Featured
-
-
Letter |
 uORF-mediated translation allows engineered plant disease resistance without fitness costs
uORF-mediated translation allows engineered plant disease resistance without fitness costs
WebIn both laboratory and field studies, engineering translational control of immune mediator production in Arabidopsis and rice confers disease resistance, without compromising plant fitness.
- Guoyong Xu
- , Meng Yuan
- & Xinnian Dong
-
Letter |
 Chemical intervention in plant sugar signalling increases yield and resilience
Chemical intervention in plant sugar signalling increases yield and resilience
Treatment with signalling precursors of trehalose-6-phosphate allows light-triggered release of trehalose-6-phosphate in Arabidopsis thaliana and increases the yield and drought resistance of spring wheat (Triticum aestivum).
- Cara A. Griffiths
- , Ram Sagar
- & Benjamin G. Davis
-
Letter
| Open Access The genome of the seagrass Zostera marina reveals angiosperm adaptation to the sea
The genome of the seagrass Zostera marina reveals angiosperm adaptation to the sea
Whole-genome sequencing of the seagrass Zostera, representing the first marine angiosperm genome to be fully sequenced, provides insight into the evolutionary changes associated with a transition to a marine environment in this angiosperm lineage.
- Jeanine L. Olsen
- , Pierre Rouzé
- & Yves Van de Peer
-
Letter |
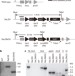 A faster Rubisco with potential to increase photosynthesis in crops
A faster Rubisco with potential to increase photosynthesis in crops
The plant enzyme Rubisco is the main enzyme converting atmospheric carbon dioxide into biological compounds, however, this enzymatic process is inefficient in vascular plants; this study demonstrates that tobacco plants can be engineered to fix carbon with a faster cyanobacterial Rubisco, thus potentially improving plant photosynthesis.
- Myat T. Lin
- , Alessandro Occhialini
- & Maureen R. Hanson
-
Letter |
 The protein kinase Pstol1 from traditional rice confers tolerance of phosphorus deficiency
The protein kinase Pstol1 from traditional rice confers tolerance of phosphorus deficiency
A gene that is present in phosphate-deficiency-tolerant rice but absent from modern rice varieties is characterized and named phosphorus-starvation tolerance 1 (PSTOL1); overexpression of PSTOL1 in rice species that naturally lack this gene confers tolerance to low phosphorus conditions, a finding that may have implications for agricultural productivity in rice-growing countries.
- Rico Gamuyao
- , Joong Hyoun Chin
- & Sigrid Heuer
-
Article |
 Coupled chaperone action in folding and assembly of hexadecameric Rubisco
Coupled chaperone action in folding and assembly of hexadecameric Rubisco
Form I Rubisco, one of the most abundant proteins in nature, catalyses the fixation of atmospheric CO2 in photosynthesis. The limited catalytic efficiency of Rubisco has sparked extensive efforts to re-engineer the enzyme to enhance agricultural productivity. To bring this goal closer, the formation of cyanobacterial form I Rubisco is now analysed by in vitro reconstitution and cryo-electron microscopy.
- Cuimin Liu
- , Anna L. Young
- & Manajit Hayer-Hartl

 Export of defensive glucosinolates is key for their accumulation in seeds
Export of defensive glucosinolates is key for their accumulation in seeds