagulation together with heparin. Nine patients were mobilised at this moment. In three of these 13 patients additional risk factors for DVT were identified: heterozygous protein S deficiency in one patient and local immobility and inflammation in two patients due to acute HO in the hip region concerned. In both patients diagnosis of HO preceded DVT by two weeks, however, the overall association of HO and DVT was not significant (P=0.67).
HO was diagnosed 31 times, correlating with an incidence of 1.82% (Figure 2) with 22 of them occurring during first rehabilitation (incidence of 8.00%) and nine during re-rehabilitation (incidence of 0.63%) (Table 2), this difference is statistically significant (P<0.001). The mean interval between acute SCI and HO was 111.3 days (36–213) (Figure 1). Nineteen patients had a DVT prophylaxis when the HO occurred, 10 of them had LMWH, six an oral anticoagulation and three both. Patients mobilised at this moment numbered 27 and in four of them the nursing staff reported an exaggerated training of the lower limbs by the patient or by his family. The laboratory parameters at the time of HO diagnosis showed in most cases a normal leukocyte count and an elevated CRP of non-infectious aetiology (Table 3ab) and were similar to those observed in DVT (Table 4a, b). The combination of leukocytosis (>10 G/L) and very high CRP (>100 mg/L) was identified in two patients with HO and florid urinary tract infection. Five first-rehabilitation and two re-rehabilitation patients with the diagnosis of HO (Table 3a,) had CRP levels within normal range. Four of these first-rehabilitation patients were already treated with anti-inflammatory drugs (indomethacin, diclofenac) and the two re-rehabilitation patients showed radiologically ‘mature’ later stages of HO.
Development of heterotopic ossification in a 17-year-old paraplegic male with history of L1 fracture 4 months earlier. (a) Coronal T2-weighted short inversion time inversion-recovery (STIR) image of the pelvis. The high signal intensity in the right rectus femoris and sartorious muscle corresponds to edema. (b) 3 weeks later the coronal STIR image of the same region shows huge inhomogeneous mass. (c) Coronal T1-weighted fat saturated contrast enhanced image. The mass is of low signal intensity with rim enhancement. Such signal intensity alterations correspond to early-stage heterotopic ossifications
In first-rehabilitation patients there is no significant difference between the peak incidence of DVT and HO (P=0.75) as shown in Figure 1. If we exclude the latest two DVT, the difference is more significant (P=0.29).
Discussion
Earlier studies showed different incidences of DVT in SCI, ranging from 15% up to 100%.1,2,3 This high incidence in SCI is in contrast to that observed in pedestrians without SCI, and is related to an elevated venous resistance and a diminished venous blood flow, both raising the risk of DVT.23 Additionally the fibrinolytic activity of the afflicted extremity is reduced.24 The classical signs and symptoms of an ongoing DVT such as local swelling, redness and elevation of temperature are often absent, or, if present, related to an infectious process.7
DVT is routinely diagnosed by Doppler ultrasonography and, in particular situations, confirmed by phlebography.25 In pedestrians without SCI the simultaneously performed quantitative determination of d-dimers is helpful,4 whereas in acute SCI patients the d-dimers are often elevated because of the injury and the subsequent orthopaedic surgery.
In SCI, DVT may be prevented with anticoagulants, elastic stockings, physiotherapy, early mobilisation and optimal hydration. During the early phase of SCI the most efficient drug for thromboembolic prophylaxis is LMWH.11 At the SPC Nottwil we use Fragmin® (5000 IE once daily subcutaneously) as a standard medication. Fragmin® is administered as early as possible because the thrombotic process starts immediately after SCI. Thus, with consequent antithrombotic prophylaxis we were able to reduce the incidence of DVT in acute SCI to 6.55%. This is an expected percentage in comparison to recent LMWH prophylaxis data of two smaller series with an incidence of 7.5 and 4.5%, respectively.25,26
Of particular interest is the fact that in nearly half (48.1%) of our patients with DVT the thrombotic event occurred in patients on prophylactic therapy and that classical risk factors (deficiency of protein-C, protein-S or AT III) had been excluded, except one patient with a protein-S deficiency. Additional clinical risk factors, ie immobility and local inflammation, were present in two patients, where acute HO preceded ipsilateral DVT by two weeks. We assume that in the remaining 10 patients additional, recently identified risk factors for DVT, like high factor VIII and XI levels or point mutations in the genes coding for factor V and factor II,27,28,29 may trigger DVT, therefore, in this group of patients further clinical investigations are mandatory. These patients may benefit from completion of hypercoagulability screening including recently detected risk factors, and, in case of positive findings, from continued anticoagulation.
