Featured
-
-
Article
| Open Access Chiral twisting in a bacterial cytoskeletal polymer affects filament size and orientation
Chiral twisting in a bacterial cytoskeletal polymer affects filament size and orientation
The actin homolog MreB directs cell-wall insertion and maintains cell shape in many rod-shaped bacteria. Here, Shi et al. perform molecular dynamics simulations for MreB to extract mechanical parameters for inputs into a coarse-grained biophysical polymer model that predicts MreB filament properties.
- Handuo Shi
- , David A. Quint
- & Kerwyn Casey Huang
-
Article
| Open Access The lipoprotein Pal stabilises the bacterial outer membrane during constriction by a mobilisation-and-capture mechanism
The lipoprotein Pal stabilises the bacterial outer membrane during constriction by a mobilisation-and-capture mechanism
The lipoprotein Pal participates in the coordination of outer-membrane constriction with septation in Gram-negative bacteria. Here, the authors show that this coordination is mediated by active mobilisation-and-capture of Pal at division septa by the Tol system.
- Joanna Szczepaniak
- , Peter Holmes
- & Colin Kleanthous
-
Article
| Open Access Complete structure of the chemosensory array core signalling unit in an E. coli minicell strain
Complete structure of the chemosensory array core signalling unit in an E. coli minicell strain
Motile bacteria sense chemical gradients with transmembrane receptors organised in supramolecular signalling arrays. Here authors introduce an E. coli strain that forms small minicells possessing extended and highly ordered chemosensory arrays that are visualized by cryo-electron tomography.
- Alister Burt
- , C. Keith Cassidy
- & Irina Gutsche
-
Article
| Open Access Structural basis of proton-coupled potassium transport in the KUP family
Structural basis of proton-coupled potassium transport in the KUP family
KUP transporters facilitate potassium uptake by the co-transport of protons and are key players in potassium homeostasis. Here authors identify the potassium importer KimA from Bacillus subtilis as a new member of the KUP transporter family and show the cryo-EM structure of KimA in an inward-occluded, trans-inhibited conformation.
- Igor Tascón
- , Joana S. Sousa
- & Inga Hänelt
-
Article
| Open Access The C. difficile toxin B membrane translocation machinery is an evolutionarily conserved protein delivery apparatus
The C. difficile toxin B membrane translocation machinery is an evolutionarily conserved protein delivery apparatus
Large Clostridial toxins infiltrate host cells using a translocation domain (LCT-T). Here, using a genomics-driven approach and functional assays, the authors uncover the presence of distant LCT-T homologs in bacteria outside clostridia and provide evidence for a toxic effector function in the gammaproteobacterium Serratia marcescens.
- Kathleen E. Orrell
- , Michael J. Mansfield
- & Roman A. Melnyk
-
Article
| Open Access Encapsulation mechanisms and structural studies of GRM2 bacterial microcompartment particles
Encapsulation mechanisms and structural studies of GRM2 bacterial microcompartment particles
Bacterial microcompartments (BMCs) consist of a protein shell and an encapsulated enzymatic core. Here, Kalnins et al. study a BMC from Klebsiella pneumoniae, show that the enzymatic core is encapsulated in a hierarchical manner, and solve the cryo-EM structure of a pT = 4 quasi-symmetric icosahedral shell particle.
- Gints Kalnins
- , Eva-Emilija Cesle
- & Kaspars Tars
-
Article
| Open Access Structural basis of denuded glycan recognition by SPOR domains in bacterial cell division
Structural basis of denuded glycan recognition by SPOR domains in bacterial cell division
SPOR domains are present in bacterial proteins that recognize cell-wall peptidoglycan strands stripped of the peptide stems. Here, Alcorlo et al. show that, indeed, the presence of peptide stems abrogates binding to a SPOR domain, and provide insights into the molecular basis for recognition.
- Martín Alcorlo
- , David A. Dik
- & Juan A. Hermoso
-
Article
| Open Access Core architecture of a bacterial type II secretion system
Core architecture of a bacterial type II secretion system
Bacterial type II secretion systems (T2SSs) translocate virulence factors, toxins and enzymes across the cell outer membrane. Here, Chernyatina and Low use negative stain and cryo-electron microscopy to reveal the core architecture of an assembled T2SS from the pathogen Klebsiella pneumoniae.
