Featured
-
-
Article
| Open Access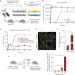 The extracellular chaperone Clusterin enhances Tau aggregate seeding in a cellular model
The extracellular chaperone Clusterin enhances Tau aggregate seeding in a cellular model
Variants of the extracellular chaperone Clusterin are associated with Alzheimer’s disease (AD) and Clusterin levels are elevated in AD patient brains. Here, the authors show that Clusterin binds to oligomeric Tau, which enhances the seeding capacity of Tau aggregates upon cellular uptake. They also demonstrate that Tau/Clusterin complexes enter cells via the endosomal pathway, resulting in damage to endolysosomes and entry into the cytosol, where they induce the aggregation of endogenous, soluble Tau.
- Patricia Yuste-Checa
- , Victoria A. Trinkaus
- & F. Ulrich Hartl
-
Article
| Open Access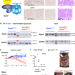 The HSP90/R2TP assembly chaperone promotes cell proliferation in the intestinal epithelium
The HSP90/R2TP assembly chaperone promotes cell proliferation in the intestinal epithelium
RPAP3 is a subunit of the R2TP complex, a co-chaperone of HSP90, with substrate proteins involved in transcription, ribosome biogenesis, DNA repair and cell growth. Here the authors report that deletion of Rpap3 abrogates cell proliferation and homeostasis in mouse intestine, partly through destabilization of PI3K-like kinases, while elevated RPAP3 levels in colorectal tumors are associated with poor prognosis.
- Chloé Maurizy
- , Claire Abeza
- & Bérengère Pradet-Balade
-
Article
| Open Access Structural basis for inhibition of the AAA-ATPase Drg1 by diazaborine
Structural basis for inhibition of the AAA-ATPase Drg1 by diazaborine
The AAA-ATPase Drg1 is a key factor in eukaryotic ribosome biogenesis that initiates cytoplasmic maturation of the large subunit. Here the authors report the structure of Drg1 in complex with its specific inhibitor diazaborine and provide insight into the mechanism of inhibition and specificity of this class of inhibitors.
- Michael Prattes
- , Irina Grishkovskaya
- & Helmut Bergler
-
Article
| Open Access Qki activates Srebp2-mediated cholesterol biosynthesis for maintenance of eye lens transparency
Qki activates Srebp2-mediated cholesterol biosynthesis for maintenance of eye lens transparency
Eye lens cells are highly enriched in cholesterol that sustains lens transparency, and disruption of cholesterol biosynthesis leads to cataracts. The authors show that cholesterol biosynthesis regulated by Qki is essential for maintenance of membrane integrity of lens cells and proper protein folding.
- Seula Shin
- , Hao Zhou
- & Jian Hu
-
Article
| Open Access Structural basis of substrate recognition and thermal protection by a small heat shock protein
Structural basis of substrate recognition and thermal protection by a small heat shock protein
Structural insights into the small heat shock proteins (sHsps) complexes with their substrates are sparse. Here, cryo-EM structure of a plastid sHsp, Hsp21, in complex with a bona fide substrate 1-deoxy-D-xylulose 5-phosphate synthase (DXPS), suggests the anti-aggregation mechanism employed by sHsps.
- Chuanyang Yu
- , Stephen King Pong Leung
- & Wilson Chun Yu Lau
-
Article
| Open Access Specificity of AMPylation of the human chaperone BiP is mediated by TPR motifs of FICD
Specificity of AMPylation of the human chaperone BiP is mediated by TPR motifs of FICD
The ER chaperone BiP is critical for the unfolded protein response and tightly regulated through reversible AMPylation by FICD, but the structural basis is unknown. Here the authors use thiol-reactive nucleotide derivatives to stabilize the transient FICD:BiP complex and determine its crystal structure.
- Joel Fauser
- , Burak Gulen
- & Aymelt Itzen
-
Article
| Open Access The landscape of molecular chaperones across human tissues reveals a layered architecture of core and variable chaperones
The landscape of molecular chaperones across human tissues reveals a layered architecture of core and variable chaperones
Tissue-specific differences in protein folding capacities are poorly understood. Here, the authors show that the human chaperone system consists of ubiquitous core chaperones and tissue-specific variable chaperones, perturbation of which leads to tissue-specific phenotypes.
