Abstract
There is conflicting evidence on the antinociceptive effects of corticotropin-releasing factor (CRF) along the neuraxis of pain transmission and the responsible anatomical sites of CRF's action at the level of the brain, spinal cord and periphery. In an animal model of tonic pain, that is, Freunds complete adjuvant (FCA) hindpaw inflammation, we systematically investigated CRF's ability to modulate inflammatory pain at those three levels of pain transmission by algesiometry following the intracerebroventricular, intrathecal, and intraplantar application of low, systemically inactive doses of CRF. At each level, CRF elicits potent antinociceptive effects, which are dose dependent and antagonized by local, but not systemic CRF receptor antagonist α-helical CRF indicating CRF receptor specificity. Consistently, we have identified by immunohistochemistry multiple brain areas, inhibitory interneurons within the dorsal horn of the spinal cord as well as immune cells within subcutaneous tissue—but not peripheral sensory neurons—that coexpress both CRF receptors and opioid peptides. In line with these anatomical findings, local administration of CRF together with the opioid receptor antagonist naloxone dose-dependently reversed CRF's antinociceptive effects at each of these three levels of pain transmission. Therefore, local application of low, systemically inactive doses of CRF at the level of the brain, spinal cord and periphery inhibits tonic inflammatory pain most likely through an activation of CRF receptors on cells that coexpress opioid peptides which results in opioid-mediated pain inhibition. Future studies have to delineate whether endogenous CRF at these three levels contributes to the body's response to cope with the stressful stimulus pain in an opioid-mediated manner.
Similar content being viewed by others
INTRODUCTION
Corticotropin-releasing factor is a 41-amino-acid peptide, which plays a major role at the level of the hypothalamus and pituitary to control the body's response mechanisms to acute and chronic stressful stimuli (Bale and Vale, 2004; Arborelius et al, 1999). Stress evokes the release of CRF into multiple areas throughout the brain (Chappell et al, 1986) and the administration of exogenous CRF mimics many of the effects of stress (Dunn and Berridge, 1990). The distribution of CRF not only along the hypothalamo–pituitary axis but also in pain-relevant anatomical sites, for example, the thalamus, suggested very early its role as a potent neuromodulator of nociception (Merchenthaler et al, 1984). As the first reports of an analgesic effect of intravenous CRF both in rats and humans (Wei et al, 1986; Hargreaves et al, 1987), there is a continuously increasing interest in the antinociceptive properties of CRF, perhaps because it may represent a new class of analgesics that has been overlooked (Lariviere and Melzack, 2000). However, several investigations that followed have led to conflicting results in the sense that some of them reported significant alterations in pain behavior, whereas others not (Lariviere and Melzack, 2000). Only two studies suggested antinociceptive effects following intrathecal (i.t.) CRF administration in visceral pain (Song and Takemori, 1991; Nijsen et al, 2005). Several other studies showed no robust results about CRF's modulation of nociception following its central or sytemic application (Lariviere and Melzack, 2000).
On the other side, the pain modulatory effect of peripheral CRF has been well established and is mostly dependent on the release of opioid peptides from immune cells during inflammatory processes (for review, see Schäfer et al, 1997). Under inflammatory and other pathological conditions, various types of immune cells have been shown to produce and contain opioid peptides like β-endorphin (END) and met-enkephalin (ENK) (Stein et al, 2003; Sibinga and Goldstein, 1988; Sharp and Linner, 1993). CRF induces the secretion of END and ENK from stimulated immune cells in vitro (Cabot et al, 2002; Kavelaars et al, 1990; Schäfer et al, 1994) and from resident immune cells in vivo resulting in potent peripheral antinociception (Cabot et al, 1997, 2002; Kavelaars et al, 1990; Schäfer et al, 1994). This release is mediated by CRF receptors on these immune cells, is calcium dependent, and mimicked by elevated extracellular concentrations of potassium (Cabot et al, 1997). In previous studies, we have shown that specific binding for radiolabeled CRF located on various immune cells (monocytes/macrophages and lymphocytes) within inflamed subcutaneous tissue was upregulated (Mousa et al, 1996). Recently, we have demonstrated that CRF activates both CRF receptor subtypes CRF-R1 and 2 located on these immune cells within inflamed subcutaneous paw tissue to release END resulting in antinociception (Mousa et al, 2003). Unfortunately, these results were misunderstood in the sense that CRF's antinociceptive properties were regarded as being exclusively in the periphery (Lariviere and Melzack, 2000).
In light of the recent increasing interest in the potential modulating effects of CRF in clinically relevant pain conditions such as visceral pain (Nozu and Kudaira, 2006; Sagami et al, 2004), postoperative joint pain (Likar et al, 2007), and fibromyalgia (McLean et al, 2006; Lund et al, 2006), we set out to investigate systematically the antinociceptive effects of CRF at the three main levels of the neuraxis of pain transmission, that is, at the level of the brain, spinal cord, and periphery. As it was suggested that antinociceptive effects of CRF are identified rather in tonic than in phasic pain (Lariviere and Melzack, 2000), we have chosen an animal model of tonic pain, that is, Freunds complete adjuvant (FCA) hindpaw inflammation. The aims of our current study were to investigate systematically CRF's ability to modulate inflammatory pain at the three levels of pain transmission, the brain, spinal cord, and periphery, by algesiometric testing following the intracerebroventricular (i.c.v.), i.t., and intraplantar (i.pl.) application of low, systemically inactive doses of CRF. We further examined whether CRF-mediated alterations in inflammatory pain were specifically reversed by a CRF receptor antagonist at the anatomical site of CRF's application. As some previous studies suggested a possible involvement of endogenous opioid peptides (Hargreaves et al, 1987; Song and Takemori, 1990), we also investigated whether CRF's antinociception is attenuated by the local application of the opioid receptor antagonist naloxone. In parallel to our behavioral experiments, we aimed at identifying potential, pain-relevant anatomical sites of CRF's action, which coexpress both CRF receptors and opioid peptides within the brain, the dorsal horn of the spinal cord and within peripheral tissue using immunohistochemistry.
MATERIALS AND METHODS
Animals
Experiments were conducted in male Wistar rats (170–200 g) (Charité—Universitätsmedizin Berlin, Campus Benjamin Franklin, Berlin, Germany). Rats were housed individually in cages and maintained on a 12 h light/dark schedule with food pellets and water ad libitum. Room temperature was maintained at 22±0.5°C and at a relative humidity between 60 and 65%. All experiments were performed during the light phase. Experiments and animal care followed the guidelines of the International Association for the Study of Pain (Zimmermann, 1983), and were approved by the local animal care committee of the Senate of Berlin, Germany (Landesamt für Arbeitsschutz, Gesundheitsschutz und Technische Sicherheit, Berlin). All efforts were made to minimize the number of animals used and their suffering.
Induction of Inflammation
Rats sedated by brief isoflurane (Willy Rüsch GmbH, Böblingen, Germany) anesthesia received an i.pl. injection of 0.15 ml FCA into the right hind paw. This treatment consistently produces a localized inflammation of the inoculated paw confirmed by an increase in paw volume, paw temperature and infiltration with various types of immune cells.
Surgery to Implant i.c.v. or i.t. Guide Cannula
The i.c.v. cannula was placed as described elsewhere (Nagaraja et al, 2004). Rats were handled and trained in the test situation for 3 days before cannulation. Anesthesia was induced and maintained with isoflurane via a loose-fitting plastic mask. Rats were positioned in a stereotaxic apparatus and the skull was exposed. A burr hole was drilled above the location of the right lateral ventricle (coordinates: AP 0.25 mm, lateral 1.6 mm, ventral 4.0 mm related to bregma) (Paxinos and Watson, 1997). A stainless-steel cannula guide pedestal was fixed to the skull over the burr hole for subsequent i.c.v. infusion of either saline or rat/human CRF using two stainless-steel screws. Then, the entire assembly was held in place with dental cement. The cannula guide extended into the burr hole 1 mm below the pedestal but did not touch the surface of the cortex. After surgery, a stainless-steel blocker was inserted into the i.c.v. cannula. After the experiments cannula placements were assessed in all animals by infusion of (1%) methylene blue dye and verification of dye in the ventricular system. Position and patency were confirmed by the presence of blue dye in the lateral ventricle on postmortem craniotomy. At least 5 days were allowed for recovery from surgery before behavioral testing was started.
