Abstract
In our previous positron emission tomography studies striatal binding for both [11C]SCH23390 and [11C]N-methylspiperone (NMSP) were decreased in the rat brain on the last day of chronic (14 days) binge cocaine administration. We have found that [11C]SCH23390 binding to dopamine D1 receptors returns to saline control levels within ten days withdrawal from chronic binge cocaine and remains at control levels after 21 days withdrawal. An 18% decrease in [11C]NMSP binding to dopamine D2 receptors was observed after ten days withdrawal. However, importantly, after 21 days withdrawal [11C]NMSP binding was at saline control levels. Changes of in vivo [11C]NMSP binding required a longer abstinence period for normalization than [11C]SCH23390 binding. The apparent recovery of dopamine D2 receptors after prolonged abstinence from chronic cocaine and the different rates of normalization for dopamine D1 versus D2 receptors may be critical information for development of pharmacotherapies for cocaine dependent patients.
Similar content being viewed by others
Main
Cocaine binds to the dopamine transporter blocking dopamine reuptake into presynaptic terminals causing increased dopamine concentrations in the synaptic cleft and extracellular space (Ritz et al. 1987; Kuhar et al. 1991). Microdialysis studies have shown that 22 hours following the last administration of chronic binge cocaine, baseline dopamine levels are significantly decreased, whereas cocaine continues to cause immediate elevations in striatal dopamine levels after each injection (Maisonneuve and Kreek 1994; Maisonneuve et al. 1995). Increased activation of dopamine receptors by the increased concentrations of dopamine during cocaine administration may mediate many of cocaine's reinforcing effects (Ritz et al. 1987; Clark and White 1987).
Several studies of the effects of cocaine on D1 and/or D2 dopamine receptors have led to results that vary with the techniques used. Cocaine has been reported to increase dopamine D1 and D2 receptor binding in vitro (Goeders and Kuhar 1987; Unterwald et al. 1994; Kuhar and Pilotte 1996; Hammer et al. 1997). Cocaine has also been reported to increase both D1 and D2 dopamine receptor mRNA levels in rat brain (Laurier et al. 1994). However, studies using in vivo positron emission tomography (PET) techniques in humans, primates, and rats have consistently reported decreased dopamine D2 receptor binding following chronic cocaine exposure (Volkow et al. 1990, 1993; Nader et al. 1998; Tsukada et al. 1996; Kreuter et al. 1998). Our previous PET studies conducted in the rat brain also have shown decreases in dopamine D1 receptor binding following chronic but not acute binge cocaine administration (Tsukada et al. 1996).
We have found that, whereas D1 receptor binding normalizes within 10 days of abstinence from chronic binge cocaine, decreased D2 receptor binding persists past 10 days of withdrawal (Kreuter et al. 1998). The length of time for recovery of D2 dopamine receptors, or even whether recovery ever occurs may be critical information for development of more effective pharmacotherapies for cocaine dependent persons. Therefore, the present study was designed and executed to determine if longer withdrawal after chronic binge cocaine would allow normalization of in vivo D2 dopamine receptor binding to occur.
METHODS
Animals and Drug Administration
Male Sprague-Dawley rats (250–280 g, Japan SLC, Hamamatsu, Japan) were caged individually and provided free access to food and water. Rats were allowed to acclimate to a 12 hr light/dark cycle (with lights on at 7:30 AM) for one week before initiation of the experiment. The cocaine binge paradigm (Branch et al. 1992; Spangler et al. 1993) started 30 min after lights on and consisted of three intraperitoneal (i.p.) injections of cocaine HCl (15 mg/kg; Shionogi Pharmaceutical, Osaka, Japan) or saline (1 ml/kg) at hourly intervals (i.e., 8, 9, and 10 AM) for 14 days. Rats were assigned to one of six groups: Group 1), binge saline for 14 days; Group 2), binge cocaine for 14 days; Group 3), binge saline for 14 days, followed by 10 days of no injections; Group 4), binge cocaine for 14 days, followed by 10 days of no injections; Group 5), binge saline for 14 days, followed by 21 days of no injections; Group 6), binge cocaine for 14 days, followed by 21 days of no injections. In this study, rats were not weighed or otherwise handled during the withdrawal period.
