Summary:
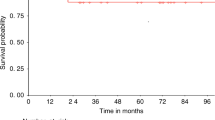
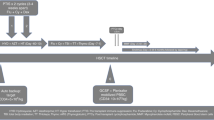
Hurler syndrome (MPS1H) is a progressive inborn error of mucopolysaccharide metabolism leading to premature death. Allogeneic hematopoietic cell transplantation (HCT) can achieve stabilization and improve long-term survival. However, large studies have shown that preparative regimen-related toxicity (RRT) and graft failure rates have been relatively high. We transplanted five Hurler children with a fludarabine-based conditioning regimen, consisting of fludarabine/busulphan/ATG for matched family donor (MFD), with the addition of melphalan for mismatched family donor and matched unrelated donor (MUD) transplantations. Median age at HCT was 27 months (range 10–36). The source of stem cells was bone marrow in one MFD and CD34-selected PBSC in four patients. Median CD34+ cell dose was 25 × 106/kg (range 11.5–54). No RRT >grade II was observed. All patients are surviving at a median of 32 months (range 14–41) and show sustained donor engraftment with 3/5 having full donor chimerism, and 2/5 mixed chimerism (>85%). We conclude that this regimen is feasible and has low toxicity in Hurler children. In combination with high doses of CD34+ selected cells (>10 × 106/kg) and donor lymphocyte infusions, stable engraftment could be achieved in unrelated and mismatched related transplantations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Beck M . Variable clinical presentation in lysosomal storage disorders. J Inherit Metab Dis 2001; 24 (Suppl 2): 47–51.
Peters C, Balthazor M, Shapiro EG et al. Outcome of unrelated donor bone marrow transplantation in 40 children with Hurler syndrome. Blood 1996; 87: 4894–4902.
Peters C, Shapiro EG, Anderson J et al. Hurler syndrome: II. Outcome of HLA-genotypically identical sibling and HLA-haploidentical related donor bone marrow transplantation in fifty-four children. The Storage Disease Collaborative Study Group. Blood 1998; 91: 2601–2608.
Peters C, Shapiro EG, Krivit W . Neuropsychological development in children with Hurler syndrome following hematopoietic stem cell transplantation. Pediatr Transplant 1998; 2: 250–253.
Grewal SS, Krivit W, Defor TE et al. Outcome of second hematopoietic cell transplantation in Hurler syndrome. Bone Marrow Transplant 2002; 29: 491–496.
McDonald GB, Slattery JT, Bouvier ME et al. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood 2003; 101: 2043–2048.
Chun HG, Leyland-Jones B, Cheson BD . Fludarabine phosphate: a synthetic purine antimetabolite with significant activity against lymphoid malignancies. J Clin Oncol 1991; 9: 175–188.
Keating MJ, O'Brien S, Lerner S et al. Long-term follow-up of patients with chronic lymphocytic leukemia (CLL) receiving fludarabine regimens as initial therapy. Blood 1998; 92: 1165–1171.
Nieto Y, Vaughan WP . Pharmacokinetics of high-dose chemotherapy. Bone Marrow Transplant 2004; 33: 259–269.
Schmidt J, Fleissner S, Heimann-Weitschat I et al. Effect of corticosteroids, cyclosporin A, and methotrexate on cytokine release from monocytes and T-cell subsets. Immunopharmacology 1994; 27: 173–179.
Scott HS, Bunge S, Gal A et al. Molecular genetics of mucopolysaccharidosis type I: diagnostic, clinical, and biological implications. Hum Mutat 1995; 6: 288–302.
Yogalingam G, Guo XH, Muller VJ et al. Identification and molecular characterization of alpha-L-iduronidase mutations present in mucopolysaccharidosis type I patients undergoing enzyme replacement therapy. Hum Mutat 2004; 24: 199–207.
Guffon N, Souillet G, Maire I et al. Follow-up of nine patients with Hurler syndrome after bone marrow transplantation. J Pediatr 1998; 133: 119–125.
Vassal G, Fischer A, Challine D et al. Busulfan disposition below the age of three: alteration in children with lysosomal storage disease. Blood 1993; 82: 1030–1034.
Jacobson P, Park JJ, Defor TE et al. Oral busulfan pharmacokinetics and engraftment in children with Hurler syndrome and other inherited metabolic storage diseases undergoing hematopoietic cell transplantation. Bone Marrow Transplant 2001; 27: 855–861.
Krivit W, Aubourg P, Shapiro E, Peters C . Bone marrow transplantation for globoid cell leukodystrophy, adrenoleukodystrophy, metachromatic leukodystrophy, and Hurler syndrome. Curr Opin Hematol 1999; 6: 377–382.