The i.t. catheterization was performed as described elsewhere with slight modifications (Schmitt et al, 2003; Størkson et al, 1996). Briefly, an incision was made at the L3–L4 level. The needle through which the catheter was set up was inserted at the L5–L6 vertebra. Keeping the angle of the needle parallel with the dorsal surface, the catheter was carefully pushed upward to reach L4 at the lumbar enlargement. The needle was then carefully removed and the catheter was sealed with glue to the tissue to secure it. Then, saline was injected intrathecally in a volume of 10 μl to flush the catheter. Another skin incision was made at the neck of the animal and the catheter was tunnelled under the skin and pulled out at the neck after which incisions were sutured. Animals showing signs of neurological damage were immediately excluded from the study. The i.t. location of the catheter was confirmed by administration of 10 μl of lidocaine 2% flushed with 10 μl of saline. Lidocaine but not saline caused reversible bilateral hindlimb paresis. The animals were allowed 5 days to recover. Drugs were injected intrathecally in a volume of 10 μl followed by 10 μl of vehicle to flush the catheter. All rats were investigated for correct catheter position in relation to the spinal cord on postmortem laminectomy.
Drugs
The following drugs were used: rat/human CRF (Sigma–Aldrich, St Louis, MO); CRF antagonist α-helical CRF (α-CRF (9–41) antagonist, see Rivier et al, 1986) (Sigma); naloxone hydrochloride (Sigma). Doses were calculated as the free base, and drugs were dissolved in the following vehicles: sterile isotonic saline (naloxone), sterile water (CRF, α-helical CRF). Routes and volumes of drug administration were always i.c.v. (10 μl), i.t. (10 μl), and i.pl. (100 μl). For each dose a separate group of animals (n=6) was used. Drugs were administered during brief isoflurane anesthesia.
Algesiometric Testing
Nociceptive thresholds were assessed by paw pressure test (modified Randall–Selitto test). Animals (n=6 per group) were gently restrained under paper wadding and incremental pressure was applied via a wedge-shaped, blunt piston onto the dorsal surface of the hind paw by means of an automated gauge (Ugo Basile). The pressure required to elicit paw withdrawal, the paw-pressure threshold (PPT), was determined. A cutoff of 250 g was used. Three consecutive trials, separated by intervals of 10 s, were conducted and the average was determined. Baseline PPT was tested before and 4 days after inoculation with FCA. The same procedure was performed on the contralateral side; the sequence of sides was alternated between subjects to preclude order effects.
Dose–Response Relationships
The antinociceptive effects of i.c.v., i.t. or i.pl. CRF treatments were examined as a function of dose. After baseline measurements separate groups of animals for each dose and injection technique (n=6 per group) received i.c.v., i.t. or i.pl. administrations of different doses of CRF (i.c.v.: 25, 50, 100, 500 ng; i.t.: 2, 4, 10, 20 ng; or i.pl.: 0.5, 1, 1.5, 2 ng). Control animals (n=6 per group) received vehicle treatment. Five minutes later PPT were reassessed in ipsi- and contralateral hindpaws. To exclude possible systemic effects, the highest effective dose of all CRF doses tested was also given subcutaneously (s.c.) at the neck of a separate group of animals. The experimenter performing the PPT assessments following i.c.v., i.t., and i.pl. injections was blinded to the drugs and doses applied.
Receptor Specificity
The highest effective dose of i.c.v., i.t. or i.pl. CRF was administered together with different doses of α-helical CRF (i.c.v.: 0, 30, 60 ng; i.t.: 0, 1, 3, 5 ng; or i.pl.: 0, 0.5, 1.5, 2 ng) in separate groups of animals to determine the receptor specificity of CRF-mediated antinociceptive effects.
To examine whether CRF-elicited antinociception is opioid mediated, the highest effective dose of i.c.v., i.t. or i.pl. CRF agonist was administered in separate groups of animals together with the opioid receptor antagonist naloxone (i.c.v.: 0, 1, 5, 10 μg; i.t.: 0, 0.5, 1, 2 μg; or i.pl.: 0, 0.4, 1, 10 μg).
CRF and α-helical CRF receptor antagonist or naloxone were coadministered i.c.v., i.t. or i.pl. in a total volume of 10 μl (i.c.v.), 10 μl (i.t.) or 100 μl (i.pl.) in separate groups of animals.
Tissue Preparation
Four days after FCA inoculation, rats were deeply anesthetized with halothane and transcardially perfused with 100 ml warm saline, followed by 300 ml 4% (w/v) paraformaldehyde in 0.16 M phosphate buffer solution (pH 7.4). After perfusion, brain, spinal cord (L4-5) and the ipsilateral L4-5 dorsal root ganglia (DRG), the sciatic nerve (0.5 cm), and subcutaneous paw tissue were removed, postfixed in the same fixatives for 90 min, and then cryoprotected overnight at 4°C in phosphate-buffered saline (PBS) containing 10% sucrose. The tissues were then embedded in tissue-Tek compound (OCT, Miles Inc., Elkhart, IN) and frozen. Brain was serially cut at 40 μm on cryostat and every third section of brain was collected in PBS (floating sections). However, spinal cord, DRG, sciatic nerve or subcutaneous tissue sections (10 μm thick) were mounted onto gelatin-coated slides.
Immunofluorescence Staining
For single or double immunofluorescence, tissue sections were processed as described previously (Mousa et al, 2002). Briefly, coronal brain floating tissue sections were incubated with the following antibodies: rabbit polyclonal antibody against the C terminus of CRF receptor (H-215) reactive with both CRF receptor subtypes 1 and 2 (1:100; Santa Cruz Biotechnology Inc., CA), mouse monoclonal antibody against ENK (1:1000, Chemicon International, MA) or mouse monoclonal anti-panopioid 3E7 against the amino terminal H-Tyr-Gly-Gly-Phe sequence of END, which also has a high cross-reactivity with homologs of identical sequence such as ENK (10 μg/ml; Gramsch Laboratories, Schwabhausen, Germany). Coronal or parasagittal spinal cord sections were incubated with the following antibodies: rabbit polyclonal antibody against CRF receptor (1:100) alone or in combination with guinea pig polyclonal antibody against CGRP (1:1000, Peninsula Laboratories, Belmont, CA), mouse monoclonal antibody against ENK (1:1000), mouse monoclonal antipan opioid 3E7 (10 μg/ml) or guinea pig polyclonal antibody against MOR (1:1000, Chemicon International, MA) as well as with guinea pig polyclonal antibody against CGRP in combination with rabbit anti-rat MOR or DOR (dilution of 1:1000). For DRG, sciatic nerve or subcutaneous paw tissue mounted tissue sections were incubated with the following antibodies: rabbit polyclonal antibody against CRF receptor in combination with guinea pig polyclonal antibody against CGRP. For subcutaneous paw tissue sections were incubated with antibody against CRF receptor in combination with mouse monoclonal antibody against ENK or panopioid 3E7. After incubation with primary antibodies, the tissue sections were washed with PBS and then incubated with the appropriate secondary antibodies; texas red conjugated goat anti-rabbit antibody alone or in combination with FITC-conjugated donkey anti-mouse or anti-guinea pig antibody. Thereafter, sections were washed with PBS, mounted in vectashield (Vector Laboratories) and viewed under a Zeiss 510 confocal laser scanning microscope.
Specificity Controls
To demonstrate specificity of staining, the following controls were included as mentioned in detail elsewhere (Mousa et al, 2003, 2004; Brack et al, 2004): (1) preabsorption of diluted antibody against ENK, 3E7, CRF receptor, MOR or DOR with a synthetic peptide for ENK, END (Peninsula Laboratories), CRF receptor (Santa Cruz Biotechnology), MOR or DOR (Gramsch Laboratories), respectively. (2) Omission of either the primary antisera or the secondary antibodies.