PET Scan
Chloral hydrate (400 mg/kg, i.p., Sigma, St. Louis, Missouri, USA) was administered 30 min after the second injection of saline or cocaine for animals that did not undergo withdrawal on the day of each PET scan. A cannula was implanted into the tail vein of one saline control and one cocaine-treated animal for administration of radiolabelled tracer and continuous anesthesia (chloral hydrate, 100 mg/kg/hr intravenously (i.v.)). Two sets of two animals, with one saline treated control and one cocaine treated animal in each set, were scanned on each day of PET imaging. In order that more than two animals could be scanned per day, a second set of animals received their binge pattern injection four hours later than on previous days (i.e., 12, 1, and 2 PM) on the day of their scan. Animals in withdrawal received no injections other than chloral hydrate (400 mg/kg, i.p., 1.5 hrs before the start of the scan) on the day of the scan. All PET sessions commenced at 11 AM and 3 PM on each scan day, or one hour after the time of the last injection for animals that did not undergo withdrawal. During each session, two animals, one saline control and one cocaine treated were simultaneously scanned. Each set of animals received two consecutive 64 min PET scans. In each case, the first scan, starting one hour after the last cocaine injection for animals that did not undergo withdrawal, utilized [11C]SCH23390, a dopamine D1 receptor antagonist, to evaluate dopamine D1 receptor binding. The second scan, starting 3.5 hrs after the last injection for animals that did not undergo withdrawal, utilized [11C]N-methylspiperone (NMSP), a dopamine D2 receptor antagonist, to evaluate dopamine D2 receptor binding.
The specific radioactivity of the compounds used ranged from 27 to 88 Gbq/μmol, and the radioactive purities of [11C]SCH23390 and [11C]NMSP were greater than 98%. All PET scans were performed using the SHR-2000 PET camera (Hamamatsu Photonics K.K., Hamamatsu, Japan; Watanabe et al. 1992), which has a transaxial resolution of 3.0 mm full width at half maximum and a center to center distance of 3.25 mm with Z-motion of the rat at every specific time frame. The PET camera used records seven simultaneous slices; 14 slices per animal were obtained by moving the platform holding the rats in the PET gantry. Each PET scan lasted 64 min with 16 time frames at 1 min intervals (total PET scanning time of 16 min), and 16 time frames at 3 min intervals (total time of 48 min). Recording of emissions commenced immediately after administration of each radioligand. The brain regions of interest (ROIs), striatum and cerebellum, were identified using the rat brain atlas, and time activity curves in ROIs were obtained.
Kinetic Analysis of In Vivo Receptor Binding
A kinetic analysis method was applied for quantitation of in vivo dopamine D1 receptor binding (Huang et al. 1986). Because of its low abundance of dopamine receptors, the cerebellum was used as a reference region. Total radioactivity in the cerebellum was used as an input function. Specific binding in the striatum was determined by subtraction of radioactivity in cerebellum from radioactivity in striatum. The rate constants of association (k3) or dissociation (k4) were calculated by a nonlinear least squares fitting procedure. The binding potential of [11C]SCH23390 for the dopamine D1 receptors was expressed as the ratio of the estimated k3 value to the estimated k4 value.
A graphical analysis method was applied for quantitation of in vivo dopamine D2 receptor binding (Huang et al. 1986). Again, the cerebellum was used as a reference region as discussed above. The estimated k3 value, equal to the product of the bimolecular association constant (kon) and the number of receptors (Bmax), indicates the binding capacity of the ligand with the specific receptors.
Statistical Analysis
Two-way analyses of variance (ANOVA), drug condition × withdrawal period, followed by Newman-Keuls post hoc tests were used, when appropriate, to determine significant differences in binding parameters between groups.
