Featured
-
-
Article
| Open Access Living GenoChemetics by hyphenating synthetic biology and synthetic chemistry in vivo
Living GenoChemetics by hyphenating synthetic biology and synthetic chemistry in vivo
Coupling synthetic biology and chemical reactions in cells is a challenging task. The authors engineer bacteria capable of generating bromo-metabolites, develop a mild Suzuki-Miyaura cross-coupling reaction compatible with cell growth and carry out the cross-coupling chemistry in live cell cultures.
- Sunil V. Sharma
- , Xiaoxue Tong
- & Rebecca J. M. Goss
-
Article
| Open Access De novo active sites for resurrected Precambrian enzymes
De novo active sites for resurrected Precambrian enzymes
The emergence of novel catalytic functions in ancient proteins likely played a role in the evolution of modern enzymes. Here, the authors use protein sequences from Precambrian beta-lactamases and demonstrate that a single hydrophobic-to-ionizable amino acid mutation can lead to substantial Kemp eliminase activity.
- Valeria A. Risso
- , Sergio Martinez-Rodriguez
- & Jose M. Sanchez-Ruiz
-
Article
| Open Access Nano-palladium is a cellular catalyst for in vivo chemistry
Nano-palladium is a cellular catalyst for in vivo chemistry
Palladium (Pd) is a well-known catalyst in organic chemistry but its use in nanomedicine is limited. Here, the authors design a Pd nanoparticle that triggers the activation of an antitumour prodrugin vivo, which shows efficacy and improves toxicity compared to traditional solvent- and nanoparticle-drug formulations.
- Miles A. Miller
- , Bjorn Askevold
- & Ralph Weissleder
-
Article
| Open Access An L-threonine transaldolase is required for L-threo-β-hydroxy-α-amino acid assembly during obafluorin biosynthesis
An L-threonine transaldolase is required for L-threo-β-hydroxy-α-amino acid assembly during obafluorin biosynthesis
Obafluorin is a β-lactone antibiotic produced byPseudomonas fluorescens. Here the authors present the biosynthetic gene cluster and biosynthetic pathway of obafluorin, which is characterized by a central transaldolase step catalysed by a rare L-threonine transaldolase.
- Thomas A. Scott
- , Daniel Heine
- & Barrie Wilkinson
-
Article
| Open Access Single-mutation fitness landscapes for an enzyme on multiple substrates reveal specificity is globally encoded
Single-mutation fitness landscapes for an enzyme on multiple substrates reveal specificity is globally encoded
Systematically understanding the sequence determinants to substrate specificity for enzymes has implications in areas from evolutionary biology to biocatalysis. Here, Whitehead and colleagues generate and analyse near-comprehensive single-mutation fitness landscapes for an amidase with three different substrates.
- Emily E. Wrenbeck
- , Laura R. Azouz
- & Timothy A. Whitehead
-
Article
| Open Access Enzyme catalysed Pictet-Spengler formation of chiral 1,1’-disubstituted- and spiro-tetrahydroisoquinolines
Enzyme catalysed Pictet-Spengler formation of chiral 1,1’-disubstituted- and spiro-tetrahydroisoquinolines
The Pictet-Spengler condensation of β-arylethylamine and carbonyl compounds is an important step in the synthesis of bioactive alkaloids. Here, the authors report a Pictet-Spengler reaction between dopamine and unactivated ketones catalysed by norcoclaurine synthase and its engineered variants.
- Benjamin R. Lichman
- , Jianxiong Zhao
- & John M. Ward
-
Article
| Open Access A redox-mediated Kemp eliminase
A redox-mediated Kemp eliminase
The majority of enzymatic Kemp elimination reactions proceed via a well-established acid-base mechanism. Here, the authors show that cytochrome P450 is able to metabolize the leflunomide drug via a redox Kemp elimination, offering new insights into enzyme catalysis.
- Aitao Li
- , Binju Wang
- & Manfred T. Reetz
-
Article
| Open Access Proximity does not contribute to activity enhancement in the glucose oxidase–horseradish peroxidase cascade
Proximity does not contribute to activity enhancement in the glucose oxidase–horseradish peroxidase cascade
The activity enhancement of the glucose oxide and horseradish peroxidase enzymatic cascade on DNA scaffolds has been linked to proximity effects. Here, the authors challenge this view and suggest that the activity improvement is rather due to the pH near the DNA surface.