Previous studies demonstrated a comorbidity of HO and DVT18,19 which also presented in two of our patients. We agree with these authors that the expanding ectopic mass of HO associated with inflammation will compress vascular structures and irritate endothelial cells. As shown for the hip, this will result in hypercoagulability (elevated d-dimer levels) followed19 or not20 by DVT. In accordance with these findings, acute HO involving the hip appears to increase the risk for development of secondary ipsilateral DVT through local presence of generally accepted risk factors as compression, inflammation and immobilisation. Thus, the pathogenetic events leading to DVT after onset of HO are evident and secondary to the changes associated with acute HO. Seven patients showed, at a first glance, the surprising finding of normal CRP levels when the diagnosis of HO was confirmed by MRT or CT. However, four first-rehabilitation patients were already treated for several days with anti-inflammatory drugs for clinically suspected HO, and the two re-rehabilitation patients showed radiologically inactive, late stages of HO.
Acknowledging that the pathogenesis of HO is still unknown, we identified some clinical characteristics in the formation of HO, underscoring an independent pathogenetic mechanism: (i) HO and DVT have different time points of onset, (ii) DVT is an early complication appearing shortly after the primary event or during immobilisation after orthopaedic surgery, (iii) HO has a later onset when the patient is integrated in the process of the rehabilitation with intense physiotherapy, and (iv) the benefit of LMWH in prevention of DVT was not observed in prevention of HO.
Thus, an exaggerated mechanical activity by the patient himself or by his family (our physiotherapists are aware of this danger and inform the patients about potential risks of forced mechanical activity) might trigger the development of HO, ie the combination of microtrauma and spasticity of the muscles could play an important role.15,17 An additional inflammatory component involved in the pathogenesis of HO16 is supported by our laboratory parameters. It has been suggested that inflammation together with a high local concentration of Ca2+ would favour transformation of undifferentiated cells of the connective tissue into chondroblasts and osteoblasts leading finally to ossification in the muscle.15 In this process, bone morphogenetic protein (BMP) appears to be primordial based on experimental data.21 Indeed elevated levels of osteoblast stimulating factors have been identified in SCI with heterotopic ossification30 and osteoprogenitor cells have been shown to reside within skeletal muscle.31
Our findings support the concept of HO as a complex, primarily inflammatory process possibly triggered by mechanical (microtraumatic) events. To define the pathogenetic mechanisms responsible for HO, molecular studies (ie gene expression studies) of involved tissues appear necessary.
References
Myllynen P et al. Deep venous thrombosis and pulmonary embolism in patients with acute spinal cord injury: a comparison with nonparalyzed patients immobilized due to spinal fractures. J Trauma 1985; 25: 541–543.
Waring WP & Karunas RS . Acute spinal cord injuries and the incidence of clinically occurring thromboembolic disease. Paraplegia 1991; 29: 8–16.
Yelnik A et al. Systematic lower limb phlebography in acute spinal cord injury in 147 patients. Paraplegia 1991; 29: 253–260.
Bounameaux H, Bongard O & Huber O . Epidemologie und Risikofaktoren der venösen Thromboembolie. Therapeutische Umschau 1992; 49: 799–802.
Myllynen P et al. The blood F VIII: Ag/F VIII:C ratio as an early indicator of deep venous thrombosis during post-traumatic immobilization. J Trauma 1987; 27: 287–290.
Rossi EC et al. Sequential changes in factor VIII and platelets preceding deep vein thrombosis in patients with spinal cord injury. Brit J Haematol 1980; 45: 143–151.
Mäder M et al. Guidelines der DMGP (Deutsche Medizinische Gesellschaft für Paraplegie) zur Antikoagulation der frischen Querschnittverletzung. German Medical Society for Paraplegia 1998; 1–24.
Merli GJ, Crabbe S, Paluzzi RG & Fritz D . Etiology, incidence and prevention of deep vein thrombosis in acute spinal cord injury. Arch Phys Med Rehabil 1993; 74: 1199–1205.
