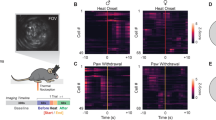
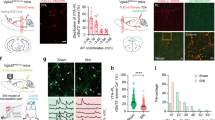
Abstract
Both peripheral and central corticotropin-releasing factor (CRF) systems have been implicated in regulating pain sensation. However, compared with the peripheral, the mechanisms underlying central CRF system in pain modulation have not yet been elucidated, especially at the neural circuit level. The corticoaccumbal circuit, a structure rich in CRF receptors and CRF-positive neurons, plays an important role in behavioral responses to stressors including nociceptive stimuli. The present study was designed to investigate whether and how CRF signaling in this circuit regulated pain sensation under physiological and pathological pain conditions. Our studies employed the viral tracing and circuit-, and cell-specific electrophysiological methods to label the CRF-containing circuit from the medial prefrontal cortex to the nucleus accumbens shell (mPFCCRF-NAcS) and record its neuronal propriety. Combining optogenetic and chemogenetic manipulation, neuropharmacological methods, and behavioral tests, we were able to precisely manipulate this circuit and depict its role in regulation of pain sensation. The current study found that the CRF signaling in the NAc shell (NAcS), but not NAc core, was necessary and sufficient for the regulation of pain sensation under physiological and pathological pain conditions. This process was involved in the CRF-mediated enhancement of excitatory synaptic transmission in the NAcS. Furthermore, we demonstrated that the mPFCCRF neurons monosynaptically connected with the NAcS neurons. Chronic pain increased the protein level of CRF in NAcS, and then maintained the persistent NAcS neuronal hyperactivity through enhancement of this monosynaptic excitatory connection, and thus sustained chronic pain behavior. These findings reveal a novel cell- and circuit-based mechanistic link between chronic pain and the mPFCCRF → NAcS circuit and provide a potential new therapeutic target for chronic pain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper or the supplementary materials.
References
Walsh JJ, Friedman AK, Sun H, Heller EA, Ku SM, Juarez B, et al. Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nat Neurosci. 2014;17:27–29.
Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–57.
Henckens MJ, Deussing JM, Chen A. Region-specific roles of the corticotropin-releasing factor-urocortin system in stress. Nat Rev Neurosci. 2016;17:636–51.
Lv Y, Chen P, Shan QH, Qin XY, Qi XH, Zhou JN. Regulation of Cued Fear Expression via Corticotropin-Releasing-Factor Neurons in the Ventral Anteromedial Thalamic Nucleus. Neurosci Bull. 2021;37:217–28.
Engelke DS, Zhang XO, O’Malley JJ, Fernandez-Leon JA, Li S, Kirouac GJ, et al. A hypothalamic-thalamostriatal circuit that controls approach-avoidance conflict in rats. Nat Commun. 2021;12:2517.
Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3–14.
Binder EB, Nemeroff CB. The CRF system, stress, depression and anxiety-insights from human genetic studies. Mol Psychiatry. 2010;15:574–88.
Chen P, Lou S, Huang ZH, Wang Z, Shan QH, Wang Y, et al. Prefrontal Cortex Corticotropin-Releasing Factor Neurons Control Behavioral Style Selection under Challenging Situations. Neuron. 2020;106:301–15. e307
Abdallah CG, Geha P. Chronic Pain and Chronic Stress: Two Sides of the Same Coin? Chronic Stress. 2017;1:2470547017704763.
Yu W, Caira CM, Del RRSN, Moseley GA, Kash TL. Corticotropin-releasing factor neurons in the bed nucleus of the stria terminalis exhibit sex-specific pain encoding in mice. Sci Rep. 2021;11:12500.
Bourbia N, Ansah OB, Pertovaara A. Corticotropin-releasing factor in the rat amygdala differentially influences sensory-discriminative and emotional-like pain response in peripheral neuropathy. J Pain. 2010;11:1461–71.