- Yifei Zhang
- , Stanislav Tsitkov
- & Henry Hess
-
Article
| Open Access The in vivo hydrocarbon formation by vanadium nitrogenase follows a secondary metabolic pathway
The in vivo hydrocarbon formation by vanadium nitrogenase follows a secondary metabolic pathway
Nitrogenases reduce inorganic nitrogen to organic ammonia in a crucial step of the nitrogen cycle. Here the authors show that the vanadium-nitrogenase ofAzotobacter vinelandii can also catalyse the in vivoconversion of carbon monoxide to hydrocarbons in a secondary non-biosynthetic pathway.
- Johannes G. Rebelein
- , Chi Chung Lee
- & Markus W. Ribbe
-
Article
| Open Access Biocatalytic trifluoromethylation of unprotected phenols
Biocatalytic trifluoromethylation of unprotected phenols
The introduction of fluorine into organic molecules often requires prefunctionalised molecules or protection of reactive functional groups. Here, the authors report a biocatalytic method for the trifluoromethylation of unprotected phenols under mild conditions.
- Robert C. Simon
- , Eduardo Busto
- & Wolfgang Kroutil
-
Article
| Open Access Heme biomolecule as redox mediator and oxygen shuttle for efficient charging of lithium-oxygen batteries
Heme biomolecule as redox mediator and oxygen shuttle for efficient charging of lithium-oxygen batteries
Natural biomolecules including heme-like porphyrins can be applied as sustainable chemical catalysts in lithium-oxygen batteries. Here, the authors show that the heme molecule can function as a soluble redox catalyst and oxygen shuttle for efficient oxygen evolution in non-aqueous lithium-oxygen cells.
- Won-Hee Ryu
- , Forrest S. Gittleson
- & André D. Taylor
-
Article
| Open Access Elucidation of the biosynthesis of carnosic acid and its reconstitution in yeast
Elucidation of the biosynthesis of carnosic acid and its reconstitution in yeast
Diterpenes are plant products with high antioxidant properties and potential application as food additives and therapeutics. Here, the authors describe the complete biosynthetic pathway of carnosic acid and reconstruct it in yeast, opening the way to metabolic engineering of phenolic diterpenes.
- Ulschan Scheler
- , Wolfgang Brandt
- & Alain Tissier
-
Article
| Open Access Forward design of a complex enzyme cascade reaction
Forward design of a complex enzyme cascade reaction
Building multi-component enzymatic processes in one pot is challenged by the inherent complexity of each biochemical system. Here, the authors use online mass spectroscopy and engineering systems theory to achieve forward design of a ten-membered reaction cascade.
- Christoph Hold
- , Sonja Billerbeck
- & Sven Panke
-
Article
| Open Access Arginine demethylation is catalysed by a subset of JmjC histone lysine demethylases
Arginine demethylation is catalysed by a subset of JmjC histone lysine demethylases
While reversal of lysine methylation on histone tails is a well-established mechanism to tune gene expression, the existence of a similar arginine demethylation process is controversial. Here, the authors show that some jumonji enzymes possess both lysine and arginine demethylase activity in vitro.
- Louise J. Walport
- , Richard J. Hopkinson
- & Christopher J. Schofield
-
Article
| Open Access Printable enzyme-embedded materials for methane to methanol conversion
Printable enzyme-embedded materials for methane to methanol conversion
There is a need for small-scale reactors that convert methane emissions to more valuable products to reduce climate impacts. Here, the authors show that printing 3D structures of the pMMO enzyme enables continuous methane conversion under ambient conditions and reduces mass transfer limitations.
- Craig D. Blanchette
- , Jennifer M. Knipe
- & Sarah E. Baker
-
Article
| Open Access Highly regio- and enantioselective multiple oxy- and amino-functionalizations of alkenes by modular cascade biocatalysis
Highly regio- and enantioselective multiple oxy- and amino-functionalizations of alkenes by modular cascade biocatalysis
Biocatalysis can perform highly selective multi-step synthesis in one pot, but with a limited range of non-natural reactions and products. Here, the authors report regio- and enantioselective bio-cascades, able to convert styrenes into a number of nitrogen and oxygen containing chiral molecules.
