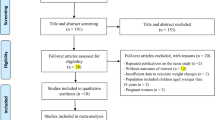
Abstract
Context:
The metabolic syndrome is a complex and multifactorial disorder often associated with type 2 diabetes mellitus and cardiovascular diseases. The liver X receptor α (NR1H3) plays numerous roles in metabolic pathways involved in metabolic syndrome.
Objective:
In the search for susceptibility genes to metabolic syndrome, we hypothesized that common genetic variation in NR1H3 gene influences metabolic syndrome susceptibility.
Design:
Two large French population-based studies (n=1130 and 1160) including overall 664 individuals with and 1626 individuals without metabolic syndrome were genotyped for three polymorphisms (rs12221497, rs11039155 and rs2279239) of NR1H3.
Results:
We found that the −6A allele of rs11039155 was consistently associated with a 30% reduction in risk of metabolic syndrome in the two independent population samples (adjusted OR (95% CI)=0.68 (0.53–0.86), P=0.001 for the combined sample). Moreover, it was associated with an increase in plasma HDL-cholesterol concentrations (P=0.02 for the combined sample). Neither rs12221497 nor rs11039155, both polymorphisms located in the 5′ region of NR1H3, had significant influence on NR1H3 and ATP-binding cassette transporter A1 (ABCA1) gene expression in primary human macrophages.
Conclusions:
These results suggest that NR1H3 plays an important role in the HDL-cholesterol metabolism and in the genetic susceptibility to metabolic syndrome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA et al. Diagnosis and management of the metabolic syndrome. An american heart association/national heart, lung, and blood institute scientific statement. Executive summary. Cardiol Rev 2005; 13: 322–327.
Dallongeville J, Helbecque N, Cottel D, Amouyel P, Meirhaeghe A . The Gly16 → Arg16 and Gln27 → Glu27 polymorphisms of beta2-adrenergic receptor are associated with metabolic syndrome in men. J Clin Endocrinol Metab 2003; 88: 4862–4866.
Meirhaeghe A, Cottel D, Amouyel P, Dallongeville J . Association between peroxisome proliferator-activated receptor gamma haplotypes and the metabolic syndrome in French men and women. Diabetes 2005; 54: 3043–3048.
Willy PJ, Umesono K, Ong ES, Evans RM, Heyman RA, Mangelsdorf DJ . LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev 1995; 9: 1033–1045.
Laffitte BA, Joseph SB, Chen M, Castrillo A, Repa J, Wilpitz D et al. The phospholipid transfer protein gene is a liver X receptor target expressed by macrophages in atherosclerotic lesions. Mol Cell Biol 2003; 23: 2182–2191.
Luo Y, Tall AR . Sterol upregulation of human CETP expression in vitro and in transgenic mice by an LXR element. J Clin Invest 2000; 105: 513–520.
Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ, Argyropoulos G, Walts B et al. The human obesity gene map: the 2005 update. Obesity (Silver Spring) 2006; 14: 529–644.
Dahlman I, Nilsson M, Jiao H, Hoffstedt J, Lindgren CM, Humphreys K et al. Liver X receptor gene polymorphisms and adipose tissue expression levels in obesity. Pharmacogenet Genomics 2006; 16: 881–889.
Ecological analysis of the association between mortality and major risk factors of cardiovascular disease. The world health organization MONICA project. Int J Epidemiol 1994; 23: 505–516.
Marques-Vidal P, Ruidavets JB, Amouyel P, Ducimetiere P, Arveiler D, Montaye M et al. Change in cardiovascular risk factors in France, 1985–1997. Eur J Epidemiol 2004; 19: 25–32.
Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997; 20: 1183–1197.
Abecasis GR, Cookson WO . GOLD – graphical overview of linkage disequilibrium. Bioinformatics 2000; 16: 182–183.
Tregouet DA, Escolano S, Tiret L, Mallet A, Golmard JL . A new algorithm for haplotype-based association analysis: the Stochastic-EM algorithm. Ann Hum Genet 2004; 68: 165–177.
Tregouet DA, Barbaux S, Escolano S, Tahri N, Golmard JL, Tiret L et al. Specific haplotypes of the P-selectin gene are associated with myocardial infarction. Hum Mol Genet 2002; 11: 2015–2023.
Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ . An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature 1996; 383: 728–731.
Costet P, Luo Y, Wang N, Tall AR . Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J Biol Chem 2000; 275: 28240–28245.
Venkateswaran A, Laffitte BA, Joseph SB, Mak PA, Wilpitz DC, Edwards PA et al. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc Natl Acad Sci USA 2000; 97: 12097–12102.
Repa JJ, Turley SD, Lobaccaro JA, Medina J, Li L, Lustig K et al. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science 2000; 289: 1524–1529.
Rigamonti E, Helin L, Lestavel S, Mutka AL, Lepore M, Fontaine C et al. Liver X receptor activation controls intracellular cholesterol trafficking and esterification in human macrophages. Circ Res 2005; 97: 682–689.
Mak PA, Laffitte BA, Desrumaux C, Joseph SB, Curtiss LK, Mangelsdorf DJ et al. Regulated expression of the apolipoprotein E/C-I/C-IV/C-II gene cluster in murine and human macrophages. A critical role for nuclear liver X receptors alpha and beta. J Biol Chem 2002; 277: 31900–31908.
Zhang Y, Repa JJ, Gauthier K, Mangelsdorf DJ . Regulation of lipoprotein lipase by the oxysterol receptors, LXRalpha and LXRbeta. J Biol Chem 2001; 276: 43018–43024.
Lund EG, Peterson LB, Adams AD, Lam MH, Burton CA, Chin J et al. Different roles of liver X receptor alpha and beta in lipid metabolism: effects of an alpha-selective and a dual agonist in mice deficient in each subtype. Biochem Pharmacol 2006; 71: 453–463.
Jensen MK, Pai JK, Mukamal KJ, Overvad K, Rimm EB . Common genetic variation in the ATP-binding cassette transporter A1, plasma lipids, and risk of coronary heart disease. Atherosclerosis 2007, in press.
Sviridov D, Nestel PJ . Genetic factors affecting HDL levels, structure, metabolism and function. Curr Opin Lipidol 2007; 18: 157–163.
Kozak M . At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J Mol Biol 1987; 196: 947–950.
Acknowledgements
The Lille and Toulouse MONICA population studies were supported by Grants from the Conseil Régional du Nord-Pas de Calais, ONIVINS, Parke-Davis Laboratory, the Mutuelle Générale de l'Education Nationale (MGEN), the Groupe Fournier, the Réseau National de Santé Publique, the Direction Générale de la Santé, the Institut National de la Santé et de la Recherche Médicale (INSERM), the Institut Pasteur de Lille and the Unité d'Evaluation du Centre Hospitalier et Universitaire de Lille. This study was part of the CRESCENDO (Consortium for Research into Nuclear Receptors in Development and Aging) consortium funded by the Commission's Sixth Framework Programme (integrated project LSHM-CT-2005-018652). We thank Dr Rébecca Dièvart and Lionel Helin for technical experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on International Journal of Obesity website (http://www.nature.com/ijo)
Supplementary information
Rights and permissions
About this article
Cite this article
Legry, V., Cottel, D., Ferrières, J. et al. Association between liver X receptor α gene polymorphisms and risk of metabolic syndrome in French populations. Int J Obes 32, 421–428 (2008). https://doi.org/10.1038/sj.ijo.0803705
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0803705
Keywords
This article is cited by
-
Significant association of LXRβ (NR1H2) polymorphisms (rs28514894, rs2303044) with type 2 diabetes mellitus and laboratory characteristics
Journal of Diabetes & Metabolic Disorders (2021)
-
Emerging role of liver X receptors in cardiac pathophysiology and heart failure
Basic Research in Cardiology (2016)
-
Liver X receptors: from cholesterol regulation to neuroprotection—a new barrier against neurodegeneration in amyotrophic lateral sclerosis?
Cellular and Molecular Life Sciences (2016)
-
Genetic variations in SREBP-1 and LXRα are not directly associated to PCOS but contribute to the physiological specifics of the syndrome
Molecular Biology Reports (2012)
-
A common polymorphism in NR1H2 (LXRbeta) is associated with preeclampsia
BMC Medical Genetics (2011)