McLean SA, Williams DA, Stein PK, Harris RE, Lyden AK, Whalen G, et al. Cerebrospinal fluid corticotropin-releasing factor concentration is associated with pain but not fatigue symptoms in patients with fibromyalgia. Neuropsychopharmacology. 2006;31:2776–82.
Larauche M, Moussaoui N, Biraud M, Bae WK, Duboc H, Million M, et al. Brain corticotropin-releasing factor signaling: Involvement in acute stress-induced visceral analgesia in male rats. Neurogastroenterol Motil. 2019;31:e13489.
Lariviere WR, Melzack R. The role of corticotropin-releasing factor in pain and analgesia. Pain. 2000;84:1–12.
Vit JP, Clauw DJ, Moallem T, Boudah A, Ohara PT, Jasmin L. Analgesia and hyperalgesia from CRF receptor modulation in the central nervous system of Fischer and Lewis rats. Pain. 2006;121:241–60.
Williams DW Jr., Lipton JM, Giesecke AH Jr. Influence of centrally administered peptides on ear withdrawal from heat in the rabbit. Peptides. 1986;7:1095–1100.
Thompson JM, Neugebauer V. Cortico-limbic pain mechanisms. Neurosci Lett. 2019;702:15–23.
Zhu X, Zhou W, Jin Y, Tang H, Cao P, Mao Y, et al. A Central Amygdala Input to the Parafascicular Nucleus Controls Comorbid Pain in Depression. Cell Rep. 2019;29:3847–58. e3845
Zhou W, Jin Y, Meng Q, Zhu X, Bai T, Tian Y, et al. A neural circuit for comorbid depressive symptoms in chronic pain. Nat Neurosci. 2019;22:1649–58.
Takahashi D, Asaoka Y, Kimura K, Hara R, Arakaki S, Sakasai K, et al. Tonic Suppression of the Mesolimbic Dopaminergic System by Enhanced Corticotropin-Releasing Factor Signaling Within the Bed Nucleus of the Stria Terminalis in Chronic Pain Model Rats. J Neurosci. 2019;39:8376–85.
Castro DC, Bruchas MR. A Motivational and Neuropeptidergic Hub: Anatomical and Functional Diversity within the Nucleus Accumbens Shell. Neuron. 2019;102:529–52.
Liu D, Tang QQ, Yin C, Song Y, Liu Y, Yang JX, et al. Brain-derived neurotrophic factor-mediated projection-specific regulation of depressive-like and nociceptive behaviors in the mesolimbic reward circuitry. Pain. 2018;159:175.
An K, Zhao H, Miao Y, Xu Q, Li YF, Ma YQ, et al. A circadian rhythm-gated subcortical pathway for nighttime-light-induced depressive-like behaviors in mice. Nat Neurosci. 2020;23:869–80.
Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66:149–60.
Harris HN, Peng YB. Evidence and explanation for the involvement of the nucleus accumbens in pain processing. Neural Regen Res. 2020;15:597–605.
Guo F, Du Y, Qu FH, Lin SD, Chen Z, Zhang SH. Dissecting the Neural Circuitry for Pain Modulation and Chronic Pain: Insights from Optogenetics. Neurosci Bull. 2022;38:440–52.
Zhang H, Qian YL, Li C, Liu D, Wang L, Wang XY, et al. Brain-Derived Neurotrophic Factor in the Mesolimbic Reward Circuitry Mediates Nociception in Chronic Neuropathic Pain. Biol Psychiatry. 2017;82:608–18.
Makary MM, Polosecki P, Cecchi GA, DeAraujo IE, Barron DS, Constable TR, et al. Loss of nucleus accumbens low-frequency fluctuations is a signature of chronic pain. Proc Natl Acad Sci USA. 2020;117:10015–23.
Seminowicz DA, Remeniuk B, Krimmel SR, Smith MT, Barrett FS, Wulff AB, et al. Pain-related nucleus accumbens function: modulation by reward and sleep disruption. Pain. 2019;160:1196–207.