RESULTS
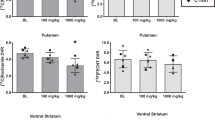
Computer derived PET images of the density of [11C]SCH23390 binding at dopamine D1 receptors are shown in Figure 1 for a representative animal of each withdrawal timepoint and treatment group. As is suggested by the panels in Figure 1, D1 receptor binding was decreased on the last day of chronic binge cocaine, but recovered within 10 days withdrawal and remained at control levels after 21 days withdrawal. Time activity curves from each treatment group (n = 5 or 6/group) showing mean [11C]SCH23390 binding potential ± SEM are shown in Figure 2 . The mean values ± SEM for total striatal radioactivity, derived from the time activity curves of [11C]SCH23390 binding potential, are shown in Figure 3 . A two-way ANOVA of the data shown in Figure 3 showed a main effect of chronic binge cocaine on [11C]SCH23390 binding potential in the striatum [F(2,29) = 6.89; p < .005]. The striatal dopamine D1 receptor binding potential of the cocaine treated animals was reduced 14% compared to saline controls on the last day of chronic binge cocaine (Groups 1 and 2; Newman-Keuls post hoc test, p < .0005). No differences between saline and cocaine treated groups were observed after 10 days of withdrawal from chronic binge cocaine. [11C]SCH23390 binding potential remained at control levels after 21 days of withdrawal from chronic binge cocaine.
Computer derived PET images of the density of [11C]NMSP binding at dopamine D2 receptors are shown in Figure 4 for a representative animal of each withdrawal timepoint and treatment group. As is suggested by the panels in Figure 4, D2 receptor binding was decreased on the last day of chronic binge cocaine, recovered partially but remained decreased after 10 days withdrawal, and most importantly, returned to saline control levels after 21 days withdrawal. Time activity curves from each treatment group (n = 5 or 6/group) of mean [11C]NMSP binding ± SEM are shown in Figure 5 . The mean values ± SEM for total striatal radioactivity, derived from the [11C]NMSP binding time activity curves, are shown in Figure 6 . A two-way ANOVA showed a main effect of chronic binge cocaine on [11C]NMSP binding potential in the striatum [F(2,29) = 29.14; p < .0001]. The striatal dopamine D2 receptor binding of [11C]NMSP in the cocaine treated animals was reduced 37% compared to saline controls on the last day of chronic binge cocaine (Groups 1 and 2; Newman-Keuls post hoc test, p < .0005). After 10 days withdrawal, cocaine treated animals showed increased [11C]NMSP binding as compared to cocaine treated animals with no withdrawal (Groups 2 and 4; Newman-Keuls post hoc test, p < .0005), but [11C]NMSP binding was still significantly reduced by 18% when compared to the saline controls (Groups 3 and 4; Newman-Keuls post hoc tests, p < .0005). However, by 21 days of withdrawal from chronic binge cocaine, [11C]NMSP binding had returned to saline control levels.
DISCUSSION
This study confirms our earlier findings that chronic binge cocaine decreases both dopamine D1 and D2 receptor binding in vivo (Tsukada et al. 1996; Kreuter et al. 1998). Also, the data reproduced our recent finding that dopamine D1 receptors recovered within 10 days, whereas dopamine D2 receptors did not recover to saline control levels after 10 days abstinence from 14 days of binge cocaine (Kreuter et al. 1998). A study in rats using dopamine D2 receptor antagonists showed that lower dopamine D2 receptor availability does not alter cocaine self-administration (Caine and Koob 1994). However, the difference in recovery times for dopamine D1 receptor binding versus dopamine D2 receptor binding may lead to abnormalities in the putative opposing and/or synergistic relationships between dopamine D1 and D2 receptor subtypes (Clark and White 1987; Self et al. 1996; Wang and McGinty 1997).