Winemiller MH, Stolp-Smith KA, Silverstein MD & Therneau TM . Prevention of venous thromboembolism in patients with spinal cord injury: effects of sequential pneumatic compression and heparin. J Spinal Cord Med 1999; 22: 182–191.
Green D et al. Prevention of thromboembolism in spinal cord injury: role of low molecular weight heparin. Arch Phys Med Rehabil 1994; 75: 290–292.
Green D . Current trends in the use of heparins in thromboprophylaxis. Seminars in Thrombosis and Hemostasis 1999; 25 (Suppl. 1): 29–35.
Banovac K & Gonzalez F . Evaluation and management of heterotopic ossification in patients with spinal cord injury. Spinal Cord 1997; 35: 158–162.
Shehab D, Elgazzar AH & Collier BD . Heterotopic ossification. J Nucl Med 2002; 43: 346–353.
Subbarao JV & Garrison SJ . Heterotopic ossification: diagnosis and management, current concepts and controversies. J Spinal Cord Med 1999; 22: 273–283.
Wang D, Berström E & Garnder B . The pathogenesis of heterotopic ossification in spinal cord injury (a preliminary report), 40th Annual Scientific Meeting Nottwil, Switzerland, 12th–15th September 2001, Abstract book, page 73.
Haselkorn JK, Britell CW & Cardenas DD . Diagnostic imaging of heterotopic ossification with coexistent deep-venous thrombosis in flaccid paraplegia. Arch Phys Med Rehabil 1991; 72: 227–229.
Lotta S, Scelsi L & Scelsi R . Microvascular changes in the lower extremities of paraplegics with heterotopic ossification. Spinal Cord 2001; 39: 595–598.
Colachis SC & Clinchot DM . The association between deep venous thrombosis and heterotopic ossification in patients with acute spinal cord injury. Paraplegia 1993; 31: 507–512.
Perkash A et al. Persistent hypercoagulation associated with heterotopic ossification in patients with spinal cord injury long after injury has occurred. Paraplegia 1993; 31: 653–659.
Yin KS, James J, Lew K & Little JW . Refractory heterotopic ossification with complications. J Spinal Cord Med 2001; 24: 119–122.
Volek-Smith H & Urist MR . Recombinant human bone morphogenetic protein (rhBMP) induces heterotopic bone development in vivo and in vitro. Proc Soc Exp Biol Med 1996; 211: 265–272.
Koshizuka Y et al. Isolation of novel mouse genes associated with ectopic ossification by differential display method using ttw, a mouse model for ectopic ossification. Cytogenet Cell Genet 2001; 94: 163–168.
Miranda AR & Hassouna HI . Mechanisms of thrombosis in spinal cord injury. Hematol Oncol Clin North Am 2000; 14: 401–416.
Boudaoud L et al. Endothelial fibrinolytic reactivity and the risk of deep venous thrombosis after spinal cord injury. Spinal Cord 1997; 35: 151–157.
Powell M, Kirschblum S & O'Conner KC . Duplex ultrasound screening for deep vein thrombosis in spinal cord injured patients at rehabilitation admission. Arch Phys Med Rehabil 1999; 80: 1044–1046.
Lohmann U, Glaser E, Braun BE & Botel U . Prevention of thromboembolism in spinal fractures with spinal cord injuries. Standard heparin versus low-molecular-weight heparin in acute paraplegia. Zentralbl Chir 2001; 126: 385–390.
Koster T et al. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet 1995; 345: 152–155.
Meijers JCM et al. High levels of coagulation factor XI as a risk factor for venous thrombosis. N Engl J Med 2000; 342: 696–701.
Rosendaal FR . Venous thrombosis: a multicausal disease. Lancet 1999; 353: 1167–1173.
Kurer MHJ, Khoker MA & Dandona P . Human osteoblast stimulation by sera from paraplegic patients with heterotopic ossification. Paraplegia 1992; 30: 165–168.
Bosch P et al. Osteoprogenitor cells within skeletal muscle. J Orthopaedic Research 2000; 18: 933–944.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Riklin, C., Baumberger, M., Wick, L. et al. Deep vein thrombosis and heterotopic ossification in spinal cord injury: a 3 year experience at the Swiss Paraplegic Centre Nottwil. Spinal Cord 41, 192–198 (2003). https://doi.org/10.1038/sj.sc.3101421
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101421