- Anastasia A. Chernyatina
- & Harry H. Low
-
Article
| Open Access Heme and hemoglobin utilization by Mycobacterium tuberculosis
Heme and hemoglobin utilization by Mycobacterium tuberculosis
Iron is essential for growth of Mycobacterium tuberculosis, but most of the iron in the human body is stored in heme within hemoglobin. Here, Mitra et al. identify two heme uptake mechanisms in M. tuberculosis, one dependent on the inner-membrane Dpp importer and the other dependent on host albumin.
- Avishek Mitra
- , Ying-Hui Ko
- & Michael Niederweis
-
Article
| Open Access Molecular basis for metabolite channeling in a ring opening enzyme of the phenylacetate degradation pathway
Molecular basis for metabolite channeling in a ring opening enzyme of the phenylacetate degradation pathway
The bacterial enzyme PaaZ is involved in the breakdown of environmental pollutants via the aerobic-anaerobic hybrid pathway but its substrate transfer mechanism is not fully understood. Here, the authors present cryoEM structures of free and ligand-bound PaaZ that suggest a mechanism for internal substrate channeling.
- Nitish Sathyanarayanan
- , Giuseppe Cannone
- & Kutti R. Vinothkumar
-
Article
| Open Access The complex of ferric-enterobactin with its transporter from Pseudomonas aeruginosa suggests a two-site model
The complex of ferric-enterobactin with its transporter from Pseudomonas aeruginosa suggests a two-site model
Bacteria produce small iron-binding molecules called siderophores, which are recognised by outer-membrane transporters. Here, the authors show that a Pseudomonas transporter recognises the siderophore enterobactin using extracellular loops distant from the pore, and propose that there is a second binding site deeper inside the structure.
- Lucile Moynié
- , Stefan Milenkovic
- & James H. Naismith
-
Article
| Open Access Zinc-binding to the cytoplasmic PAS domain regulates the essential WalK histidine kinase of Staphylococcus aureus
Zinc-binding to the cytoplasmic PAS domain regulates the essential WalK histidine kinase of Staphylococcus aureus
WalKR is an essential two-component regulator that controls peptidoglycan synthesis in the human pathogen Staphylococcus aureus. Here, the authors provide biochemical, structural, and functional evidence supporting that the binding of a zinc ion inhibits autophosphorylation and thus alters WalKR regulatory activity.
- Ian R. Monk
- , Nausad Shaikh
- & Timothy P. Stinear
-
Article
| Open Access Structural basis for the homotypic fusion of chlamydial inclusions by the SNARE-like protein IncA
Structural basis for the homotypic fusion of chlamydial inclusions by the SNARE-like protein IncA
Chlamydia trachomatis forms membrane-bound inclusions inside the host cell that are decorated with IncA, a SNARE-like protein that promotes the fusion of inclusions. Here, Cingolani et al. show that the protein folds into a non-canonical four-helix bundle and identify an intramolecular clamp required for membrane fusion.
- Gino Cingolani
- , Michael McCauley
- & Fabienne Paumet
-
Article
| Open Access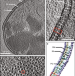 In situ structure and assembly of the multidrug efflux pump AcrAB-TolC
In situ structure and assembly of the multidrug efflux pump AcrAB-TolC
Multidrug efflux pumps actively expel a wide range of toxic substrates from bacteria and play a major role in drug resistance. Here authors show the in situ structure of the efflux pump AcrAB-TolC obtained by electron cryo-tomography and subtomogram averaging.
- Xiaodong Shi
- , Muyuan Chen
- & Zhao Wang
-
Article
| Open Access Unique structural features of a bacterial autotransporter adhesin suggest mechanisms for interaction with host macromolecules
Unique structural features of a bacterial autotransporter adhesin suggest mechanisms for interaction with host macromolecules
Autotransporter proteins are localised to the bacterial surface and promote colonisation of host epithelial surfaces. Here, the authors present the crystal structure of autotransporter UpaB and show evidence for distinct binding sites for glycosaminoglycans and host fibronectin.