Mallory GW, Abulseoud O, Hwang SC, Gorman DA, Stead SM, Klassen BT, et al. The nucleus accumbens as a potential target for central poststroke pain. Mayo Clin Proc. 2012;87:1025–31.
Jing PB, Chen XH, Lu HJ, Gao YJ, Wu XB. Enhanced function of NR2C/2D-containing NMDA receptor in the nucleus accumbens contributes to peripheral nerve injury-induced neuropathic pain and depression in mice. Mol Pain. 2022;18:17448069211053255.
Faramarzi G, Zendehdel M, Haghparast A. D1- and D2-like dopamine receptors within the nucleus accumbens contribute to stress-induced analgesia in formalin-related pain behaviours in rats. Eur J Pain. 2016;20:1423–32.
Schank JR, Nelson BS, Damadzic R, Tapocik JD, Yao M, King CE, et al. Neurokinin-1 receptor antagonism attenuates neuronal activity triggered by stress-induced reinstatement of alcohol seeking. Neuropharmacology. 2015;99:106–14.
Smith NK, Kondev V, Hunt TR, Grueter BA. Neuropeptide Y modulates excitatory synaptic transmission and promotes social behavior in the mouse nucleus accumbens. Neuropharmacology. 2022;217:109201.
Morales-Mulia S, Magdaleno-Madrigal VM, Nicolini H, Genis-Mendoza A, Morales-Mulia M. Orexin-A up-regulates dopamine D2 receptor and mRNA in the nucleus accumbens Shell. Mol Biol Rep. 2020;47:9689–97.
Xia SH, Yu J, Huang X, Sesack SR, Huang YH, Schlüter OM, et al. Cortical and Thalamic Interaction with Amygdala-to-Accumbens Synapses. J Neurosci. 2020;40:7119–32.
Baumgartner HM, Schulkin J, Berridge KC. Activating Corticotropin-Releasing Factor Systems in the Nucleus Accumbens, Amygdala, and Bed Nucleus of Stria Terminalis: Incentive Motivation or Aversive Motivation? Biol Psychiatry. 2021;89:1162–75.
Marcinkiewcz CA, Prado MM, Isaac SK, Marshall A, Rylkova D, Bruijnzeel AW. Corticotropin-releasing factor within the central nucleus of the amygdala and the nucleus accumbens shell mediates the negative affective state of nicotine withdrawal in rats. Neuropsychopharmacology. 2009;34:1743–52.
Lemos JC, Wanat MJ, Smith JS, Reyes BA, Hollon NG, Van Bockstaele EJ, et al. Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature. 2012;490:402–6.
Novoa J, Rivero CJ, Pérez-Cardona EU, Freire-Arvelo JA, Zegers J, Yarur HE, et al. Social isolation of adolescent male rats increases anxiety and K(+) -induced dopamine release in the nucleus accumbens: Role of CRF-R1. Eur J Neurosci. 2021;54:4888–905.
Kai Y, Li Y, Sun T, Yin W, Mao Y, Li J, et al. A medial prefrontal cortex-nucleus acumens corticotropin-releasing factor circuitry for neuropathic pain-increased susceptibility to opioid reward. Transl Psychiatry. 2018;8:100.
Chen YW, Rada PV, Bützler BP, Leibowitz SF, Hoebel BG. Corticotropin-releasing factor in the nucleus accumbens shell induces swim depression, anxiety, and anhedonia along with changes in local dopamine/acetylcholine balance. Neuroscience. 2012;206:155–66.
Watanabe M, Narita M. Brain Reward Circuit and Pain. Adv Exp Med Biol. 2018;1099:201–10.
Serafini RA, Pryce KD, Zachariou V. The Mesolimbic Dopamine System in Chronic Pain and Associated Affective Comorbidities. Biol Psychiatry. 2020;87:64–73.
Elman I, Borsook D. Common Brain Mechanisms of Chronic Pain and Addiction. Neuron. 2016;89:11–36.
Ambroggi F, Ghazizadeh A, Nicola SM, Fields HL. Roles of nucleus accumbens core and shell in incentive-cue responding and behavioral inhibition. J Neurosci. 2011;31:6820–30.