Quantification of Immunolabeling
The method of quantification for CRF or ENK immunoreactivity at the dorsal horn of the spinal cord has been described previously (Mousa et al, 2002; Frank and Tilby, 2003). Briefly, images of red fluorescence for CRF and green fluorescence for ENK were obtained from five sections for each animal (n=5) using a Zeiss laser scanning confocal microscope. Then, advanced image analysis softwares (LSM510 Software 3.2) was applied to quantify changes in fluorescent intensity as described in detail elsewhere (Mousa et al, 2002, 2007; Frank and Tilby, 2003). A standardized box was positioned over the dorsal horn of the spinal cord of all groups and the product of the area and density of pixels within the threshold value were calculated. Data represent the following differences: ipsilateral–contralateral.
Analysis of Data
Data were analyzed using one-way analysis of variance (ANOVA) followed by Student–Newman–Keuls or Dunnett's post hoc test. For data not normally distributed, Kruskal–Wallis one-way ANOVA on ranks was performed, followed by Dunnett's or Tukey post hoc test. Dose–response curves were analyzed by one-way ANOVA followed by linear regression. Differences were considered significant if P<0.05. All tests were performed using Sigma Stat 2.03 (SPSS Science, Chicago, IL) software. Data are expressed as means±SEM.
RESULTS
Complete Freund's Adjuvant-Induced Hindpaw Inflammation
Four days after i.pl. injection of FCA rats developed a hindpaw inflammation which was confined to the inoculated paw and characterized by ipsilateral hyperalgesia (decreased thresholds to noxious pressure: 57±4.7 vs 69±5.4 vs 72±4.5 g; inflamed paws vs noninflamed paws vs paws of naïve animals; P<0.05; Paired t-test), swelling (increased paw volume: 2.5±0.07 vs 1.2±0.03 ml; inflamed vs noninflamed paws; P<0.05; Paired t-test), and hyperthermia (elevated paw temperature: 32±0.3 vs 28±0.4°C; inflamed vs noninflamed paws) (P<0.001; Paired t-test).
Opioid-Mediated Antinociceptive Effects Following i.c.v. CRF
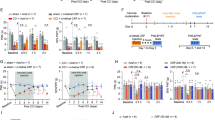
I.c.v. injection of CRF (25, 50, 100, 500 ng) dose-dependently produced significant elevations of PPT in inflamed and in contralateral noninflamed hind paws of FCA-treated rats (P<0.05, ANOVA and Dunnett's test) (Figure 1a). These PPT elevations increased beyond baseline PPT in naive rats (69±5.4 g) (see above) and were significantly different from baseline values of FCA-treated rats for 50, 100, and 500 ng CRF, whereas in noninflamed paws only for 100 ng CRF (Figure 1a). The highest effective dose of CRF given s.c. did not alter PPT (data not shown).
(a) In Wistar rats with 4 days FCA hindpaw inflammation, effects of i.c.v. injections of low doses of CRF on ipsi- (closed bars) and contralateral (open bars) nociceptive PPT were measured by algesiometry. (a) I.c.v. injections of CRF significantly increased PPT in a dose-dependent manner ipsi- and contralateral to the FCA-inflamed paw (P<0.05, * indicates significant differences from 0=saline treated group). (b) Dose-dependent antagonism of i.c.v. CRF's (100 ng) antinociception by coadministered α-helical CRF receptor antagonist was significant ipsi- and contralateral to the FCA-inflamed paw (P<0.05, ! indicates significant differences from baseline (BL), * indicates significant differences from 0=vehicle-treated group). (c) Dose-dependent attenuation of i.c.v. CRF's (100 ng) antinociception by coadministered opioid receptor antagonist naloxone was significant ipsi- and contralateral to the FCA-inflamed paw (P<0.05, ! indicates significant differences from baseline (BL), * indicates significant differences from 0=vehicle-treated group).
I.c.v. coadministration of different doses of α-helical CRF antagonist with 100 ng CRF dose-dependently and significantly reversed CRF-induced increases in PPT (ANOVA and Dunnett's test) (Figure 1b). In addition, i.c.v. coadministration of different doses of the opioid receptor antagonist naloxone with 100 ng CRF dose-dependently and significantly attenuated CRF-induced PPT elevations (ANOVA and Dunnett's test) (Figure 1c). The highest effective dose of either α-helical CRF antagonist or naloxone given s.c. did not alter i.c.v. CRF-elicited PPT elevations (data not shown).
Brain Areas of CRF Receptor and Opioid Peptide Coexpression
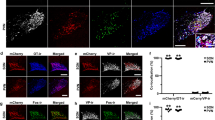
The double-immunofluorescence confocal microscopy of brain sections of FCA-treated animals revealed that CRF receptors are expressed in opioid peptide positive (3E7+) neurons within multiple pain relevant brain areas such as the rostral agranular insular cortex (RAIC), hippocampal formation, thalamus, hypothalamus, periaquaductal gray, and locus coeruleus (LC) (Figures 2 and 3). This immunoreactivity was always bilateral and, apparently, not different between both sides.
Double-immunofluorescence staining of CRF receptor (a, d, g, h) and opioid peptides (3E7+) in the RAIC (a–c), hippocampal CA1 pyramidal cells (d-f), the dentate gyrus granule cell layer of the hippocampus (g–i) and thalamus (j–l). CRF receptor (red; a, d, g, j) colocalized with opioid peptides (green; b, e, h, k). Note that most of CRF receptor–ir neurons (red) express opioid peptides (green) (double arrow) with few neurons containing only CRF receptor (red; arrow) or opioid peptides (green; arrowhead). Bar=50 μm.
Double-immunofluorescence staining of CRF receptor (a, AA, d-f, g, j) and opioid peptides (3E7+) (b, BB, e, f, h, k) or ENK (d) in the hypothalamus regions (a–f), periaquaductal grey area (g–i) and LC (j–l). CRF receptor (red; a, AA, d–f, g, j) colocalize with opioid peptides (green; b, BB, d, e, f, h, k). Note that most of CRF receptor–ir neurons (red) express opioid peptides (green) (double arrow) with few neurons containing only CRF receptor (red; arrow) in the median eminence (ME). (d) Arcuate nucleus contains an abundant population of ENK-ir neurons in close contact with dense nerve fibers positive with CRF receptor-ir fibers with few fibers showing overlap. In the paraventricular (PVN) (e) and supraoptic nuclei (SON) (f) within the hypothalamus there is an abundant population of CRF receptor-ir neurons (red), which are in close contact with dense nerve fibers positive for opioid peptides (green). In the periaquaductal gray area (g–i) and LC, the majority of CRF receptor-ir neurons express opioid peptides (double arrow) with few neurons containing only CRF receptor (arrow) or opioid peptides (arrowhead). Bar=50 μm (for a–i) and 25 (AA, BB, CC) μm.
In the RAIC, nearly all CRF receptor-ir neurons expressed opioid peptides, but not vice versa. The neurons coexpressing CRF receptor and opioid peptides had the appearance of pyramidal neurons with a typical apical dendrite (Figure 2a and b).
In the CA1–CA3 regions of the hippocampus, CRF receptor-ir neurons within the pyramidal cell layer coexpressed opioid peptides (Figure 2d–f). Also, all CRF receptor-ir neurons within the dentate gyrus granule cell layer of the hippocampus were immunoreactive for opioid peptides (Figure 2g–i). Opioid peptides and CRF receptors colocalized within the thalamus. Some neurons within the thalamus were immunoreactive for opioid peptides alone (Figure 2j–l). The hypothalamus showed a large number of CRF receptor-ir fibers being located primarily in the internal layer of the median eminence (Figure 3a–d). Within these regions, both ENK- and 3E7-ir neurons were positive for CRF receptors. Interestingly, these neurons coexpressed CRF receptor and 3E7 but not ENK within the median eminence (Figure 3AA–CC) indicating that CRF receptor-ir neurons expressed only END but not ENK. Within the PVN, and the supraoptic nuclei (SON) in the hypothalamus, abundant CRF receptor-ir neurons were in close contact with dense 3E7-ir nerve fibers (Figure 3e and f). The arcuate nucleus showed abundant ENK-ir neurons in close contact with dense nerve fibers positive for CRF receptor but with some fibers overlapping (Figure 3d). Within the periaquaeductal gray area, the majority of CRF receptor-ir neurons expressed opioid peptides, but few neurons showed immunoreactivity of either CRF receptor or opioid peptides (Figure 3g–i). In the LC, we demonstrated that CRF receptor-ir neurons coexpressed opioid peptides (Figure 3j–l).