- Netta Shemesh
- , Juman Jubran
- & Esti Yeger-Lotem
-
Article
| Open Access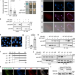 UXT chaperone prevents proteotoxicity by acting as an autophagy adaptor for p62-dependent aggrephagy
UXT chaperone prevents proteotoxicity by acting as an autophagy adaptor for p62-dependent aggrephagy
p62/SQSTM1 acts as a key mediator in the selective autophagy of protein aggregates, or aggrephagy. Here the authors identify the prefoldin-like chaperone UXT as an autophagy adaptor of p62 dependent aggrephagy and show that ectopic UXT expression delays motor neuron degeneration in a Xenopus model.
- Min Ji Yoon
- , Boyoon Choi
- & Chungho Kim
-
Article
| Open Access Regulatory inter-domain interactions influence Hsp70 recruitment to the DnaJB8 chaperone
Regulatory inter-domain interactions influence Hsp70 recruitment to the DnaJB8 chaperone
The Hsp70/Hsp40 system plays an important role in maintaining cellular proteostasis but so far it is not well understood how Hsp70 proteins are recruited to specific Hsp40 co-chaperones. Here, the authors combine biochemical and biophysical approaches to characterise the oligomeric mammalian Hsp40 DnaJB8. They identify an intra-oligomer DnaJB8 interaction between the N-terminal J-Domain and the C-terminal domain that occludes the J-Domain surface that binds Hsp70 and propose a model for DnaJB8-Hsp70 recruitment.
- Bryan D. Ryder
- , Irina Matlahov
- & Lukasz A. Joachimiak
-
Article
| Open Access Mechanism of the small ATP-independent chaperone Spy is substrate specific
Mechanism of the small ATP-independent chaperone Spy is substrate specific
Spy is an ATP independent chaperone that can act as both a holdase and a foldase towards topologically simple substrates. Assessing the interaction of Spy and apoflavodoxin, a complex client, the authors show that Spy’s activity is substrate specific. Spy binds partially unfolded states of apoflavodoxin tightly, which limits the possibility of folding and converts Spy to a pure holdase.
- Rishav Mitra
- , Varun V. Gadkari
- & James C. A. Bardwell
-
Article
| Open Access Structural elements in the flexible tail of the co-chaperone p23 coordinate client binding and progression of the Hsp90 chaperone cycle
Structural elements in the flexible tail of the co-chaperone p23 coordinate client binding and progression of the Hsp90 chaperone cycle
p23 is a co-chaperone of Hsp90 but its mode of action is mechanistically not well understood. Here, the authors combine in vitro and yeast in vivo assays, biochemical measurements and NMR experiments to characterize p23 and identify two conserved helical elements in the intrinsically disordered C-terminal tail of p23 that together with the folded domain of p23 regulate the Hsp90 ATPase activity and affect the binding and maturation of Hsp90 clients.
- Maximilian M. Biebl
- , Abraham Lopez
- & Johannes Buchner
-
Article
| Open Access Ribosome-bound Get4/5 facilitates the capture of tail-anchored proteins by Sgt2 in yeast
Ribosome-bound Get4/5 facilitates the capture of tail-anchored proteins by Sgt2 in yeast
The guided entry of tail-anchored proteins (GET) pathway assists in the delivery of such proteins to the ER. Here, the authors reveal that the pathway components Get4/5 probe a region near the ribosomal exit tunnel. Upon emergence of a client protein, Get4/5 recruits Sgt2 and initiates the targeting phase of the pathway.
- Ying Zhang
- , Evelina De Laurentiis
- & Sabine Rospert
-
Article
| Open Access Microthermal-induced subcellular-targeted protein damage in cells on plasmonic nanosilver-modified surfaces evokes a two-phase HSP-p97/VCP response
Microthermal-induced subcellular-targeted protein damage in cells on plasmonic nanosilver-modified surfaces evokes a two-phase HSP-p97/VCP response
Existing methods for inflicting cellular heat shock are limited by the time delay in achieving the desired temperature and the spatial precision that can be achieved. Here the authors report a method to induce focused thermal protein damage using plasmonic silver nanoparticles.