Lisman J, Cooper K, Sehgal M, Silva AJ. Memory formation depends on both synapse-specific modifications of synaptic strength and cell-specific increases in excitability. Nat Neurosci. 2018;21:309–14.
Bradley C, Nydam AS, Dux PE, Mattingley JB. State-dependent effects of neural stimulation on brain function and cognition. Nat Rev Neurosci. 2022;23:459–75.
Authement ME, Langlois LD, Shepard RD, Browne CA, Lucki I, Kassis H, et al. A role for corticotropin-releasing factor signaling in the lateral habenula and its modulation by early-life stress. Sci Signal. 2018;11:eaan6480.
Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12:623–37.
Itoga CA, Chen Y, Fateri C, Echeverry PA, Lai JM, Delgado J, et al. New viral-genetic mapping uncovers an enrichment of corticotropin-releasing hormone-expressing neuronal inputs to the nucleus accumbens from stress-related brain regions. J Comp Neurol. 2019;527:2474–87.
Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–79.
Wang H, Qian T, Zhao Y, Zhuo Y, Wu C, Osakada T, et al. A tool kit of highly selective and sensitive genetically encoded neuropeptide sensors. Science. 2023;382:eabq8173.
Xu X, Holmes TC, Luo MH, Beier KT, Horwitz GD, Zhao F, et al. Viral Vectors for Neural Circuit Mapping and Recent Advances in Trans-synaptic Anterograde Tracers. Neuron. 2020;107:1029–47.
Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–79.
Dedic N, Kühne C, Jakovcevski M, Hartmann J, Genewsky AJ, Gomes KS, et al. Chronic CRH depletion from GABAergic, long-range projection neurons in the extended amygdala reduces dopamine release and increases anxiety. Nat Neurosci. 2018;21:803–7.
Sanford CA, Soden ME, Baird MA, Miller SM, Schulkin J, Palmiter RD, et al. A Central Amygdala CRF Circuit Facilitates Learning about Weak Threats. Neuron. 2017;93:164–78.
Chen W, Taché Y, Marvizón JC. Corticotropin-Releasing Factor in the Brain and Blocking Spinal Descending Signals Induce Hyperalgesia in the Latent Sensitization Model of Chronic Pain. Neuroscience. 2018;381:149–58.
Rouwette T, Klemann K, Gaszner B, Scheffer GJ, Roubos EW, Scheenen WJ, et al. Differential responses of corticotropin-releasing factor and urocortin 1 to acute pain stress in the rat brain. Neuroscience. 2011;183:15–24.
Holland PR, Bartsch T. Involvement of corticotrophin-releasing factor and orexin-A in chronic migraine and medication overuse headache: findings from cerebrospinal fluid. Cephalalgia. 2008;28:681–2.
Cabot PJ, Carter L, Gaiddon C, Zhang Q, Schäfer M, Loeffler JP, et al. Immune cell-derived beta-endorphin. Production, release, and control of inflammatory pain in rats. J Clin Invest. 1997;100:142–8.
Andreoli M, Marketkar T, Dimitrov E. Contribution of amygdala CRF neurons to chronic pain. Exp Neurol. 2017;298:1–12.
Mazzitelli M, Yakhnitsa V, Neugebauer B, Neugebauer V. Optogenetic manipulations of CeA-CRF neurons modulate pain- and anxiety-like behaviors in neuropathic pain and control rats. Neuropharmacology. 2022;210:109031.
Kita A, Imano K, Nakamura H. Involvement of corticotropin-releasing factor in the antinociception produced by interleukin-1 in mice. Eur J Pharmacol. 1993;237:317–22.
Deussing JM, Chen A. The Corticotropin-Releasing Factor Family: Physiology of the Stress Response. Physiological reviews. 2018;98:2225–86.
Vita N, Laurent P, Lefort S, Chalon P, Lelias JM, Kaghad M, et al. Primary structure and functional expression of mouse pituitary and human brain corticotrophin releasing factor receptors. FEBS Lett. 1993;335:1–5.