Opioid-Mediated Antinociceptive Effects Following i.t. CRF
I.t. injection of CRF (2, 4, 10, 20 ng) dose-dependently produced significant elevations of PPT in inflamed paws (P<0.05, ANOVA and Dunnett's test) but not in contralateral noninflamed hind paws of FCA-treated rats (P>0.05, ANOVA and Dunnett's test) (Figure 4a). These PPT elevations increased beyond baseline PPT in naive rats (69±5.4 g) (see above). The highest effective dose of CRF given s.c. did not alter PPT (data not shown).
In Wistar rats with 4 days FCA hindpaw inflammation, effects of i.t. injections of low doses of CRF on ipsi- (closed bars) and contralateral (open bars) nociceptive paw pressure thresholds (PPT) were measured by algesiometry. (a) I.t. injections of CRF significantly increased PPT in a dose-dependent manner ipsi- but not contralateral to the FCA-inflamed paw (P<0.05, * indicates significant differences from 0=saline-treated group). (b) Dose-dependent antagonism of i.t. CRF's (10 ng) antinociception by coadministered α-helical CRF receptor antagonist was significant ipsilateral to the FCA-inflamed paw (P<0.05, ! indicates significant differences from baseline (BL), * indicates significant differences from 0=vehicle-treated group). (c) Dose-dependent attenuation of i.t. CRF's (10 ng) antinociception by coadministered opioid receptor antagonist naloxone was significant ipsilateral to the FCA-inflamed paw (P<0.05, ! indicates significant differences from baseline (BL), * indicates significant differences from 0=vehicle-treated group).
I.t. coadministration of different doses off α-helical CRF antagonist with 10 ng CRF dose-dependently and significantly attenuated CRF-induced PPT increases in inflamed hindpaws (P<0.05, ANOVA and Dunnett's test) (Figure 4b). However, this treatment did not affect PPT on the contralateral side (data not shown). In addition, i.t. coadministration of different doses of the opioid receptor antagonist naloxone with 10 ng CRF dose-dependently and significantly attenuated CRF-induced PPT elevations in inflamed hindpaws (ANOVA and Dunnett's test) (Figure 4c), but did not alter PPT on the contralateral side (data not shown). The highest effective dose of either α-helical CRF antagonist or naloxone given s.c. did not alter i.t. CRF-elicited PPT elevations (data not shown).
Spinal Cord Areas of CRF Receptor and Opioid Peptide Coexpression
In the L4–L5 segments of the spinal cord of rats with FCA-induced hindpaw inflammation, CRF receptor immunoreactivity was mainly distributed in the superficial laminae of dorsal horn (Figure 5a, e, f, j). Also, CRF receptor immunoreactivity was detected in the parasympathetic nucleus of the lumber spinal cord (Figure 5j). A few labeled nerve fibers were distributed in the gray matter of the lumbar spinal cord. As most ENK-ir neurons within the dorsal horn specifically characterized inhibitory interneurons (Millan, 1999; Schulte et al, 2003; Llewellyn-Smith et al, 2005), we identified most CRF receptor-ir nerve fibers of the dorsal horn overlapping at the confocal level with ENK immunoreactivity (Figure 5a–c). In contrast, the marker for primary afferent central nerve endings (CGRP) showed no colocalization with CRF receptor immunoreactivity (Figure 5e and f). However, opioid receptor MOR and DOR immunoreactivity overlapped with CGRP (Figure 5g–i). As behavioral data significantly changed only on the ipsi-, but not contralateral side, we investigated quantitative changes in CRF receptor- or ENK-immunoreactivity within the superficial laminae of spinal cord. We found a significant increase in the density of CRF receptors (37.1±5.4%, P<0.05) or ENK-ir (53.0±10.4%, P<0.05) immunostaining on the superficial laminae of the spinal cord ipsilateral to the inflamed vs untreated side (Figure 5j–k). The immunostaining had a similar distribution on both sides but the density increased ipsilateral to the inflamed side. This change was restricted to the lumbar segments (ie, L4–L5) that are innervated by the sciatic nerve.
Double-immunofluorescence staining of CRF receptor (a, d–f) and ENK (b, d), CGRP (e) or MOR (f) in the superficial laminae of dorsal horn of L4-L5 spinal cord of the rat with unilateral FCA hindpaw inflammation. Note that most of CRF receptor-ir fibers express ENK (double arrow) but do not express CGRP (e) or MOR (f) in the dorsal horn of coronal (b, e, f) and parasagittal (d) sections of L4–L5 spinal cord of the rat; however, few fibers contain only CRF receptor (arrow) or ENK (arrowhead) (c, d). Note that the network of CRF receptor-ir fibers is extending into the lateral gray matter and in the parasympathetic nucleus of the lumber spinal cord (arrows) ((e, f). (g–i) Double-immunofluorescence staining of DOR (g) and CGRP (h) showing that DOR overlaps with CGRP (i). (j, k) Single immunostaining of CRF receptor (j) or ENK (k) show that the intensity of CRF receptor (j) or ENK (k) immunoreactivity is increased in ipsi- (ipsi) compared to contralateral (contral) spinal cord dorsal horns following FCA hindpaw inflammation. Bar=50 μm (for a–i) and 100 (j, k) μm.
Opioid-Mediated Antinociceptive Effects Following i.pl. CRF
I.pl. injection of CRF (0.5, 1, 1.5 and 2 ng) dose-dependently produced significant elevations of PPT in inflamed paws (P<0.05, ANOVA and Dunnett's test) (Figure 6a) but not in contralateral noninflamed hind paws (P>0.05, ANOVA and Dunnett's test). These PPT elevations increased beyond baseline PPT in naive rats (69±5.4 g) (see above). The highest effective dose of CRF given s.c. did not alter PPT (data not shown). I.pl. coadministration of different doses of α-helical CRF antagonist with 1.5 ng CRF dose-dependently and significantly attenuated CRF-induced PPT increases in inflamed hindpaws (P<0.05, ANOVA and Dunnett's test) (Figure 6b). However, this treatment did not affect PPT on the contralateral side (data not shown). In addition, i.pl. coadministration of different doses of the opioid receptor antagonist naloxone with 1.5 ng CRF dose-dependently and significantly attenuated CRF-induced PPT elevations in inflamed hindpaws (ANOVA and Dunnett's test) (Figure 6c), but did not alter PPT on the contralateral side (data not shown). The highest effective dose of either α-helical CRF antagonist or naloxone given s.c. did not alter i.pl. CRF-elicited PPT elevations (data not shown).
In Wistar rats with 4 days FCA hindpaw inflammation, effects of i.pl. injections of low doses of CRF on ipsi- (closed bars) and contralateral (open bars) nociceptive paw pressure thresholds (PPT) were measured by algesiometry. (a) I.pl. injections of CRF significantly increased PPT in a dose-dependent manner ipsi- but not contralateral to the FCA-inflamed paw (P<0.05, * indicates significant differences from 0=saline-treated group). (b) Dose-dependent antagonism of i.pl. CRF's (1.5 ng) antinociception by coadministered α-helical CRF receptor antagonist was significant ipsilateral to the FCA-inflamed paw (P<0.05, ! indicates significant differences from baseline (BL), * indicates significant differences from 0=vehicle-treated group). (c) Dose-dependent attenuation of i.pl. CRF's (1.5 ng) antinociception by coadministered opioid receptor antagonist naloxone was significant ipsilateral to the FCA-inflamed paw (P<0.05, ! indicates significant differences from baseline (BL), * indicates significant differences from 0=vehicle-treated group).