- Martin Mistrik
- , Zdenek Skrott
- & Jiri Bartek
-
Article
| Open Access UPRmt scales mitochondrial network expansion with protein synthesis via mitochondrial import in Caenorhabditis elegans
UPRmt scales mitochondrial network expansion with protein synthesis via mitochondrial import in Caenorhabditis elegans
The mitochondrial network expands to accommodate cell growth, but how scaling occurs is unclear. Here, the authors show in C. elegans that ATFS-1 mitochondrial import is reduced when mitochondrial proteins are highly expressed, activating the unfolded protein response and causing expansion.
- Tomer Shpilka
- , YunGuang Du
- & Cole M. Haynes
-
Article
| Open Access Functional cooperativity between the trigger factor chaperone and the ClpXP proteolytic complex
Functional cooperativity between the trigger factor chaperone and the ClpXP proteolytic complex
ClpXP is the main ATP-dependent proteolytic complex in bacteria, is essential for maintaining cellular protein homeostasis and is also critical for bacterial pathogenesis. Here, the authors establish a functional link between ClpXP and trigger actor, a chaperone involved in the early stages of protein folding.
- Kamran Rizzolo
- , Angela Yeou Hsiung Yu
- & Walid A. Houry
-
Article
| Open Access LONP1 and mtHSP70 cooperate to promote mitochondrial protein folding
LONP1 and mtHSP70 cooperate to promote mitochondrial protein folding
Most mitochondrial proteins are imported from the cytosol and must fold in the mitochondria. Here, the authors show that the mitochondrial protease LONP1 plays a critical role in the mtHSP70 chaperone system independently of its protease activity.
- Chun-Shik Shin
- , Shuxia Meng
- & David C. Chan
-
Article
| Open Access Sis1 potentiates the stress response to protein aggregation and elevated temperature
Sis1 potentiates the stress response to protein aggregation and elevated temperature
Identifying factors that enable cells to induce a potent stress response to amyloid-like aggregation can provide further insight into the mechanism of stress regulation. Here, the authors express polyglutamine-expanded Huntingtin as a model disease protein in yeast cells and perform a genetic screen for chaperone factors that allow yeast cells to activate a potent stress response. They identify Sis1, an essential Hsp40 co-chaperone of Hsp70, as a critical sensor of proteotoxic stress and further show that both Sis1 and its mammalian homolog DnaJB6 regulate the magnitude of the cellular heat stress response, indicating that this mechanism is conserved.
- Courtney L. Klaips
- , Michael H. M. Gropp
- & F. Ulrich Hartl
-
Article
| Open Access The Hsp70-Hsp90 co-chaperone Hop/Stip1 shifts the proteostatic balance from folding towards degradation
The Hsp70-Hsp90 co-chaperone Hop/Stip1 shifts the proteostatic balance from folding towards degradation
Hop, also known as Stip1 or Sti1, facilitates substrate transfer between the Hsp70 and Hsp90 molecular chaperones. Characterization of proteostasis-related pathways in STIP1 knock-out cell lines reveals that in eukaryotes Stip1 modulates the balance between protein folding and degradation.
- Kaushik Bhattacharya
- , Lorenz Weidenauer
- & Didier Picard
-
Article
| Open Access A ribosome-associated chaperone enables substrate triage in a cotranslational protein targeting complex
A ribosome-associated chaperone enables substrate triage in a cotranslational protein targeting complex
Biochemistry combined with biophysical measurements and mathematical modeling offer insight into the mechanism by which the cotranslational chaperone, nascent polypeptide-associated complex (NAC), modulates substrate selection by signal recognition particle (SRP) and reduces aberrant, nonspecific targeting of ribosomes to the ER.
- Hao-Hsuan Hsieh
- , Jae Ho Lee
- & Shu-ou Shan
-
Article
| Open Access Distinct metabolic states of a cell guide alternate fates of mutational buffering through altered proteostasis
Distinct metabolic states of a cell guide alternate fates of mutational buffering through altered proteostasis
Changes in osmotic homeostasis alter metabolites and therefore chemical milieu of the cells. Here, the authors show that altering metabolites in E. coli also change the cellular capacity for buffering mutations that impair protein folding and influences proteostasis irrespective of molecular chaperones
- Kanika Verma
- , Kanika Saxena
- & Kausik Chakraborty
-
Article
| Open Access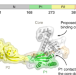 Inter-domain dynamics in the chaperone SurA and multi-site binding to its outer membrane protein clients
Inter-domain dynamics in the chaperone SurA and multi-site binding to its outer membrane protein clients
The chaperone SurA is involved in outer membrane protein (OMP) biogenesis in Gram-negative bacteria, but its mechanism of action is not fully understood. Combining mass spectrometric, biophysical and computational approaches, the authors here show how the conformational dynamics of SurA facilitate OMP binding.