- Jason J. Paxman
- , Alvin W. Lo
- & Begoña Heras
-
Article
| Open Access Structural and biochemical evidence supporting poly ADP-ribosylation in the bacterium Deinococcus radiodurans
Structural and biochemical evidence supporting poly ADP-ribosylation in the bacterium Deinococcus radiodurans
Poly-ADP-ribosylation (PARylation) is a well-known regulatory event in eukaryotes but has not yet been observed in bacteria. Here, the authors solve the structure of a bacterial PAR-glycohydrolase and provide evidence for a prokaryotic PARylation machinery involved in the response to genotoxic stress.
- Chao-Cheng Cho
- , Chia-Yu Chien
- & Chun-Hua Hsu
-
Article
| Open Access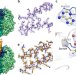 Cryo-EM structure of the homohexameric T3SS ATPase-central stalk complex reveals rotary ATPase-like asymmetry
Cryo-EM structure of the homohexameric T3SS ATPase-central stalk complex reveals rotary ATPase-like asymmetry
Many Gram-negative bacteria rely on a type III secretion system (T3SS) for their pathogenicity. Here authors present the cryo-EM structure of the E.coli T3SS ATPase-central stalk complex, which forms a homohexameric, asymmetric pore with different functional states.
- Dorothy D. Majewski
- , Liam J. Worrall
- & Natalie C. J. Strynadka
-
Article
| Open Access The cell cycle regulator GpsB functions as cytosolic adaptor for multiple cell wall enzymes
The cell cycle regulator GpsB functions as cytosolic adaptor for multiple cell wall enzymes
GpsB is a cytosolic protein that modulates bacterial cell wall synthesis by interacting with cytoplasmic domains of peptidoglycan synthases. Here, Cleverley et al. describe structural features that are important for these interactions, and identify new interacting partners of GpsB in three bacterial species.
- Robert M. Cleverley
- , Zoe J. Rutter
- & Richard J. Lewis
-
Article
| Open Access Identification and characterization of a large family of superbinding bacterial SH2 domains
Identification and characterization of a large family of superbinding bacterial SH2 domains
SH2 domains bind to tyrosine-phosphorylated proteins and play crucial roles in signal transduction in mammalian cells. Here, Kaneko et al. identify a large family of SH2 domains in the bacterial pathogen Legionella that bind to mammalian phosphorylated proteins, in some cases with very high affinity.
- Tomonori Kaneko
- , Peter J. Stogios
- & Shawn S.-C. Li
-
Article
| Open Access Structural basis of cell wall anchoring by SLH domains in Paenibacillus alvei
Structural basis of cell wall anchoring by SLH domains in Paenibacillus alvei
Gram-positive bacterial envelopes comprise proteinaceous surface layers (S-layers) important for survival and virulence that are often anchored to the cell wall through secondary cell wall polymers. Here the authors use a structural and biophysical approach to define the molecular mechanism of this important interaction.
- Ryan J. Blackler
- , Arturo López-Guzmán
- & Stephen V. Evans
-
Article
| Open Access Structures of chaperone-substrate complexes docked onto the export gate in a type III secretion system
Structures of chaperone-substrate complexes docked onto the export gate in a type III secretion system
Bacterial flagella are composed of proteins secreted by a type III secretion system (T3SS), which requires the action of dedicated chaperones. Here, Xing et al. report the structures of two ternary complexes among flagellar chaperones, flagellar protein substrates, and the export gate platform protein.
- Qiong Xing
- , Ke Shi
- & Charalampos G. Kalodimos
-
Article
| Open Access Crystal structure of lipid A disaccharide synthase LpxB from Escherichia coli
Crystal structure of lipid A disaccharide synthase LpxB from Escherichia coli
LpxB is a membrane-associated glycosyltransferase required for bacterial lipopolysaccharide biosynthesis. Here, Bohl et al. solve the crystal structure of a soluble LpxB variant, showing an intertwined C-terminally swapped dimer, and residues likely mediating association with lipidic substrates or the membrane.