CRF Receptor and Opioid Peptide Expression in DRG, Sciatic Nerve and Inflamed Subcutaneous Paw Tissue
Within inflamed subcutaneous tissue double-immunofluorescence confocal microscopy demonstrated that most of opioid peptides-ir immune cells contained CRF receptors. Only few immune cells immunolabeled for either opioid peptides or CRF receptor alone (Figure 7g–i). Double-immunofluorescence staining of inflamed subcutaneous paw tissue using CRF receptor and CGRP antisera identified CRF receptor and CGRP immunoraectivity on immune cells and on subcutaneous nerve fibers, respectively, without overlap (Figure 7f).
Double-immunofluorescence staining of CRF receptor (red; a, d) and CGRP (green; b, e) in L4-L5 DRG and sciatic nerve ipsilateral to the FCA inflamed hindpaw. Note only CGRP-ir neurons or fibers within DRG or sciatic nerve, respectively, but no CRF receptor immunoreactivity was detected. (f) Inflamed subcutaneous paw tissue double-immunostained for CRF receptor (red) and CGRP (green) shows CRF receptor immunoreactivity located on immune cells (arrow) and CGRP immunoreactivity located on nerve fibers (arrowhead). (g, h, i) Inflamed subcutaneous paw tissue double-immunostained for CRF receptor (red; g) and opioid peptides (3E7+) (green; h) shows that most of CRH receptor-ir cells contain opioid peptides (double arrow) with some cells containing CRF receptor only (red) (arrow). Bar=50 μm.
In DRG as well as sciatic nerve sections innervate either inflamed or noninflamed paws, CRF receptor or CGRP double-immunofluorescence labeling revealed no staining for CRF receptor, but strong immunoreactivity for CGRP in primary afferent DRG neurons and nerve fibers within the sciatic nerve (Figure 7a–e).
DISCUSSION
This investigation sought to investigate systematically i.c.v., i.t., and i.pl. CRF's effects on nociception in a standardized model of tonic pain, that is, FCA-induced hindpaw inflammation. The major finding of this study is that application of low, systemically inactive doses of CRF inhibits inflammatory pain not only at peripheral but also at spinal and supraspinal sites by the involvement of endogenous opioid peptides. This is established in several ways: (i) i.c.v. administration of low doses of CRF elicits potent antinociception ipsi- as well as contralateral to the inflamed site which is dose-dependent and attenuated by i.c.v. but not systemically administered α-helical CRF or naloxone; (ii) consistently, we identified multiple pain relevant brain areas which show colocalization of CRF receptors with opioid peptides; (iii) i.t. administration of low doses of CRF elicits potent antinociception ipsi- but not contralateral to the inflamed site which is dose-dependent and attenuated by i.t. but not systemically applied α-helical CRF or naloxone; (iv) consistently, we identified CRF receptors mostly on ENK-ir inhibitory dorsal horn interneurons—but neither on primary afferent central nerve endings (CGRP-ir) nor on excitatory dorsal horn neurons (MOR-ir nociceptive neurons as well as interneurons); dorsal horn CRF receptor-ir as well as ENK-ir was highly upregulated ipsi- but not contralateral to the inflamed painful site; (v) i.pl. administration of low doses of CRF elicits potent antinociception in ipsilateral inflamed, but not contralateral noninflamed hindpaws which is dose-dependent and attenuated by i.pl. but not systemically administered α-helical CRF or naloxone and (vi) consistently, we identified CRF receptors exclusively on opioid peptide containing immune cells, but not within DRG cell bodies, sciatic axons, or peripheral nerve fibers within inflamed subcutaneous tissue.
To this end, our findings report about identified areas of CRH receptor and opioid peptide colocalization in pain relevant areas along the neuraxis of pain transmission. This may support previous evidence for a new class of CRF-related analgesics that has been overlooked (Lariviere and Melzack, 2000).
Opioid-Mediated Inhibition of Inflammatory Pain Following i.c.v. CRF
In our tonic pain animal model of FCA hindpaw inflammation, i.c.v. but not systemic application of low doses of CRF elicits potent, dose-dependent antinociception which is attenuated by coadministration of the CRF receptor antagonist α-helical CRF. This observation is in agreement with previous studies showing that i.c.v. administration of CRF produced dose-dependent antinociception at low doses (ie, 40–500 ng) (Vit et al, 2006; Bianchi and Panerai, 1995). Instead, in other studies using higher doses (0.3–30 μg) i.c.v. CRF was not effective (Ayesta and Nikolarakis, 1989; Poree et al, 1989; Sherman and Kalin, 1987; Britton et al, 1985). Several previous investigations of the pain modulating effects of intracranial CRF have led to conflicting results (Lariviere and Melzack, 2000; Taché et al, 2004). In studies examining somatic pain, the influence of intracranial CRF has ranged from pain inhibition (Vit et al, 2006; Cui et al, 2004; Bianchi et al, 1991, 1995) over no pain modulation (Ayesta and Nikolarakis, 1989; Poree et al, 1989; Sherman and Kalin, 1988) to increased pain sensitivity (Williams et al, 1986). Some of these discrepancies may be explained by differences in (i) the animal models (tonic vs phasic pain); (ii) the doses of CRF (low vs high dose, single dose vs dose–response); and (iii) various intracerebral locations and techniques of application. As Lariviere and Melzack (2000) suggested that antinociceptive effects of CRF are identified rather in tonic than in phasic pain, we have chosen an animal model of tonic pain, that is, FCA hindpaw inflammation, and have examined not just a single dose but a full dose range of i.c.v. CRF. Interestingly, we have also observed elevated PPT contralateral to the inflamed hindpaw, although they were less pronounced and significantly different only for a single dose. This might be explained by the fact that alterations in neuroplasticity following ipsilateral tonic pain show some degree of convergence at supraspinal levels (Melzack, 1999). In studies examining visceral pain, most of them suggest a pain enhancing effect during colonic distension—although not a direct pain stimulating effect—of i.c.v. CRF in a dose range of 5–20 μg/kg (Gué et al, 1997; Schwetz et al, 2005). However, one study reported about pain inhibiting effects in the mouse writhing assay, which were maximal with 100–200 ng i.c.v. CRF (Kita et al, 1993).
CRF has been suggested to affect pain behavior through the hypothalamo–pituitary–adrenal (HPA) axis from the release of corticosterone (Pavcovich and Valentino, 1997; Lariviere and Melzack, 2000; Wang et al, 2004). A recent study by Vit et al (2006) does not support this idea by showing that the inhibitory effects of i.c.v. CRF on nociception in response to paw pinch are independent of its control of the HPA axis, as corticosterone levels did not correlate with pain behavior and adrenalectomized Fischer rats retained normal nociceptive thresholds as well as unchanged antinociceptive effects following i.c.v. CRF. In our study the use of low, systemically inactive doses of i.c.v. CRF, the demonstration of dose-dependent increases and the antagonism by i.c.v. but not systemic α-helical CRF indicate that the inhibition of inflammatory pain is owing to a local CRF receptor specific effect within the brain. CRF receptors occur throughout the brain (Potter et al, 1994 and Van Pett et al, 2000) and offer many targets where CRF might act to change pain behavior. Following i.c.v. administration, CRF reaches high concentrations in pain relevant regions such as the thalamus, hypothalamus, LC, and periaqueductal gray (Bittencourt and Sawchenko, 2000). It has previously been noted that CRF modulates electrophysiological activity within the hippocampus (Aldenhoff et al, 1983; Siggins et al, 1985) and LC (Lejeune and Millan, 2003; Valentino et al, 1987). CRF elicits excitatory actions within the cortex and the hypothalamus (Ehlers et al, 1983; Siggins et al, 1985) or inhibitory actions on the electrophysiological activity of the thalamus and the paraventricular nucleus of the hypothalamus (Siggins et al, 1985), all of which have been shown to be involved in pain processing (Bouckoms, 1994; Melzack and Wall, 1996). Interestingly, our studies revealed that the antinociceptive effect of i.c.v. CRF is attenuated by i.c.v. but not systemic coadministration of the opioid receptor antagonist naloxone. These findings suggest that ic.v. CRF's antinociception seems to be mediated by activation of opioid receptors through opioid peptides within the brain. In line with our behavioral data, double-immunofluorescence confocal microscopy of brain sections of FCA-treated animals identified multiple areas of colocalization of opioid peptides with CRF receptors such as the cerebral cortex, hippocampal formation, thalamus, hypothalamus, periaquaductal gray and LC, which suggest a possible explanation of our behavioral findings. Further support for this explanation comes from the evidence that pain modulating opioid receptors have been identified in the same brain regions (Arvidsson et al, 1995). However, there might be also other mechanisms responsible for the antinociceptive effects of i.c.v. CRF. For example, CRF affects the tonic electrophysiological activity of the LC (Lejeune and Millan, 2003; Valentino and Foote, 1987; Borsody and Weiss, 1996), which is involved in the tonic descending inhibitory control of spinal cord circuits (Besson and Chaouch, 1987). Taken together, our findings provide evidence that in the tonic pain animal model of FCA hindpaw inflammation low, systemically ineffective doses of i.c.v. CRF elicit antinociception by activation of opioid receptors through released opioid peptides within pain relevant multiple brain regions. This opioid peptide release is mediated by CRF receptors at the brain level and results in potent antinociception.