- Shuke Wu
- , Yi Zhou
- & Zhi Li
-
Article
| Open Access Ligand-induced substrate steering and reshaping of [Ag2(H)]+ scaffold for selective CO2 extrusion from formic acid
Ligand-induced substrate steering and reshaping of [Ag2(H)]+ scaffold for selective CO2 extrusion from formic acid
Designing catalysts and understanding the influence of ligands for particular transformations remains a highly challenging task. Here, the authors show that bisphosphine ligands can alter the geometry of the active site in silver catalysts, driving protonation and ultimately extrusion of carbon dioxide from formic acid.
- Athanasios Zavras
- , George N. Khairallah
- & Richard A. J. O’Hair
-
Article
| Open Access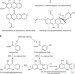 Bifunctional CYP81AA proteins catalyse identical hydroxylations but alternative regioselective phenol couplings in plant xanthone biosynthesis
Bifunctional CYP81AA proteins catalyse identical hydroxylations but alternative regioselective phenol couplings in plant xanthone biosynthesis
Xanthones are pharmacologically and biosynthetically intriguing compounds. Here, the authors identify two cytochrome P450 enzymes, which hydroxylate and cyclize the benzophenone precursor to either 1,3,7- or 1,3,5-trihydroxyxanthones, and pinpoint residues that determine the alternative regioselectivities.
- Islam El-Awaad
- , Marco Bocola
- & Ludger Beerhues
-
Article
| Open Access Crystal structure of glycogen debranching enzyme and insights into its catalysis and disease-causing mutations
Crystal structure of glycogen debranching enzyme and insights into its catalysis and disease-causing mutations
Debranching of glycogen is an important step in its use as an energy source. Here, the authors describe the crystal structures of glycogen debranching enzyme alone and in complex with oligosaccharides and provide molecular insights into the function, and into associated diseases.
- Liting Zhai
- , Lingling Feng
- & Song Xiang
-
Article
| Open Access Collaboration between primitive cell membranes and soluble catalysts
Collaboration between primitive cell membranes and soluble catalysts
Early cells likely consisted of fatty acid vesicles enclosing magnesium-dependent ribozymes. Here, the authors show that fatty acid derivatives can form vesicles that, unlike those formed from only unmodified fatty acids, are stable in the presence of magnesium and could support ribozyme catalysis.
- Katarzyna P. Adamala
- , Aaron E. Engelhart
- & Jack W. Szostak
-
Article
| Open Access A unified mechanism for proteolysis and autocatalytic activation in the 20S proteasome
A unified mechanism for proteolysis and autocatalytic activation in the 20S proteasome
The proteasome, an essential molecular machine, is a threonine protease, but the evolution and the components of its proteolytic centre are unclear. Here, the authors use structural biology and biochemistry to investigate the role of proteasome active site residues on maturation and activity.
- Eva M. Huber
- , Wolfgang Heinemeyer
- & Michael Groll
-
Article
| Open Access Nanocaged enzymes with enhanced catalytic activity and increased stability against protease digestion
Nanocaged enzymes with enhanced catalytic activity and increased stability against protease digestion
Cells compartmentalize enzymes for enhanced efficiency of their metabolic pathways. Here, the authors describe a self-assembly approach to construct DNA nanocaged enzymes for enhancing catalytic activity and stability, and observe an inversed correlation between the protein size and the activity enhancement.
- Zhao Zhao
- , Jinglin Fu
- & Hao Yan
-
Article
| Open Access Native characterization of nucleic acid motif thermodynamics via non-covalent catalysis
Native characterization of nucleic acid motif thermodynamics via non-covalent catalysis
DNA hybridisation thermodynamics parameters underlie rational design of oligonucleotides for diagnostics and nanotechnology. Here, the authors present an accurate method to measure the free energy of a given DNA structure at specific temperature and buffer conditions.
- Chunyan Wang
- , Jin H. Bae
- & David Yu Zhang
-
Article
| Open Access Structure of the polyisoprenyl-phosphate glycosyltransferase GtrB and insights into the mechanism of catalysis
Structure of the polyisoprenyl-phosphate glycosyltransferase GtrB and insights into the mechanism of catalysis
Polyisoprenyl-glycosyltransferases (PI-GTs) catalyse the addition of sugar to lipid carriers, which is the first step in the production of sugar donors for glycosylation. Here Ardiccioni et al.present the structure of a bacterial PI-GT and propose a mechanistic basis for sugar transfer.