The difference between our published in vitro data, which showed an increase in rostral caudate putamen and nucleus accumbens dopamine D2 receptor densities after 7 days, but not after 14 days of chronic binge cocaine, and increases in nucleus accumbens dopamine D1 receptor density following the same administration, and our in vivo data is intriguing since the experimental paradigm is essentially identical to that used in these studies, except for the strain of rats used (Unterwald et al. 1994; Tsukada et al. 1996; Kreuter et al. 1998). The apparent contradiction may be due to the differences in technology and/or methodology utilized. In vitro autoradiography studies, which are performed on tissue sections, measure not only receptors on the cell membrane but also receptors that are present in the cytosol. In contrast, in vivo PET technology, when used to study cell membrane receptors, measures only those receptors which are, at the time of the study, on cell membranes. Perhaps the absolute numbers of dopamine receptors increase within the cell after prolonged cocaine use, but the numbers of receptors actually located and functioning on the in vivo cell membrane is reduced.
Dopamine readily displaces SCH23390 at dopamine D1 receptors. However, in our earlier PET studies we found that the first day of binge cocaine administration did not alter SCH23390 binding (Tsukada et al. 1996). The first day is an acute timepoint at which microdialysis studies from our group and others have shown that single dose or binge cocaine increases extracellular fluid dopamine levels in the striatum to levels that are all significantly higher than dopamine levels in saline treated controls (Maisonneuve and Kreek 1994; Maisonneuve et al. 1995; Kuhar and Pilotte 1996; Hammer et al. 1997). However, similar microdialysis studies following 14 days of chronic binge cocaine administration showed significantly lower cocaine-induced elevations in extracellular fluid dopamine levels as compared to the levels achieved during the first day of binge cocaine administration (Maisonneuve et al. 1995). Therefore, if extracellular fluid dopamine's ability to displace SCH23390 were playing a predominant role in the observed SCH23390 binding potential as observed in this study, one would expect greater SCH23390 binding to dopamine receptors after 14 days binge cocaine as compared to the binding following one day binge cocaine. In our two earlier studies and in this study, this was not the case; lower binding levels of SCH23390 were found after 14 days chronic binge cocaine as compared with one day of binge cocaine (Tsukada et al. 1996; Kreuter et al. 1998). Of special note, D1 receptor binding remained significantly reduced after one day withdrawal from chronic binge cocaine at a time when there was no cocaine present (Maisonneuve et al. 1995). Rosetti et al. (1992) also found a reduction in extracellular fluid dopamine levels during the withdrawal period from chronic cocaine. In fact, our previous studies have also shown that basal extracellular fluid dopamine levels in animals which have received binge cocaine for 14 days are lower than saline treated control animals (Maisonneuve et al 1995). Thus, after one day withdrawal from chronic cocaine, one might have anticipated seeing increased SCH23390 binding rather than the significant reduction observed. In both our other recent withdrawal study and this study, SCH23390 binding returned to normal within 10 days following cocaine withdrawal (Kreuter et al. 1998).
NMSP is not displaced by dopamine (Young et al. 1991) and dissociates from dopamine receptors slowly. As a result, we performed all SCH23390 PET scans before PET scans using NMSP. Although concerns can be raised about the possible confounding effects caused by the general anesthesia, essential for conducting PET studies in the rat, the same anesthesia was used in both saline and cocaine treated animals. Furthermore, the data show profound differences between cocaine and saline control animals immediately after and over the course of withdrawal from cocaine administration. Therefore, changes due to anesthesia are probably not hindering our ability to measure the effect of binge cocaine administration on dopamine receptor binding.
Decreased in vivo dopamine D2 receptor binding in primates and humans has been reported to persist after much longer periods of abstinence, following periods of cocaine exposure that exceeded the duration of the present study. A recent PET study, in which adult rhesus monkeys that self-administered approximately 17 g of cocaine over a period of two to three years, reported that dopamine D2 receptor binding did not recover even after 227 days of abstinence (Nader et al. 1998). Another study, working with human cocaine addicted patients, showed decreased dopamine D2 receptor binding immediately following cocaine exposure. This decrease persisted even after 3 or 4 months of abstinence from cocaine (Volkow et al. 1993). These results indicate that dopamine D2 receptors may not recover, or will recover only very slowly after prolonged cocaine administration.