Opioid-Mediated Inhibition of Inflammatory Pain Following i.t. CRF
We also examined in our animal model of FCA hindpaw inflammation the pain modulating effects of i.t. CRF. I.t. but not systemic application of low, systemically inactive doses of CRF elicits potent antinociception on the ipsi- but not contralateral noninflamed side which increases dose-dependently and is attenuated by coadministration of the CRF receptor antagonist α-helical CRF. An antinociceptive effect of i.t. CRF is in agreement with other studies showing that i.t. CRF inhibited the writhing response in mice (Song and Takemori, 1990, 1991) as well as the visceromotor response following duodenal distension (Nijsen et al, 2005). However, these studies examined exclusively visceral pain and no study so far investigated the influence of i.t. CRF on somatic pain. In our model of inflammatory somatic pain, we demonstrated dose-dependent antinociception of i.t. CRF, which is antagonized by α-helical CRF and, therefore, specifically mediated by spinal CRF receptors. The substantia gelatinosa within the spinal cord, a part of the dorsal horn that receives pain-related afferent signals, also contains receptors for CRF throughout its length (Bell and De Souza, 1988; Skofitsch et al, 1985). Our findings are in agreement with previous studies by Song and Takemori (1990, 1991) showing that analgesia induced by i.t. administration of CRF is blocked by i.t. administration of the CRF receptor antagonist. To exclude further a possible site of action of i.t. CRF outside the spinal cord, we found that systemic administration of the highest effective doses of CRF or CRF antagonist α-helical CRF had no effect. Furthermore, i.t. CRF's antinociception was attenuated by i.t. but not systemic coadministration of the opioid receptor antagonist naloxone, suggesting activation of opioid receptors through opioid peptides within the spinal cord.
In support of these findings, our double-immunofluorescence staining showed that most of CRF receptor-ir nerve fibers overlap with ENK-ir interneurons but not with primary afferent (CGRP-ir) central endings or MOR-ir nociceptive neurons in the superficial dorsal horn of the spinal cord. Consistent with a putative release of opioid peptides, ENK-ir interneurons are in close proximity of clusters of MOR-ir nociceptive neurons (Arvidsson et al, 1995) whose activation may contribute to presynaptic inhibition of sensory neuron neurotransmitter release (Kondo et al, 2005) and/or postsynaptic hyperpolarization of excitatory neurons (Trafton et al, 2000). Interestingly, i.t. CRF produces antinociception exclusively in inflamed but not in contralateral noninflamed hind paws. Accordingly, quantification of our immunolabeling demonstrates that FCA-induced inflammation enhances the expression of CRF receptors and ENK in interneurons within the superficial laminae of the spinal cord. This finding is consistent with previous studies (Ji et al, 1994; Spetea et al, 2002; Calza et al, 1998) demonstrating an upregulation in the expression of ENK after FCA-induced inflammation. However, another explanation for the differences in ipsi- and contralateral antinociceptive effects of i.t. CRF might be that tonic ongoing pain is needed to detect CRF's inhibitory actions on pain; only on the ipsilateral, but not on the contralateral side is continuous afferent barrage or firing towards the spinal cord (Ikeda et al, 2003).
Taken together, the possible explanation of mechanism of CRF-induced antinociception within the spinal cord is that inflammatory pain induces upregulation of CRF receptors and ENK within substantia gelatinosa in the spinal cord. Then, i.t. CRF administration activates its receptors on inhibitory interneurons and directly and/or indirectly induces ENK release from interneurons activating inhibitory circuits within the spinal cord.
Opioid-Mediated Inhibition of Inflammatory Pain Following i.pl. CRF
Extending and confirming our previous study (Mousa et al, 2003), we investigated the functional role of peripheral CRF receptor and endogenous opioid peptides within inflammatory pain using CRF and opioid receptor antagonists. We tried to investigate the relative contributions of CRF receptors on sensory neurons vs immune cells to the pain inhibiting effects of i.pl. CRF. In agreement with our previous studies, we found potent antinociceptive effects following the administration of CRF into inflamed paw tissue. However, CRF did not have any effects in noninflamed paws or when the same dose administered subcutaneously at a remote site (Schäfer et al, 1994; Mousa et al, 2003), indicating a local site of action within the inflamed paw. Kiang and Wei (1987) as well as Hargreaves et al (1989) also provide support for a peripheral antinociceptive mechanism following local administration of CRF in two other inflammatory conditions, carrageenan-induced hyperalgesia and thermal injury of the rat hindpaw. Apparently, inflammation is a prerequisite for local CRF to produce analgesia (migration of inflammatory cells containing opioids). The antinociception induced by i.pl. CRF was attenuated by CRF receptor antagonists. Similarly, i.pl. but not systemic injection of naloxone, eliminated the analgesic effect of CRF. These findings suggest the involvement of CRF receptor and endogenous opioid peptides in CRF-induced antinociception. In support of this finding, our double-immunofluorescence confocal microscopy demonstrated colocalization of opioid peptides with CRF receptors in immune cells within inflamed subcutaneous paw tissue. In contrast, the immunostaining of DRG, sciatic nerve and subcutaneous paw tissue using CRF receptor antibodies showed no staining suggesting that CRF receptors are not present in primary afferent neurons. This anatomical distribution of CRF receptor is in line with our previous studies (Mousa et al, 1996, 2003).
SUMMARY
In an animal model of tonic pain, that is, FCA-induced hindpaw inflammation, we observed that the local application of low, systemically inactive doses of CRF at the three main levels of pain transmission, that is, the brain, spinal cord, and peripheral sensory neuron, elicits potent antinociception. At each of these levels, CRF-induced elevated PPT are well beyond baseline PPT of naïve animals, are dose-dependent and are antagonized by local CRF receptor antagonist α-helical CRF, indicating truly antinociceptive effects and CRF receptor specificity. Consistently, we have identified multiple brain areas, inhibitory neurons within the dorsal horn of the spinal cord as well as immune cells—but not peripheral sensory neurons—that coexpress CRF receptors and opioid peptides. In line with these anatomical findings, local administration of CRF together with the opioid receptor antagonist naloxone dose-dependently reversed CRF's antinociceptive effects at each of these three levels of pain transmission. The present results have highlighted the need for further studies to investigate the contribution of each CRF receptor subtype in central CRF-induced antinociception during inflammatory pain and to delineate whether endogenous CRF at these three levels contributes to the body's response to cope with the stressful stimulus pain in an opioid-mediated manner.
References
Aldenhoff JB, Gruol DL, Rivier J, Vale W, Siggins GR (1983). Corticotropin releasing factor decreases postburst hyperpolarizations and excites hippocampal neurons. Science 221: 875–877.
Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB (1999). The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol 160: 1–12.
Arvidsson U, Riedl M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ et al (1995). Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. J Neurosci 15: 3328–3341.