- Antonio N. Calabrese
- , Bob Schiffrin
- & Sheena E. Radford
-
Article
| Open Access The ribosome-associated complex RAC serves in a relay that directs nascent chains to Ssb
The ribosome-associated complex RAC serves in a relay that directs nascent chains to Ssb
The ribosome-associated complex (RAC), which contains the Hsp40 protein Zuo1 and the non-canonical Hsp70 protein Ssz1 forms a chaperone triad with the fungal-specific Hsp70 protein Ssb. Here the authors combine X-ray crystallography, crosslinking and biochemical experiments and present the structure of the Zuo1 N-terminus bound to Ssz1 and demonstrate that Ssz1 is an active chaperone for nascent chains.
- Ying Zhang
- , Genís Valentín Gesé
- & Irmgard Sinning
-
Article
| Open Access Conformational dynamics modulate the catalytic activity of the molecular chaperone Hsp90
Conformational dynamics modulate the catalytic activity of the molecular chaperone Hsp90
The chaperone Hsp90 uses the free energy from ATP hydrolysis to control the folding of client proteins in eukaryotic cells. Here the authors provide mechanistic insights into how its catalytic activity is coupled to conformational changes by combining large-scale molecular simulations with NMR, FRET and SAXS experiments.
- Sophie L. Mader
- , Abraham Lopez
- & Ville R. I. Kaila
-
Article
| Open Access A methylated lysine is a switch point for conformational communication in the chaperone Hsp90
A methylated lysine is a switch point for conformational communication in the chaperone Hsp90
Methylation of a lysine residue in Hsp90 is a recently discovered post-translational modification but the mechanistic effects of this modification have remained unknown so far. Here the authors combine biochemical and biophysical approaches, molecular dynamics (MD) simulations and functional experiments with yeast and show that this lysine is a switch point, which specifically modulates conserved Hsp90 functions including co-chaperone regulation and client activation.
- Alexandra Rehn
- , Jannis Lawatscheck
- & Johannes Buchner
-
Article
| Open Access Arginine π-stacking drives binding to fibrils of the Alzheimer protein Tau
Arginine π-stacking drives binding to fibrils of the Alzheimer protein Tau
Tau fibril formation is a hallmark of Alzheimer’s disease. Here the authors reveal an aggregation-dependent protein interaction pattern of Tau and further show that π-stacking of the arginine side-chains drives aberrant protein binding to Tau fibrils.
- Luca Ferrari
- , Riccardo Stucchi
- & Stefan G. D. Rüdiger
-
Article
| Open Access Chaperone mediated detection of small molecule target binding in cells
Chaperone mediated detection of small molecule target binding in cells
Quantitative profiling of small molecule-protein binding in cells can aid basic biochemical research and drug discovery. Here, the authors develop the Heat Shock Protein Inhibition Protein Stability Assay (HIPStA) as a high-throughput method to assess cellular target engagement and identify new drug targets.
- Kelvin F. Cho
- , Taylur P. Ma
- & Robert A. Blake
-
Article
| Open Access Chaperone-mediated ordered assembly of the SAGA and NuA4 transcription co-activator complexes in yeast
Chaperone-mediated ordered assembly of the SAGA and NuA4 transcription co-activator complexes in yeast
Transcription initiation involves the coordinated assembly and activity of large multimeric complexes. Here the authors report on the chaperone-mediated ordered assembly of the SAGA and NuA4 transcription co-activator complexes in fission yeast, providing insight into the de novo assembly of transcriptional complexes and the contribution of dedicated chaperones to this process.