- Chiara Ardiccioni
- , Oliver B. Clarke
- & Filippo Mancia
-
Article
| Open Access Rational engineering of a mesohalophilic carbonic anhydrase to an extreme halotolerant biocatalyst
Rational engineering of a mesohalophilic carbonic anhydrase to an extreme halotolerant biocatalyst
Halophilic organisms thrive in high salt conditions and express proteins that display desirable characteristics for industrial applications. Here, the authors use a rational design approach to transform wild-type carbonic anhydrase into a strongly halophilic enzyme.
- Andrew C. Warden
- , Michelle Williams
- & Victoria S. Haritos
-
Article
| Open Access Structure–function characterization reveals new catalytic diversity in the galactose oxidase and glyoxal oxidase family
Structure–function characterization reveals new catalytic diversity in the galactose oxidase and glyoxal oxidase family
Auxilliary Activity Family 5 (AA5) comprises mononuclear copper radical oxidases with catalytic diversity that is not well characterised. Here, structural, phylogenetic and biochemical analyses advance our understanding of the potential biological and biotechnology functions of these proteins.
- DeLu (Tyler) Yin
- , Saioa Urresti
- & Harry Brumer
-
Article
| Open Access Ultrahigh-throughput discovery of promiscuous enzymes by picodroplet functional metagenomics
Ultrahigh-throughput discovery of promiscuous enzymes by picodroplet functional metagenomics
Environmental DNA from unculturable microorganisms contains genes with useful functions that remain difficult to identify and isolate. Here Colin, Kintses et al.demonstrate the screening of millions of samples in pL volumes to directly identify new enzymatic activities and complements sequence-based approaches.
- Pierre-Yves Colin
- , Balint Kintses
- & Florian Hollfelder
-
Article
| Open Access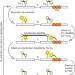 Single-molecule spectroscopy exposes hidden states in an enzymatic electron relay
Single-molecule spectroscopy exposes hidden states in an enzymatic electron relay
A major challenge in following electron transfer through dithiol/disulfide exchange is the dearth of accompanying spectroscopic effects. Here, the authors use single-molecule Förster resonance energy transfer experiments to illuminate disulfide bond rearrangements within the enzyme quiescin sulfhydryl oxidase.
- Iris Grossman
- , Haim Yuval Aviram
- & Deborah Fass
-
Article
| Open Access The thiostrepton A tryptophan methyltransferase TsrM catalyses a cob(II)alamin-dependent methyl transfer reaction
The thiostrepton A tryptophan methyltransferase TsrM catalyses a cob(II)alamin-dependent methyl transfer reaction
Cobalamin-dependent radical SAM enzymes are proposed as methyltransferases in many biosynthetic pathways. Here, the authors study a pathway involving the methylation of tryptophan, showing that methylcob(III)alamin is the most likely cofactor and propose a radical-based C-methylation mechanism.
- Alhosna Benjdia
- , Stéphane Pierre
- & Olivier Berteau
-
Article
| Open Access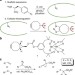 Engineering a dirhodium artificial metalloenzyme for selective olefin cyclopropanation
Engineering a dirhodium artificial metalloenzyme for selective olefin cyclopropanation
Artificial metalloenzymes (ArMs) have the potential to improve transition metal reactivity in complex media. Here, the authors link a dirhodium catalyst to a prolyl oligopeptidase to create an ArM that catalyzes enantioselective olefin cyclopropanation in aqueous solution.
- Poonam Srivastava
- , Hao Yang
- & Jared C. Lewis
-
Article
| Open Access Single-site trinuclear copper oxygen clusters in mordenite for selective conversion of methane to methanol
Single-site trinuclear copper oxygen clusters in mordenite for selective conversion of methane to methanol
Copper-exchanged zeolites with mordenite structure can mimic the active sites in particulate methane monooxygenase. Here, the authors show that mordenite micropores can stabilize trinuclear copper-oxo clusters that exhibit a high reactivity towards activation of carbon–hydrogen bonds in methane.