Ayesta FJ, Nikolarakis KE (1989). Peripheral but not intracerebroventricular corticotropin-releasing hormone (CRH) produces antinociception which is not opioid mediated. Brain Res 503: 219–224.
Bale TL, Vale WW (2004). CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol 44: 525–557 (Review).
Bell JA, de Souza EB (1988). Functional corticotropin-releasing factor receptors in neonatal rat spinal cord. Peptides 9: 1317–1322.
Besson JM, Chaouch A (1987). Peripheral and spinal mechanisms of nociception. Physiol Rev 67: 67–186.
Bianchi M, Panerai AE (1995). CRH and the noradrenergic system mediate the antinociceptive effect of central interleukin-la in the rat, Brain Res. Bull 36: 113–117.
Bianchi M, Sacerdote P, Locatelli L, Mantegazza P, Panerai AE (1991). Corticotropin releasing hormone, interleukin-1a, and tumor necrosis factor-α share characteristics of stress mediators. Brain Res 546: 139–142.
Bittencourt JC, Sawchenko PE (2000). Do centrally administered neuropeptides access cognate receptors? An analysis in the central corticotropin-releasing factor system. J Neurosci 20: 1142–1156.
Britton KT, Morgan J, Rivier J, Vale W, Koob GF (1985). Chlordiazepoxide attenuates response suppression induced by corticotropin-releasing factor in the conflict test. Psychopharmacology (Berl) 86: 170–174.
Borsody MK, Weiss JM (1996). Influence of corticotropin-releasing hormone on electrophysiological activity of locus coeruleus neurons. Brain Res 724: 149–168.
Bouckoms AJ (1994). Limbic surgery for pain. In: Wall PD, Melzack R (eds). Textbook of Pain, 3rd edn. Churchill Livingstone: Edinburgh. pp 1171–1187.
Brack A, Rittner HL, Machelska H, Leder K, Mousa SA, Schafer M et al (2004). Endogenous peripheral antinociception in early inflammation is not limited by the number of opioid-containing leukocytes but by opioid receptor expression. Pain 108: 67–75.
Cabot P, Carter L, Gaiddon C, Zhang Q, Schäfer M, Loeffler J et al (1997). Immune cell-derived b-endorphin. J Clin Investig 100: 142–148.
Cabot PJ, Carter L, Schäfer M, Stein C (2002). Methionine–enkephalin- and dynorphin A-release from immune cells and control of inflammatory pain. Pain 93: 207–212.
Calza L, Pozza M, Zanni M, Manzini CU, Manzini E, Hokfelt T (1998). Peptide plasticity in primary sensory neurons and spinal cord during adjuvant-induced arthritis in the rat: an immunocytochemical and in situ hybridization study. Neuroscience 82: 575–589.
Chappell PB, Smith MA, Kilts CD, Bissette G, Ritchie J, Anderson C et al (1986). Alterations in corticotropin-releasing factor-like immunoreactivity in discrete rat brain regions after acute and chronic stress. J Neurosci 6: 2908–2914.
Cui XY, Lundeberg T, Yu LC (2004). Role of corticotropin-releasing factor and its receptor in nociceptive modulation in the central nucleus of amygdala in rats. Brain Res 995: 23–28.
Dunn AJ, Berridge CW (1990). Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev 15: 71–100 (Review).
Ehlers CL, Henriksen SJ, Wang M, Rivier J, Vale W, Bloom FE (1983). Corticotropin releasing factor produces increases in brain excitability and convulsive seizures in rat. Brain Res 278: 332–336.
Frank AJ, Tilby MJ (2003). Quantification of DNA adducts in individual cells by immunofluorescence: effects of variation in DNA conformation. Exp Cell Res 283: 127–134.
Hargreaves KM, Dubner R, Costello AH (1989). Corticotropin releasing factor (CRF) has a peripheral site of action for antinociception. Eur J Pharmacol 170: 275–279.
Hargreaves KM, Mueller GP, Dubner R, Goldstein D, Dionne RA. (1987). Corticotropin-releasing factor (CRF) produces analgesia in humans and rats. Brain Res 422: 154–157.
Gué M, Del Rio-Lacheze C, Eutamene H, Theodorou V, Fioramonti J, Bueno L (1997). Stress-induced visceral hypersensitivity to rectal distension in rats: role of CRF and mast cells. Neurogastroenterol Motil 9: 271–279.
Ikeda H, Kusudo K, Ryu PD, Murase K (2003). Effects of corticotropin-releasing factor on plasticity of optically recorded neuronal activity in the substantia gelatinosa of rat spinal cord slices. Pain 106: 197–207.
Ji RR, Zhang X, Wiesenfeld-Hallin Z, Hokfelt T (1994). Expression of neuropeptide Y and neuropeptide Y (Y1) receptor mRNA in rat spinal cord and dorsal root ganglia following peripheral tissue inflammation. J Neurosci 14: 6423–6434.
Kavelaars A, Berkenbosch F, Croiset G, Ballieux RE, Heijnen CJ (1990). Induction of b-endorphin secretion by lymphocytes after subcutaneous administration of corticotropin-releasing factor. Endocrinology 126: 759–764.
Kiang JG, Wei ET (1987). Corticotropin-releasing factor inhibits thermal injury. J Pharmacol Exp Ther 243: 517–520.
Kita A, Imano K, Nakamura H (1993). Involvement of corticotropin-releasing factor in the antinociception produced by interleukin-1 in mice. Eur J Pharmacol 237: 317–322.
Kondo I, Marvizon JC, Song B, Salgado F, Codeluppi S, Hua XY et al (2005). Inhibition by spinal mu- and delta-opioid agonists of afferent-evoked substance P release. J Neurosci 25: 3651–3660.
Lariviere WR, Melzack R (2000). The role of corticotropin-releasing factor in pain and analgesia. Pain 84: 1–12.
Lejeune F, Millan MJ (2003). The CRF1 receptor antagonist, DMP695, abolishes activation of locus coeruleus noradrenergic neurones by CRF in anesthetized rats. Eur J Pharmacol 464: 127–133.
Likar R, Mousa SA, Steinkellner H, Koppert W, Philippitsch G, Stein C et al (2007). Involvement of intraarticular corticotropin-releasing hormone in postoperative pain modulation. Clin J Pain 23: 136–142.
Llewellyn-Smith IJ, Dicarlo SE, Collins HL, Keast JR (2005). Enkephalin-immunoreactive interneurons extensively innervate sympathetic preganglionic neurons regulating the pelvic viscera. J Comp Neurol 488: 278–289.
Lund I, Lundeberg T, Carleson J, Sonnerfors H, Uhrlin B, Svensson E (2006). Corticotropin releasing factor in urine—a possible biochemical marker of fibromyalgia. Responses to massage and guided relaxation. Neurosci Lett 403: 166–171.
McLean SA, Williams DA, Stein PK, Harris RE, Lyden AK, Whalen G et al (2006). Cerebrospinal Fluid Corticotropin-Releasing Factor Concentration is Associated with Pain but not Fatigue Symptoms in Patients with Fibromyalgia. Neuropsychopharmacology 31: 2776–2782.
Melzack R (1999). Pain—an overview. Acta Anaesthesiol Scand 43: 880–884.
Melzack R, Wall PD (1996). The Challenge of Pain, Updated 2nd edn. Penguin Books: London. p 339.
Merchenthaler I, Vigh S, Schally AV, Stumpf WE, Arimura A (1984). Immunocytochemical localization of corticotropin releasing factor (CRF) like immunoreactivity in the thalamus of the rat. Brain Res 323: 119–122.
Millan MJ (1999). The induction of pain: an integrative review. Prog Neurobiol 57: 1–164 (Review).
Mousa SA, Bopaiah CP, Shaqura M, Fischer O, Hofmann J, Hellweg R et al (2007). Nerve growth factor increases sensory neuron μ-opioid receptors and responsiveness during inflammatory pain. Brain 130(Pt 2): 502–513.
Mousa SA, Bopaiah CP, Stein C, Schafer M (2003). Involvement of corticotropin-releasing hormone receptor subtypes 1 and 2 in peripheral opioid-mediated inhibition of inflammatory pain. Pain 106: 297–307.