- Heather O. Bohl
- , Ke Shi
- & Hideki Aihara
-
Article
| Open Access Structural basis for chitin acquisition by marine Vibrio species
Structural basis for chitin acquisition by marine Vibrio species
Chitin degrading bacteria are important for marine ecosystems. Here the authors structurally and functionally characterize the Vibrio harveyi outer membrane diffusion channel chitoporin and give mechanistic insights into chito-oligosaccharide uptake.
- Anuwat Aunkham
- , Michael Zahn
- & Bert van den Berg
-
Article
| Open Access Insights into the structure and assembly of a bacterial cellulose secretion system
Insights into the structure and assembly of a bacterial cellulose secretion system
Many Gram-negative bacteria secrete exopolysaccharides via functionally homologous synthase-dependent systems. Here the authors use electron microscopy to reveal that biofilm-promoting cellulose in E. coli is secreted by a conserved multi-component secretion system with a megadalton-sized asymmetric architecture.
- Petya Violinova Krasteva
- , Joaquin Bernal-Bayard
- & Jean-Marc Ghigo
-
Article
| Open Access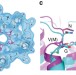 The structural basis of proton driven zinc transport by ZntB
The structural basis of proton driven zinc transport by ZntB
The bacterial zinc transporter ZntB is important for maintaining zinc homeostasis and is mechanistically not well understood. Here, the authors present the cryo-EM structure of ZntB at 4.2 Å resolution, perform transport assays and propose a model for its Zn2+ transport mechanism.
- Cornelius Gati
- , Artem Stetsenko
- & Albert Guskov
-
Article
| Open Access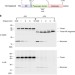 Folding of a bacterial integral outer membrane protein is initiated in the periplasm
Folding of a bacterial integral outer membrane protein is initiated in the periplasm
The Bam complex promotes the insertion of β-barrel proteins (such as UpaG, a trimeric autotransporter adhesin) into the bacterial outer membrane. Here, Sikdar et al. show that UpaG β-barrel segments fold into a trimeric structure in the periplasm before they interact with the Bam complex.
- Rakesh Sikdar
- , Janine H. Peterson
- & Harris D. Bernstein
-
Article
| Open Access A structural model of flagellar filament switching across multiple bacterial species
A structural model of flagellar filament switching across multiple bacterial species
Bacterial flagellar filaments are composed almost entirely of a single protein—flagellin—which can switch between different supercoiled states in a highly cooperative manner. Here the authors present near-atomic resolution cryo-EM structures of nine flagellar filaments, and begin to shed light on the molecular basis of filament switching.
- Fengbin Wang
- , Andrew M. Burrage
- & Edward H. Egelman
-
Article
| Open Access Molecular architecture of the PBP2–MreC core bacterial cell wall synthesis complex
Molecular architecture of the PBP2–MreC core bacterial cell wall synthesis complex
Bacterial wall biosynthesis is a complex process that requires the coordination of multiple enzymes. Here, the authors structurally characterize the PBP2:MreC complex involved in peptidoglycan elongation and cross-linking, and demonstrate that its disruption leads to loss of H. pylori shape and inability to sustain growth.
- Carlos Contreras-Martel
- , Alexandre Martins
- & Andréa Dessen
-
Article
| Open Access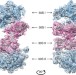 The cryo-EM structure of hibernating 100S ribosome dimer from pathogenic Staphylococcus aureus
The cryo-EM structure of hibernating 100S ribosome dimer from pathogenic Staphylococcus aureus
Under conditions of nutrient limitation, bacterial ribosomes undergo dimerization, forming a 100S complex that is translationally inactive. Here the authors present the structural basis for formation of the 100S complexes in Gram-positive bacteria, shedding light on the mechanism of translation suppression by the ribosome-silencing factors.
- Donna Matzov
- , Shintaro Aibara
- & Ada E. Yonath
-
Article
| Open Access A shape-shifting redox foldase contributes to Proteus mirabilis copper resistance
A shape-shifting redox foldase contributes to Proteus mirabilis copper resistance
Bacterial disulfide isomerases shuffle incorrect disulfide bonds. Here, the authors structurally characterize the disulfide isomerase ScsC fromProteus mirabilisand identify a functionally important shape-shifting motif that allows ScsC to adopt a diverse range of conformations and enable swarming in the presence of copper stress.