The profound differences between species with respect to physiology, energy metabolism, and lifespan may be critical for the interpretation of rodent data, especially with respect to the amount of withdrawal time needed for normalization of neurobiological systems, such as the dopamine receptor systems. It is possible that the three week abstinence period needed for the rat dopamine D2 receptors to return to control levels may translate into several years in the primate model or the human cocaine dependent patient. Nevertheless, it is also possible that if chronic binge cocaine were administered to rats for a period longer than two weeks the time required for full recovery would also be longer, or perhaps no recovery would occur. The data in human cocaine dependent patients, rhesus monkeys, and rats suggest that there may be a “point of no return” following prolonged cocaine administration, after which profound changes in dopamine D2 receptor binding may become persistent or even permanent (Volkow et al. 1993; Nader et al. 1998; Kreuter et al. 1998). The duration and/or intensity of cocaine abuse needed to reach such persistent changes is still unknown across species, as is the rate at which these persistent changes develop during the drug exposure period. Furthermore, these data indicate a putative differential plasticity between the D1 and D2 receptors. If these findings of slow recovery of D1, and even slower recovery of D2 receptor density following chronic cocaine abuse are confirmed in humans, new pharmacotherapeutic agents for cocaine dependent patients may benefit from properties that partly mimic or reactivate dopamine D1 and D2 receptor systems and take into account their differences in plasticity.
References
Branch AD, Unterwald EM, Lee SE, Kreek MJ . (1992): Quantitation of preproenkephalin mRNA levels in brain regions from male Fischer rats following chronic cocaine treatment using a recently developed solution hybridization assay. Mol Brain Res 14: 231–238
Caine SB, Koob GF . (1994): Effects of dopamine D-1 and D-2 antagonists on cocaine self-administration under different schedules of reinforcement in the rat. J Pharmacol Exp Ther 270: 209–218
Clark D, White FJ . (1987): D1 dopamine receptor—the search for a function: A critical evaluation of the D1/D2 dopamine receptor classification and its functional implications. Synapse 1: 347–388
Goeders NE, Kuhar MJ . (1987): Chronic cocaine administration induces opposite changes in dopamine receptors in the striatum and nucleus accumbens. Alcohol Drug Res 7: 207–216
Hammer RP, Egilmez Y, Emmet-Oglesby MW . (1997): Neural mechanisms of tolerance to the effects of cocaine. Behav Brain Res 84: 225–239
Huang SH, Barrio J, Phelps M . (1986): Neuroreceptor assay with positron emission tomography: Equilibrium versus dynamic approach. J Cereb Blood Flow Metab 6: 515–521
Kreuter J, Tsukada H, Schlussman SD, Kakiuchi T, Nishiyama S, Maggos CE, Unterwald EM, Kreek MJ . (1998): Effects of withdrawal from binge pattern cocaine administration on dopamine D1 and D2 receptors in the rat brain as measured by PET. Problems of Drug Dependence 1994. Proceedings of the 59th Annual Scientific Meeting, The College on Problems of Drug Dependence, NIDA Research Monograph (in press).
Kuhar MJ, Pilotte NS . (1996): Neurochemical changes in cocaine withdrawal. Trends Pharmacol Sci 17: 260–264
Kuhar MJ, Ritz MC, Boja JW . (1991): The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci 14: 299–302
Laurier LG, Corrigall WA, George SR . (1994): Dopamine receptor density, sensitivity and mRNA levels are altered following self-administration of cocaine in the rat. Brain Res 634: 31–40
Maisonneuve IM, Ho A, Kreek MJ . (1995): Chronic administration of a cocaine “binge” alters basal extracellular dopamine levels in male rats: An in vivo microdialysis study. J Pharmacol Exp Ther 272: 652–657
Maisonneuve IM, Kreek MJ . (1994): Acute tolerance to the dopamine response induced by a binge pattern of cocaine administration in male rats: An in vivo microdialysis study. J Pharmacol Exp Ther 268: 916–921
Nader M, Mach R, Ehrenkaufer R, Gage H, Morton T . (1998): Longterm downregulation of dopamine D2 receptors following cocaine self-administration in monkeys. Problems of Drug Dependence 1997. Proceedings of the 59th Annual Scientific Meeting, The College on Problems of Drug Dependence, NIDA Research Monograph (in press).
Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ . (1987): Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 237: 1219–1223
Rosetti ZL, Hmaidan Y, Gessa GL . (1992): Marked inhibition of mesolimbic dopamine release: A common feature of ethanol, morphine, cocaine, and amphetamine abstinence in rats. Eur J Pharmacol 221: 227–234
Self DW, Barnhart WJ, Lehman DA, Nestler EJ . (1996): Opposite modulation of cocaine-seeking behavior by d-1- and d-2-like dopamine receptor agonists. Science 271: 1586–1589
Spangler R, Unterwald EM, Kreek MJ . (1993): ‘Binge’ cocaine administration induces a sustained increase of prodynorphin mRNA in rat caudate-putamen. Mol Brain Res 19: 323–327
Tsukada H, Kreuter J, Maggos CE, Unterwald EM, Kakiuchi T, Nishiyama S, Futatsubashi M, Kreek MJ . (1996): Effects of binge pattern cocaine administration on dopamine D1 and D2 receptors in the rat brain: An in vivo study using positron emission tomography. J Neurosci 16: 7670–7677
Unterwald EM, Ho A, Rubenfeld JM, Kreek MJ . (1994): Time course of the development of behavioral sensitization and dopamine receptor up-regulation during binge cocaine administration. J Pharmacol Exp Ther 270: 1387–1396
Volkow ND, Fowler JS, Wolf AP, Schlyer D, Shiue CY, Alpert R, Dewey SL, Logan J, Bendriem B, Christman D, Hitzemann R, Henn F . (1990): Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am J Psychiatr 147: 719–724
Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP . (1993): Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse 14: 169–177
Wang JQ, McGinty JF . (1997): The full D1 dopamine receptor agonist SKF-82958 induces neuropeptide mRNA in the normosensitive striatum of rats: Regulation of D1/D2 interactions by muscarinic receptors. J Pharmacol Exp Ther 281: 972–982
Watanabe M, Uchida H, Okada H, Shimizu K, Sato N, Yoshikawa E, Ohmura T, Tanaka E . (1992): A high resolution PET for animal studies. IEEE Trans Nucl Sci 11: 577–580
Young LT, Wong DF, Goldman S, Minkin E, Chen C, Matsumura K, Scheffel U, Wagner HN Jr . (1991): Effects of endogenous dopamine on kinetics of [3H]N-methylspiperone and [3H]reclopride binding in the rat brain. Synapse 9: 188–194
Acknowledgements
Supported in part by NIH DA P50–05130 & NIH-DA-K05–00049.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Maggos, C., Tsukada, H., Kakiuchi, T. et al. Sustained Withdrawal Allows Normalization of In Vivo [11C]N-Methylspiperone Dopamine D2 Receptor Binding after Chronic Binge Cocaine: A Positron Emission Tomography Study in Rats. Neuropsychopharmacol 19, 146–153 (1998). https://doi.org/10.1016/S0893-133X(98)00009-8
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/S0893-133X(98)00009-8
Keywords
This article is cited by
-
Amphetamine Self-Administration Attenuates Dopamine D2 Autoreceptor Function
Neuropsychopharmacology (2014)
-
Influence of cocaine administration patterns on dopamine receptor regulation
Psychopharmacology (2014)
-
Behavioral sensitization to cocaine in rats: evidence for temporal differences in dopamine D3 and D2 receptor sensitivity
Psychopharmacology (2011)
-
The Role of Acetylcholine in Cocaine Addiction
Neuropsychopharmacology (2008)
-
Addiction-Related Alterations in D1 and D2 Dopamine Receptor Behavioral Responses Following Chronic Cocaine Self-Administration
Neuropsychopharmacology (2007)