Bale TL, Picetti R, Contarino A, Koob GF, Vale WW, Lee KF. Mice deficient for both corticotropin-releasing factor receptor 1 (CRFR1) and CRFR2 have an impaired stress response and display sexually dichotomous anxiety-like behavior. J Neurosci. 2002;22:193–9.
Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, et al. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24:410–4.
Kishimoto T, Radulovic J, Radulovic M, Lin CR, Schrick C, Hooshmand F, et al. Deletion of crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24:415–9.
Prouty EW, Waterhouse BD, Chandler DJ. Corticotropin releasing factor dose-dependently modulates excitatory synaptic transmission in the noradrenergic nucleus locus coeruleus. Eur J Neurosci. 2017;45:712–22.
Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–84.
Kuner R, Flor H. Structural plasticity and reorganisation in chronic pain. Nat Rev Neurosci. 2016;18:20–30.
Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926.
Neugebauer V, Mazzitelli M, Cragg B, Ji G, Navratilova E, Porreca F. Amygdala, neuropeptides, and chronic pain-related affective behaviors. Neuropharmacology. 2020;170:108052.
Lu VB, Biggs JE, Stebbing MJ, Balasubramanyan S, Todd KG, Lai AY, et al. Brain-derived neurotrophic factor drives the changes in excitatory synaptic transmission in the rat superficial dorsal horn that follow sciatic nerve injury. J Physiol. 2009;587:1013–32.
Yang S, Chang MC. Chronic Pain: Structural and Functional Changes in Brain Structures and Associated Negative Affective States. Int J Mol Sci. 2019;20:3130.
Holahan MR, Kalin NH, Kelley AE. Microinfusion of corticotropin-releasing factor into the nucleus accumbens shell results in increased behavioral arousal and oral motor activity. Psychopharmacology. 1997;130:189–96.
Bryce CA, Floresco SB. Alterations in effort-related decision-making induced by stimulation of dopamine D(1), D(2), D(3), and corticotropin-releasing factor receptors in nucleus accumbens subregions. Psychopharmacology. 2019;236:2699–712.
Lemos JC, Shin JH, Alvarez VA. Striatal Cholinergic Interneurons Are a Novel Target of Corticotropin Releasing Factor. J Neurosci. 2019;39:5647–61.
Kono J, Konno K, Talukder AH, Fuse T, Abe M, Uchida K, et al. Distribution of corticotropin-releasing factor neurons in the mouse brain: a study using corticotropin-releasing factor-modified yellow fluorescent protein knock-in mouse. Brain Struct Funct. 2017;222:1705–32.
Justice NJ, Yuan ZF, Sawchenko PE, Vale W. Type 1 corticotropin-releasing factor receptor expression reported in BAC transgenic mice: implications for reconciling ligand-receptor mismatch in the central corticotropin-releasing factor system. J Comp Neurol. 2008;511:479–96.
Islam J, Kc E, Kim S, Kim HK, Park YS. Stimulating GABAergic Neurons in the Nucleus Accumbens Core Alters the Trigeminal Neuropathic Pain Responses in a Rat Model of Infraorbital Nerve Injury. Int J Mol Sci. 2021;22:8421.
Yao L, Rong Y, Ma X, Li H, Deng D, Chen Y, et al. Extrasynaptic NMDA Receptors Bidirectionally Modulate Intrinsic Excitability of Inhibitory Neurons. J Neurosci. 2022;42:3066–79.
Pan G, Chen Z, Zheng H, Zhang Y, Xu H, Bu G, et al. Compensatory Mechanisms Modulate the Neuronal Excitability in a Kainic Acid-Induced Epilepsy Mouse Model. Front Neural Circuits. 2018;12:48.
Huang YH, Lin Y, Brown TE, Han MH, Saal DB, Neve RL, et al. CREB modulates the functional output of nucleus accumbens neurons: a critical role of N-methyl-D-aspartate glutamate receptor (NMDAR) receptors. J Biol Chem. 2008;283:2751–60.