- Emily J. Furlong
- , Alvin W. Lo
- & Jennifer L. Martin
-
Article
| Open Access Gating of TonB-dependent transporters by substrate-specific forced remodelling
Gating of TonB-dependent transporters by substrate-specific forced remodelling
Bacterial outer membrane TonB-dependent transporters (TBDTs) mediate the influx of several nutrients. Here the authors use single-molecule force spectroscopy to show that the interaction between TonB andEscherichia coliTBDT BtuB is mechanically resistant to the pulling that gates the BtuB channel.
- Samuel J. Hickman
- , Rachael E. M. Cooper
- & David J. Brockwell
-
Correspondence
| Open Access Correspondence: Spontaneous secondary mutations confound analysis of the essential two-component system WalKR in Staphylococcus aureus
Correspondence: Spontaneous secondary mutations confound analysis of the essential two-component system WalKR in Staphylococcus aureus
- Ian R. Monk
- , Benjamin P. Howden
- & Timothy P. Stinear
-
Article
| Open Access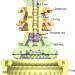 Identical folds used for distinct mechanical functions of the bacterial flagellar rod and hook
Identical folds used for distinct mechanical functions of the bacterial flagellar rod and hook
The bacterial flagellum is a motile organelle that enables bacterial movement. Here the authors explain how the structurally similar flagellum components FlgG and FlgE can give rise to distinct macrostructures—the rod and hook—through subtle differences in domain orientation attributable to a short N-terminal insertion in FlgG.
- Takashi Fujii
- , Takayuki Kato
- & Keiichi Namba
-
Article
| Open Access Complete structure of the bacterial flagellar hook reveals extensive set of stabilizing interactions
Complete structure of the bacterial flagellar hook reveals extensive set of stabilizing interactions
The bacterial flagellar hook is made up of many copies of the protein FlgE. Here, the authors report the full structure of the hook from Campylobacter jejuni and show that its overall structure is different from that of the previously published filament.
- Hideyuki Matsunami
- , Clive S. Barker
- & Fadel A. Samatey
-
Article
| Open Access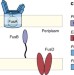 Structure of the bacterial plant-ferredoxin receptor FusA
Structure of the bacterial plant-ferredoxin receptor FusA
Many bacteria use TonB-dependent outer membrane receptors to scavenge iron from their host during infection. Here, the authors report on the structure and function of FusA, which is a bacterial receptor that is used to obtain iron from plants.
- Rhys Grinter
- , Inokentijs Josts
- & Daniel Walker
-
Article
| Open Access Structure of the Neisseria meningitidis Type IV pilus
Structure of the Neisseria meningitidis Type IV pilus
Type IV pili are present on a wide range of bacterial pathogens and mediate diverse functions. Here the authors report a high resolution crystal structure of the pilin subunit PilE, and a cryoEM reconstruction of the Type IV pilus filament from N. meningitidisthat offer insight into pilus assembly and functions.
- Subramania Kolappan
- , Mathieu Coureuil
- & Lisa Craig
-
Article
| Open Access Nucleotide binding by the widespread high-affinity cyclic di-GMP receptor MshEN domain
Nucleotide binding by the widespread high-affinity cyclic di-GMP receptor MshEN domain
Cyclic-di-GMP is a bacterial second messenger that binds to the regulatory domain of ATPases of some bacteria. Here, the authors report the crystal structure of this interaction, identify a cyclic-di-GMP binding mode, and show that this interaction might be important for bacterial biofilm formation.
- Yu-Chuan Wang
- , Ko-Hsin Chin
- & Shan-Ho Chou
-
Article
| Open Access Structural basis for haem piracy from host haemopexin by Haemophilus influenzae
Structural basis for haem piracy from host haemopexin by Haemophilus influenzae
Haemophilus influenzae requires haem, and acquires it from host haemoproteins including haemopexin. Here, the authors examine the haem transport system consisting of HxuA, HxuB and HxuC via the structures of HxuA in complex with haemopexin.