- Alberto Elías-Villalobos
- , Damien Toullec
- & Dominique Helmlinger
-
Article
| Open Access Cellular sequestrases maintain basal Hsp70 capacity ensuring balanced proteostasis
Cellular sequestrases maintain basal Hsp70 capacity ensuring balanced proteostasis
The sequestration of misfolded proteins into large assemblies by sequestrases is now considered as the third pillar in protein quality control besides chaperones and proteases. Here the authors characterise the functions of the sequestrases Hsp42 and Btn2 in the proteostasis network of S. cerevisiae and find that they protect cells from too exhaustive depletion of the Hsp70 system.
- Chi-ting Ho
- , Tomas Grousl
- & Axel Mogk
-
Article
| Open Access Protein folding while chaperone bound is dependent on weak interactions
Protein folding while chaperone bound is dependent on weak interactions
Spy is an ATP independent chaperone that allows folding of its client protein Im7 while continuously bound to Spy. Here the authors employ kinetics measurements to study the folding of another Spy client protein SH3 and find that Spy’s ability to allow a client to fold while bound is inversely related to how strongly it interacts with that client.
- Kevin Wu
- , Frederick Stull
- & James C. A. Bardwell
-
Article
| Open Access Molecular features of the UNC-45 chaperone critical for binding and folding muscle myosin
Molecular features of the UNC-45 chaperone critical for binding and folding muscle myosin
Myosin, a motor protein essential for intracellular transport to muscle contraction, requires a chaperone UNC-45 for folding and assembly. Here authors use in vitro reconstitution and structural biology to characterize the interplay between UNC-45 and muscle myosin MHC-B.
- Doris Hellerschmied
- , Anita Lehner
- & Tim Clausen
-
Article
| Open Access The molecular basis of chaperone-mediated interleukin 23 assembly control
The molecular basis of chaperone-mediated interleukin 23 assembly control
It is unclear how unassembled secretory pathway proteins are discriminated from misfolded ones. Here the authors combine biophysical and cellular experiments to study the folding of heterodimeric interleukin 23 and describe how ER chaperones recognize unassembled proteins and aid their assembly into protein complexes while preventing the premature degradation of unassembled units.
- Susanne Meier
- , Sina Bohnacker
- & Matthias J. Feige
-
Article
| Open Access HSP90 inhibitors stimulate DNAJB4 protein expression through a mechanism involving N6-methyladenosine
HSP90 inhibitors stimulate DNAJB4 protein expression through a mechanism involving N6-methyladenosine
Cells respond to heat shock with transcriptional and translational adaptations but how HSP90 inhibition alters the heat shock proteome is largely unclear. Here, the authors analyze proteome changes upon HSP90 inhibition and show that an m6A-mediated mechanism contributes to the heat shock-induced upregulation of DNAJB4.
- Weili Miao
- , Lin Li
- & Yinsheng Wang
-
Article
| Open Access The Hsp90 isoforms from S. cerevisiae differ in structure, function and client range
The Hsp90 isoforms from S. cerevisiae differ in structure, function and client range
S. cerevisiae encodes two Hsp90 isoforms, the constitutively expressed Hsc82 and stress-inducible Hsp82 that are 97% identical. Here, the authors combine a range of biophysical methods and show that they differ in their enzymatic properties, resilience to stress and client range, which suggests that they evolved to provide fine-tuned chaperone assistance under physiological and stress conditions.
- Hannah Girstmair
- , Franziska Tippel
- & Johannes Buchner
-
Article
| Open Access Hsp70 and Hsp40 inhibit an inter-domain interaction necessary for transcriptional activity in the androgen receptor
Hsp70 and Hsp40 inhibit an inter-domain interaction necessary for transcriptional activity in the androgen receptor
Hsp chaperones stabilize the inactive conformation of androgen receptor (AR) and are released upon hormone-induced AR activation. Here, the authors locate the Hsp binding region on AR, and show that Hsp70 reduces AR aggregation and promotes AR degradation in cellular and mouse models of a neuromuscular disorder.
- Bahareh Eftekharzadeh
- , Varuna C. Banduseela
- & Xavier Salvatella
-
Article
| Open Access Structural and functional analysis of the role of the chaperonin CCT in mTOR complex assembly
Structural and functional analysis of the role of the chaperonin CCT in mTOR complex assembly
β-propeller domains are an important class of folding substrates for the eukaryotic cytosolic chaperonin CTT. Here the authors find that CTT contributes to the folding and assembly of two β-propeller proteins from mTOR complexes, mLST8 and Raptor, and determine the 4.0 Å cryoEM structure of a human mLST8-CCT intermediate that shows mLST8 in a near-native state.