- Sebastian Grundner
- , Monica A.C. Markovits
- & Johannes A. Lercher
-
Article
| Open Access Non-canonical active site architecture of the radical SAM thiamin pyrimidine synthase
Non-canonical active site architecture of the radical SAM thiamin pyrimidine synthase
Most radical SAM enzymes use a [4Fe-4S] cluster to generate a 5′-deoxyadenosyl radical. Here the authors show the radical SAM thiamin pyrimidine synthase ThiC comprises an additional active site metal, probably representing an evolutionary link between the radical SAM and adenosylcobalamin-dependent enzyme superfamilies.
- Michael K. Fenwick
- , Angad P. Mehta
- & Steven E. Ealick
-
Article
| Open Access Transfer hydrogenation catalysis in cells as a new approach to anticancer drug design
Transfer hydrogenation catalysis in cells as a new approach to anticancer drug design
Organometallic complexes are effective hydrogenation catalysts for organic reactions. Here the authors report for the first time that transfer hydrogenation catalysis can take place inside the cell and could be used as a novel anticancer strategy.
- Joan J. Soldevila-Barreda
- , Isolda Romero-Canelón
- & Peter J. Sadler
-
Article
| Open Access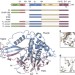 The DUSP–Ubl domain of USP4 enhances its catalytic efficiency by promoting ubiquitin exchange
The DUSP–Ubl domain of USP4 enhances its catalytic efficiency by promoting ubiquitin exchange
Ubiquitin-specific protease USP4 regulates several cellular signalling pathways. Here, Clerici et al.show that the DUSP–Ubl domain of USP4 is required for full catalytic activity, by enhancing the release of ubiquitin from the catalytic site after substrate hydrolysis.
- Marcello Clerici
- , Mark P. A. Luna-Vargas
- & Titia K. Sixma
-
Article |
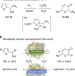 Enzymatic aerobic ring rearrangement of optically active furylcarbinols
Enzymatic aerobic ring rearrangement of optically active furylcarbinols
The Achmatowicz reaction involves the conversion of furans into dihydropyrans, classically with the use of bromine. Here, the authors couple an enzymatic oxygen activation with a chloroperoxidase-mediated oxygen transfer, providing a biocatalytic route to a formal Achmatowicz reaction.
- Daniel Thiel
- , Diana Doknić
- & Jan Deska
-
Article |
 Enzyme activity in liquid lipase melts as a step towards solvent-free biology at 150 °C
Enzyme activity in liquid lipase melts as a step towards solvent-free biology at 150 °C
Enzymatic reactions typically occur in aqueous media or with hydrated enzymes. Here, the authors form fluid enzyme-polymer conjugates with sub-solvation levels of water, and demonstrate catalytic hydrolysis in the absence of a solvent at high temperatures.
- Alex P. S. Brogan
- , Kamendra P. Sharma
- & Stephen Mann
-
Article
| Open Access Impact of residues remote from the catalytic centre on enzyme catalysis of copper nitrite reductase
Impact of residues remote from the catalytic centre on enzyme catalysis of copper nitrite reductase
Residues within the catalytic site of enzymes are important for activity, but whether more distant residues are also sensitive to mutation is unclear. Here, Leferink et al.show that mutation of residues in copper nitrate reductase that are 12Å away from the active site perturb enzyme function.
- Nicole G. H. Leferink
- , Svetlana V. Antonyuk
- & S. Samar Hasnain
-
Article
| Open Access Identification of promiscuous ene-reductase activity by mining structural databases using active site constellations
Identification of promiscuous ene-reductase activity by mining structural databases using active site constellations
Enzymes are very efficient reaction catalysts, though taking advantage of this synthetically is hampered by their notorious specificity. Here, the authors identify important arrangements of active site residues and use structural bioinformatics to successfully predict enzyme activity.
- Georg Steinkellner
- , Christian C. Gruber
- & Karl Gruber
-
Article |
 Structural basis for catalysis in a CDP-alcohol phosphotransferase
Structural basis for catalysis in a CDP-alcohol phosphotransferase
The transfer of a phosphate group from a CDP-linked donor to an acceptor alcohol is catalysed by CDP-alcohol phosphotransferases. Here, Sciara et al. report crystal structures of a CDP-alcohol phosphotransferase, define roles of conserved residues and propose a mechanism of action for this protein family.