Mousa SA, Machelska H, Schäfer M, Stein C (2002). Immunohistochemical localization of endomorphin-1 and endomorphin-2 in immune cells and spinal cord in a model of inflammatory pain. J Neuroimmunol 126: 5–15.
Mousa SA, Schafer M, Mitchell WM, Hassan AH, Stein C (1996). Local upregulation of corticotropin-releasing hormone and interleukin-1 receptors in rats with painful hindlimb inflammation. Eur J Pharmacol 311: 221–231.
Mousa SA, Shakibaei M, Sitte N, Schafer M, Stein C (2004). Subcellular pathways of beta-endorphin synthesis, processing, and release from immunocytes in inflammatory pain. Endocrinology 145: 1331–1341.
Nagaraja RY, Grecksch G, Reymann KG, Schroeder H, Becker A (2004). Group I metabotropic glutamate receptors interfere in different ways with pentylenetetrazole seizures, kindling, and kindling-related learning deficits. Naunyn Schmiedebergs Arch Pharmacol 370: 26–34.
Nijsen M, Ongenae N, Meulemans A, Coulie B (2005). Divergent role for CRF1 and CRF2 receptors in the modulation of visceral pain. Neurogastroenterol Motil 17: 423–432.
Nozu T, Kudaira M (2006). Corticotropin-releasing factor induces rectal hypersensitivity after repetitive painful rectal distention in healthy humans. J Gastroenterol 41: 740–744.
Paxinos G, Watson C (1997). The Rat Brain in Stereotaxic Coordinates. Academic Press: New York, Paperback.
Pavcovich LA, Valentino RJ (1997). Regulation of a putative neurotransmitter effect of corticotropin-releasing factor: effects of adrenalectomy. J Neurosci 17: 401–408.
Poree LR, Dickenson AH, Wei ET (1989). Corticotropin-releasing factor inhibits the response of trigeminal neurons to noxious heat. Brain Res 502: 349–355.
Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K et al (1994). Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci USA 91: 8777–8781.
Rivier C, Rivier J, Vale W (1986). Stress-induced inhibition of reproductive functions: role of endogenous corticotropin-releasing factor. Science 231: 607–609.
Sagami Y, Shimada Y, Tayama T, Satake M, Endo Y, Shoji T et al (2004). Effect of a corticotropin-releasing hormone receptor antagonist on colonic sensory and motor function in patients with irritable bowel syndrome. Gut 53: 958–964.
Schäfer M, Carter L, Stein C (1994). Interleukin 1b and corticotropin-releasing factor inhibit pain by releasing opioids from immune cells in inflamed tissue. Proc Natl Acad Sci USA 91: 4219–4223.
Schäfer M, Mousa SA, Stein C (1997). Corticotropin-releasing factor in antinociception and inflammation. Eur J Pharmacol 323: 1–10.
Schmitt TK, Mousa SA, Brack A, Schmidt DK, Rittner HL, Welte M et al (2003). Modulation of peripheral endogenous opioid analgesia by central afferent blockade. Anesthesiology 98: 195–202.
Schulte G, Robertson B, Fredholm BB, DeLander GE, Shortland P, Molander C (2003). Distribution of antinociceptive adenosine A1 receptors in the spinal cord dorsal horn, and relationship to primary afferents and neuronal subpopulations. Neuroscience 121: 907–916.
Schwetz I, McRoberts JA, Coutinho SV, Bradesi S, Gale G, Fanselow M et al (2005). Corticotropin-releasing factor receptor 1 mediates acute and delayed stress-induced visceral hyperalgesia in maternally separated Long-Evans rats. Am J Physiol Gastrointest Liver Physiol 289: G704–G712.
Sharp B, Linner K (1993). What do we know about the expression of proopiomelanocortin transcripts and related peptides in lymphoid tissue? Endocrinology 133: 1921A–1921B.
Sherman JE, Kalin NH (1987). The effects of ICV-CRH on novelty-induced behavior. Pharmacol Biochem Behav 26: 699–703.
Sherman JE, Kalin NH (1988). ICV-CRH alters stress-induced freezing behaviour without affecting pain sensitivity. Pharmacol Biochem Behav 30: 801–807.
Sibinga NE, Goldstein A (1988). Opioid peptides and opioid receptors in cells of the immune system. Annu Rev Immunol 6: 219–249.
Siggins GR, Gruol D, Aldenhoff J, Pittman Q (1985). Electrophysiological actions of corticotropin-releasing factor in the central nervous system. Fed Proc 44: 237–242.
Skofitsch G, Insel TR, Jacobowitz DM (1985). Binding sites for corticotropin releasing factor in sensory areas of the rat hindbrain and spinal cord. Brain Res Bull 15: 519–522.
Song ZH, Takemori AE (1990). Involvement of spinal kappa opioid receptors in the antinociception produced by intrathecally administered corticotropin-releasing factor in mice. J Pharmacol Exp Ther 254: 363–368.
Song ZH, Takemori AE (1991). Antagonism of morphine antinociception by intrathecally administered corticotropin-releasing factor in mice. J Pharmacol Exp Ther 256: 909–912.
Spetea M, Rydelius G, Nylander I, Ahmed M, Bileviciute-Ljungar I, Lundeberg T et al (2002). Alteration in endogenous opioid systems due to chronic inflammatory pain conditions. Eur J Pharmacol 435: 245–252.
Stein C, Schafer M, Machelska H (2003). Attacking pain at its source: new perspectives on opioids. Nat Med 9: 1003–1008.
Størkson RV, Kjorsvik A, Tjolsen A, Hole K (1996). Lumbar catheterization of the spinal subarachnoid space in the rat. J Neurosci Methods 65: 167–172.
Taché Y, Martinez V, Wang L, Million M (2004). CRF1 receptor signaling pathways are involved in stress-related alterations of colonic function and viscerosensitivity: implications for irritable bowel syndrome. Br J Pharmacol 141: 1321–1330.
Trafton JA, Abbadie C, Marek K, Basbaum AI (2000). Postsynaptic signaling via the [mu]-opioid receptor: responses of dorsal horn neurons to exogenous opioids and noxious stimulation. J Neurosci 20: 8578–8584.
Valentino RJ, Foote SL (1987). Corticotropin-releasing factor disrupts sensory responses of brain noradrenergic neurons. Neuroendocrinology 45: 28–36.
Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C et al (2000). Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 428: 191–212.
Vit JP, Clauw DJ, Moallem T, Boudah A, Ohara PT, Jasmin L (2006). Analgesia and hyperalgesia from CRF receptor modulation in the central nervous system of Fischer and Lewis rats. Pain 121: 241–260.
Wang S, Lim G, Zeng Q, Sung B, Ai Y, Guo G et al (2004). Expression of central glucocorticoid receptors after peripheral nerve injury contributes to neuropathic pain behaviors in rats. J Neurosci 24: 8595–8605.
Wei ET, Kiang JG, Buchan P, Smith TW (1986). Corticotropin-releasing factor inhibits neurogenic plasma extravasation in the rat paw. J Pharmacol Exp Ther 238: 783–787.
Williams Jr DW, Lipton JM, Giesecke Jr AH (1986). Influence of centrally administered peptides on ear withdrawal from heat in the rabbit. Peptides 7: 1095–1100.
Zimmermann M (1983). Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16: 109–110.
Acknowledgements
We are extremely grateful to Drs Stefan Schulz, Volker Höllt (Department of Pharmacology and Toxicology, Otto-von-Guericke University, Magdeburg, Germany), and R Elde (Minneapolis, MN, USA) for donating MOR and DOR antisera used in this study. Professor C Stein's continuous support and Mrs Ute Oedekoven's technical assistance is gratefully acknowledged. This study was supported by the DFG grant KFO 100.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mousa, S., Bopaiah, C., Richter, J. et al. Inhibition of Inflammatory Pain by CRF at Peripheral, Spinal and Supraspinal Sites: Involvement of Areas Coexpressing CRF Receptors and Opioid Peptides. Neuropsychopharmacol 32, 2530–2542 (2007). https://doi.org/10.1038/sj.npp.1301393
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301393