- Jorge Cuéllar
- , W. Grant Ludlam
- & José M. Valpuesta
-
Article
| Open Access Hsp90 middle domain phosphorylation initiates a complex conformational program to recruit the ATPase-stimulating cochaperone Aha1
Hsp90 middle domain phosphorylation initiates a complex conformational program to recruit the ATPase-stimulating cochaperone Aha1
Phosphorylation of Tyr313 in Hsp90 enhances the binding to its activator Aha1, but the underlying mechanism is unknown. Here, the authors study the structural consequences of Tyr313 phosphorylation, showing that it serves as a conformational switch in Hsp90 that enables Aha1 recruitment.
- Wanping Xu
- , Kristin Beebe
- & Len Neckers
-
Article
| Open Access The conserved NxNNWHW motif in Aha-type co-chaperones modulates the kinetics of Hsp90 ATPase stimulation
The conserved NxNNWHW motif in Aha-type co-chaperones modulates the kinetics of Hsp90 ATPase stimulation
Hsp90 is a molecular chaperone that acts together with co-chaperones to ensure folding and activation of many client proteins. Here authors show that a N-terminal motif in Aha-type co-chaperones modulates the apparent affinity of Hsp90 for nucleotide substrates.
- Rebecca Mercier
- , Annemarie Wolmarans
- & Paul LaPointe
-
Article
| Open Access Local unfolding of the HSP27 monomer regulates chaperone activity
Local unfolding of the HSP27 monomer regulates chaperone activity
The small heat-shock protein HSP27 occurs predominantly in oligomeric forms, which makes its structural characterisation challenging. Here the authors employ CPMG and high-pressure NMR with native mass spectrometry and biophysical assays to show that the active monomeric form of HSP27 is substantially disordered and highly chaperone-active.
- T. Reid Alderson
- , Julien Roche
- & Andrew J. Baldwin
-
Article
| Open Access Structural insights into chaperone addiction of toxin-antitoxin systems
Structural insights into chaperone addiction of toxin-antitoxin systems
SecB homologs can be associated with stress-responsive type II toxin–antitoxin (TA) systems and form tripartite toxin-antitoxin-chaperone systems (TAC). Here the authors provide structural insights into TACs by presenting the crystal structure of the M. tuberculosis TA-associated SecB chaperone in complex with the C-terminal ChAD (chaperone addiction) extension of the antitoxin HigA1.
- Valérie Guillet
- , Patricia Bordes
- & Lionel Mourey
-
Article
| Open Access MANF antagonizes nucleotide exchange by the endoplasmic reticulum chaperone BiP
MANF antagonizes nucleotide exchange by the endoplasmic reticulum chaperone BiP
The role of mesencephalic astrocyte-derived neurotrophic factor (MANF) in maintenance of protein folding homeostasis inside the ER has remained unclear. Here the authors determine the structure of the complex between MANF and the ER-localized chaperone BiP and provide evidence that MANF serves as an anti-nucleotide exchange factor for BiP.
- Yahui Yan
- , Claudia Rato
- & David Ron
-
Article
| Open Access Myopathy associated BAG3 mutations lead to protein aggregation by stalling Hsp70 networks
Myopathy associated BAG3 mutations lead to protein aggregation by stalling Hsp70 networks
BAG3 is a Hsp70 co-chaperone that is highly expressed in muscles. Here the authors show that several myofibrillar myopathy causing BAG3 mutations are not impaired in Hsp70 binding, but rather impair the ADP-ATP exchange step of the Hsp70 cycle, causing the aggregation of BAG3, Hsp70 and Hsp70 clients and leading to a collapse of protein homeostasis.
- Melanie Meister-Broekema
- , Rebecca Freilich
- & Harm H. Kampinga
-
Article
| Open Access Competing protein-protein interactions regulate binding of Hsp27 to its client protein tau
Competing protein-protein interactions regulate binding of Hsp27 to its client protein tau
Small heat shock proteins (sHSPs) limit the aggregation of proteins, such as tau. Here the authors show that Hsp27 recognizes two aggregation-prone regions of tau and that this interaction competes with Hsp27 oligomerization.