Finke J, Bertz H, Schmoor C et al. Allogeneic bone marrow transplantation from unrelated donors using in vivo anti-T-cell globulin. Br J Haematol 2000; 111: 303–313.
Dalle JH, Wall D, Theoret Y et al. Intravenous busulfan for allogeneic hematopoietic stem cell transplantation in infants: clinical and pharmacokinetic results. Bone Marrow Transplant 2003; 32: 647–651.
Nguyen L, Fuller D, Lennon S et al. I.V. busulfan in pediatrics: a novel dosing to improve safety/efficacy for hematopoietic progenitor cell transplantation recipients. Bone Marrow Transplant 2004; 33: 979–987.
Sarosy G, Leyland-Jones B, Soochan P, Cheson BD . The systemic administration of intravenous melphalan. J Clin Oncol 1988; 6: 1768–1782.
Singhal S, Powles R, Treleaven J et al. Melphalan alone prior to allogeneic bone marrow transplantation from HLA-identical sibling donors for hematologic malignancies: alloengraftment with potential preservation of fertility in women. Bone Marrow Transplant 1996; 18: 1049–1055.
Taha IA, Ahmad RA, Rogers DW et al. Pharmacokinetics of melphalan in children following high-dose intravenous injection. Cancer Chemother Pharmacol 1983; 10: 212–216.
Jacobsohn DA, Duerst R, Tse W, Kletzel M . Reduced intensity haemopoietic stem-cell transplantation for treatment of non-malignant diseases in children. Lancet 2004; 364: 156–162.
Staba SL, Escolar ML, Poe M et al. Cord-blood transplants from unrelated donors in patients with Hurler's syndrome. N Engl J Med 2004; 350: 1960–1969.
Ho VT, Weller E, Lee SJ et al. Prognostic factors for early severe pulmonary complications after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2001; 7: 223–229.
Ritchie DS, Seymour JF, Roberts AW et al. Acute left ventricular failure following melphalan and fludarabine conditioning. Bone Marrow Transplant 2001; 28: 101–103.
Reisner Y, Martelli MF . Tolerance induction by ‘megadose’ transplants of CD34+ stem cells: a new option for leukemia patients without an HLA-matched donor. Curr Opin Immunol 2000; 12: 536–541.
Lang P, Klingebiel T, Bader P et al. Transplantation of highly purified peripheral-blood CD34+ progenitor cells from related and unrelated donors in children with nonmalignant diseases. Bone Marrow Transplant 2004; 33: 25–32.
Kremens B, Basu O, Peceny R et al. Allogeneic CD34+-selected peripheral stem cell transplantation from parental donors in children with non-malignant diseases. Bone Marrow Transplant 2002; 29: 9–13.
Bader P, Klingebiel T, Schaudt A et al. Prevention of relapse in pediatric patients with acute leukemias and MDS after allogeneic SCT by early immunotherapy initiated on the basis of increasing mixed chimerism: a single center experience of 12 children. Leukemia 1999; 13: 2079–2086.
Bader P, Kreyenberg H, Hoelle W et al. Increasing mixed chimerism defines a high-risk group of childhood acute myelogenous leukemia patients after allogeneic stem cell transplantation where pre-emptive immunotherapy may be effective. Bone Marrow Transplant 2004; 33: 815–821.
Woodard P, Cunningham JM, Benaim E et al. Effective donor lymphohematopoietic reconstitution after haploidentical CD34+-selected hematopoietic stem cell transplantation in children with refractory severe aplastic anemia. Bone Marrow Transplant 2004; 33: 411–418.
Acknowledgements
We recognize the outstanding care provided by the nurses of the Mildred-Scheel Bone Marrow Transplantation unit of the Medical School, Hannover.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grigull, L., Beilken, A., Schrappe, M. et al. Transplantation of allogeneic CD34-selected stem cells after fludarabine-based conditioning regimen for children with mucopolysaccharidosis 1H (M. Hurler). Bone Marrow Transplant 35, 265–269 (2005). https://doi.org/10.1038/sj.bmt.1704786
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704786
Keywords
This article is cited by
-
New in Newborn Screening
Current Genetic Medicine Reports (2017)
-
TCRαβ CD19 depletion in allogeneic haematopoietic stem cell transplantation performed for Hurler syndrome
Bone Marrow Transplantation (2016)
-
Allogeneic blood SCT for children with Hurler's syndrome: results from the German multicenter approach MPS-HCT 2005
Bone Marrow Transplantation (2009)
-
Scheie syndrome: Enzyme replacement therapy does not prevent progression of cervical myelopathy due to spinal cord compression
Journal of Inherited Metabolic Disease (2009)
-
Allogeneic hematopoietic cell transplantation (HCT) in Hurler's syndrome using a reduced intensity preparative regimen
Bone Marrow Transplantation (2008)