- Giuliano Sciara
- , Oliver B. Clarke
- & Filippo Mancia
-
Article |
 A highly efficient cocaine-detoxifying enzyme obtained by computational design
A highly efficient cocaine-detoxifying enzyme obtained by computational design
The enzyme butyrylcholinesterase (BChE) can metabolize cocaine, albeit at relatively low speeds. Here the authors use computational methods to define mutations that increase BChE-mediated cocaine hydrolysis, achieving a catalytic activity comparable to that of one of the fastest naturally occurring enzyme.
- Fang Zheng
- , Liu Xue
- & Chang-Guo Zhan
-
Article |
 Non-enzymatic chemistry enables 2-hydroxyglutarate-mediated activation of 2-oxoglutarate oxygenases
Non-enzymatic chemistry enables 2-hydroxyglutarate-mediated activation of 2-oxoglutarate oxygenases
Studies have identified that mutations to metabolic enzymes can lead to abnormal biological activity and disease. Here, the authors show that in addition to this, non-enzymatic chemistry could also influence abnormal metabolic processes and disease development.
- Hanna Tarhonskaya
- , Anna M. Rydzik
- & Christopher J. Schofield
-
Article |
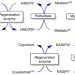 Photobiocatalytic chemistry of oxidoreductases using water as the electron donor
Photobiocatalytic chemistry of oxidoreductases using water as the electron donor
Enzymes have been considered as biocatalysts for organic chemistry, however their broad application has been somewhat hampered by the requirement for reducing cofactors. Here, the authors demonstrate that, in the presence of titania photocatalysts, water can be used as the sacrificial electron donor.
- Maria Mifsud
- , Serena Gargiulo
- & Avelino Corma
-
Article |
 Highly efficient methane biocatalysis revealed in a methanotrophic bacterium
Highly efficient methane biocatalysis revealed in a methanotrophic bacterium
Methane is a promising renewable carbon source for chemical synthesis, yet methane bio-gas is currently underutilised as a feedstock. Here the authors examine the metabolic processes of methanotrophic bacteria to assess their use for conversion of methane to value-added chemical products.
- M. G. Kalyuzhnaya
- , S. Yang
- & M. E. Lidstrom
-
Article |
 Unexpected reactivity and mechanism of carboxamide activation in bacterial N-linked protein glycosylation
Unexpected reactivity and mechanism of carboxamide activation in bacterial N-linked protein glycosylation
Oligosaccharyltransferases catalyse the transfer of lipid-anchored glycans onto acceptor asparagine residues in substrate proteins. By assaying chemically modified peptide substrate analogues, Lizak et al. rule out all but one of the currently postulated catalytic mechanisms for this enzyme.
- Christian Lizak
- , Sabina Gerber
- & Kaspar P. Locher
-
Article
| Open Access Sn-Beta zeolites with borate salts catalyse the epimerization of carbohydrates via an intramolecular carbon shift
Sn-Beta zeolites with borate salts catalyse the epimerization of carbohydrates via an intramolecular carbon shift
Epimerization of carbohydrates to rare sugars yields products that have potential applications as anti-viral drugs or chiral building blocks. Here, Sn-Beta zeolite in the presence of sodium tetraborate is shown to catalyze the selective epimerization of aldoses in aqueous media.
- William R. Gunther
- , Yuran Wang
- & Yuriy Román-Leshkov
-
Review Article |
 The biology and chemistry of high-valent iron–oxo and iron–nitrido complexes
The biology and chemistry of high-valent iron–oxo and iron–nitrido complexes
High-valent iron–oxo and –nitrido complexes are intermediates in the catalytic cycles of various metalloenzymes that activate dioxygen and dinitrogen. Hohenbergeret al. review the advances in the chemistry of model high-valent iron–oxo and –nitrido systems and relate them to our understanding of related enzymes.
- Johannes Hohenberger
- , Kallol Ray
- & Karsten Meyer

 A three enzyme system to generate the Strychnos alkaloid scaffold from a central biosynthetic intermediate
A three enzyme system to generate the Strychnos alkaloid scaffold from a central biosynthetic intermediate