- Rebecca Freilich
- , Miguel Betegon
- & Jason E. Gestwicki
-
Article
| Open Access Structure and pro-toxic mechanism of the human Hsp90/PPIase/Tau complex
Structure and pro-toxic mechanism of the human Hsp90/PPIase/Tau complex
The chaperone Hsp90 plays a key role in maintaining cellular homeostasis. Here the authors provide structural insights into substrate recognition and the pro-folding mechanism of Hsp90/co-chaperone complexes by studying the complex of Hsp90 with its co-chaperone FKBP51 and the substrate Tau bound Hsp90/FKBP51 ternary complex using a NMR based integrative approach.
- Javier Oroz
- , Bliss J. Chang
- & Markus Zweckstetter
-
Article
| Open Access Protein polarization driven by nucleoid exclusion of DnaK(HSP70)–substrate complexes
Protein polarization driven by nucleoid exclusion of DnaK(HSP70)–substrate complexes
Many bacterial proteins exhibit spatially defined localization important for function. Here the authors show that the polar localization of Shigella IpaC type III secretion substrate is mediated by its interaction with the DnaK chaperone and occlusion by the bacterial nucleoid.
- Clémence Collet
- , Jenny-Lee Thomassin
- & Guy Tran Van Nhieu
-
Article
| Open Access Rapid labelling and covalent inhibition of intracellular native proteins using ligand-directed N-acyl-N-alkyl sulfonamide
Rapid labelling and covalent inhibition of intracellular native proteins using ligand-directed N-acyl-N-alkyl sulfonamide
Chemically modifying proteins is hard to achieve selectively without purifying the target protein. Here, the authors present a method to modify proteins on lysine residues in living cells quicker than via known approaches and show that it can be used to develop protein covalent inhibitors.
- Tomonori Tamura
- , Tsuyoshi Ueda
- & Itaru Hamachi
-
Article
| Open Access A switch point in the molecular chaperone Hsp90 responding to client interaction
A switch point in the molecular chaperone Hsp90 responding to client interaction
The heat shock protein 90 (Hsp90) chaperone undergoes large conformational changes during its functional cycle. Here the authors combine in vivo, biochemical, biophysical and computational approaches and provide insights into the allosteric regulation of Hsp90 by identifying and characterizing a switch point in the Hsp90 middle domain.
- Daniel Andreas Rutz
- , Qi Luo
- & Johannes Buchner
-
Article
| Open Access TRiC controls transcription resumption after UV damage by regulating Cockayne syndrome protein A
TRiC controls transcription resumption after UV damage by regulating Cockayne syndrome protein A
An integrated network of chaperones and protein degradation machineries called the proteostasis network (PN) is required to maintain protein homeostasis. Here the authors show that one of the components of the PN, the chaperonin TRiC, interacts with the core transcription-coupled nucleotide excision repair protein CSA to ensure its assembly into the CRLCSA complex.
- Alex Pines
- , Madelon Dijk
- & Haico van Attikum
-
Article
| Open Access Aggregating sequences that occur in many proteins constitute weak spots of bacterial proteostasis
Aggregating sequences that occur in many proteins constitute weak spots of bacterial proteostasis
Aggregation is sequence-specific and nucleated by short aggregating protein segments (APR). Here authors use a multidisciplinary approach to show that in E.coli some frequently occurring APRs lead to protein aggregation and ultimately bacterial cell death, which could serve as antibacterial strategy.
- Ladan Khodaparast
- , Laleh Khodaparast
- & Joost Schymkowitz
-
Article
| Open Access UFD-2 is an adaptor-assisted E3 ligase targeting unfolded proteins
UFD-2 is an adaptor-assisted E3 ligase targeting unfolded proteins
The U-box ubiquitin ligase UFD-2 is one of the most abundant components of the ubiquitin proteasome system in muscle cells. Here the authors perform in vitro and in vivo experiments and show that UFD-2 has E3 ligase activity and that it ubiquitinates unfolded myosin using the C. elegans myosin chaperone UNC-45 as an adaptor protein.
- Doris Hellerschmied
- , Max Roessler
- & Tim Clausen

 Structures of a deAMPylation complex rationalise the switch between antagonistic catalytic activities of FICD
Structures of a deAMPylation complex rationalise the switch between antagonistic catalytic activities of FICD