Hu XT, White FJ. Glutamate receptor regulation of rat nucleus accumbens neurons in vivo. Synapse. 1996;23:208–18.
Cooper DC, White FJ. L-type calcium channels modulate glutamate-driven bursting activity in the nucleus accumbens in vivo. Brain Res. 2000;880:212–8.
Qi C, Guo B, Ren K, Yao H, Wang M, Sun T, et al. Chronic inflammatory pain decreases the glutamate vesicles in presynaptic terminals of the nucleus accumbens. Mol Pain. 2018;14:1744806918781259.
Ziółkowska B. The Role of Mesostriatal Dopamine System and Corticostriatal Glutamatergic Transmission in Chronic Pain. Brain Sci. 2021;11:1311.
Zegers-Delgado J, Aguilera-Soza A, Calderón F, Davidson H, Verbel-Vergara D, Yarur HE, et al. Type 1 Corticotropin-Releasing Factor Receptor Differentially Modulates Neurotransmitter Levels in the Nucleus Accumbens of Juvenile versus Adult Rats. Int J Mol Sci. 2022;23:1080.
Park BY, Lee JJ, Kim HJ, Woo CW, Park H. A neuroimaging marker for predicting longitudinal changes in pain intensity of subacute back pain based on large-scale brain network interactions. Sci Rep. 2020;10:17392.
Löffler M, Levine SM, Usai K, Desch S, Kandić M, Nees F, et al. Corticostriatal circuits in the transition to chronic back pain: The predictive role of reward learning. Cell Rep Med. 2022;3:100677.
Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. 2012;15:1117–9.
Waselus M, Van Bockstaele EJ. Co-localization of corticotropin-releasing factor and vesicular glutamate transporters within axon terminals of the rat dorsal raphe nucleus. Brain Res. 2007;1174:53–65.
Arrigoni E, Saper CB. What optogenetic stimulation is telling us (and failing to tell us) about fast neurotransmitters and neuromodulators in brain circuits for wake-sleep regulation. Curr Opin Neurobiol. 2014;29:165–71.
Bartfai T, Iverfeldt K, Fisone G, Serfözö P. Regulation of the release of coexisting neurotransmitters. Annu Rev Pharmacol Toxicol. 1988;28:285–310.
Acknowledgements
We would like to thank Li Yang, Dong-Yu Zhou, Wei Zheng and Prof. Cheng Xiao for technical supports. The present study was supported by the National Key R&D Program of China—the Sci-Tech Innovation 2030 Major Project (2021ZD0203100), the National Natural Science Foundation of China (82130033, 82293641, 31970937, 82271255, 82101315 and 82271263); Jiangsu Province Innovative and Entrepreneurial Team Program, the Key Project of Nature Science Foundation of Jiangsu Education Department (22KJA320006); the Innovation and Entrepreneurship Program of Xuzhou Medical University (2021CXFUZX002), China Postdoctoral Science Foundation (2022M710771 and 2022M722676), the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX21_2704); the Yangfan Plan of Shanghai Science and Technology Commission (No. 22YF1439600); the China Postdoctoral Science Foundation (No. 2022M710771); the National Natural Science Foundation of China (No. 82201372).
Author information
Authors and Affiliations
Contributions
W.Z., Y.-M.Y., X.-Y.W., S.-H.X., Y.M., H.T., M.T., H.L., Z.X., H.Z., J.-L.C., and H.-L.D. initiated and designed the research. W.Z., S.-H.X., and J.-L.C. wrote the manuscript. W.Z., X.-Y.W., Y.M., Y.-M.Y., H.T., M.T., H.L., P.W. and J.-X.Y. performed all experiments and analyzed and interpreted the results. All the authors approved the submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, W., Yu, YM., Wang, XY. et al. CRF regulates pain sensation by enhancement of corticoaccumbal excitatory synaptic transmission. Mol Psychiatry (2024). https://doi.org/10.1038/s41380-024-02488-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-024-02488-7