- Silvia Zambolin
- , Bernard Clantin
- & Philippe Delepelaire
-
Article
| Open Access Rifampin phosphotransferase is an unusual antibiotic resistance kinase
Rifampin phosphotransferase is an unusual antibiotic resistance kinase
Antibiotic resistance is a major clinical problem that threatens to undermine our ability to control infectious diseases. Here the authors present detailed structural analysis of Rifampin phosphotransferase from Listeria monocytogenes, yielding insight on how this class of enzyme inactivates its target antibiotics.
- Peter J. Stogios
- , Georgina Cox
- & Gerard D. Wright
-
Article
| Open Access Genome scale patterns of supercoiling in a bacterial chromosome
Genome scale patterns of supercoiling in a bacterial chromosome
Bacterial DNA primarily exists in a negatively supercoiled or under-wound state. Here, by mapping the supercoiling state, the authors show that there is a gradient of supercoiling across the bacterial chromosome with the terminus being more negatively supercoiled than the origin.
- Avantika Lal
- , Amlanjyoti Dhar
- & Sankar Adhya
-
Article
| Open Access Structure and mechanism of the essential two-component signal-transduction system WalKR in Staphylococcus aureus
Structure and mechanism of the essential two-component signal-transduction system WalKR in Staphylococcus aureus
The WalKR signal transduction system is involved in extracellular signal recognition, but the details of this function are not well established. Here, the authors report the crystal structure of this two-component system alongside the characterisation of a small-molecule activator.
- Quanjiang Ji
- , Peter J. Chen
- & Chuan He
-
Article
| Open Access Dynamic capsule restructuring by the main pneumococcal autolysin LytA in response to the epithelium
Dynamic capsule restructuring by the main pneumococcal autolysin LytA in response to the epithelium
Pneumococci produce a carbohydrate capsule that protects them against components of the host immune system but sensitizes them to host antimicrobial peptides. Here, Kietzman et al.show that pneumococci respond to antimicrobial peptides by capsule shedding, which requires the main autolysin LytA.
- Colin C. Kietzman
- , Geli Gao
- & Elaine I. Tuomanen
-
Article
| Open Access Structure and substrate selectivity of the 750-kDa α6β6 holoenzyme of geranyl-CoA carboxylase
Structure and substrate selectivity of the 750-kDa α6β6 holoenzyme of geranyl-CoA carboxylase
The structures of the biotin-dependent carboxylases have revealed details of their function. Here, the authors describe the first structure of Pseudomonasgeranyl-CoA carboxylase, and compare it with the previously characterised homologous 3-methylcrotonyl-CoA carboxylase.
- Ashley R. Jurado
- , Christine S. Huang
- & Liang Tong
-
Article
| Open Access Mechanistic insights into metal ion activation and operator recognition by the ferric uptake regulator
Mechanistic insights into metal ion activation and operator recognition by the ferric uptake regulator
The regulation of iron levels is an important physiological process as excess cellular iron is highly toxic. Here the authors present several structures of a bacterial ferric uptake regulator (Fur) in complex with the Fe2+transport protein operator and Fur box, shedding light on how iron promotes DNA recognition by Fur.
- Zengqin Deng
- , Qing Wang
- & Zhongzhou Chen
-
Article |
 The bacterial tubulin FtsZ requires its intrinsically disordered linker to direct robust cell wall construction
The bacterial tubulin FtsZ requires its intrinsically disordered linker to direct robust cell wall construction
The bacterial division protein FtsZ recruits cell wall synthesis enzymes to the cytokinetic ring. Sundararajan et al.show that FtsZ deletion variants alter peptidoglycan structure without detectable effects on enzyme recruitment, suggesting an additional role in the regulation of cell wall metabolism.
- Kousik Sundararajan
- , Amanda Miguel
- & Erin D. Goley
-
Article
| Open Access Anammox Planctomycetes have a peptidoglycan cell wall
Anammox Planctomycetes have a peptidoglycan cell wall
Planctomycetes are unusual bacteria with complex intracellular compartments and an apparent lack of peptidoglycan in their cell walls. Here, van Teeseling et al. show that the cell wall of an anammox planctomycete does contain peptidoglycan, and propose to redefine planctomycetes as Gram-negative bacteria.
- Muriel C.F. van Teeseling
- , Rob J. Mesman
- & Laura van Niftrik
-
Article
| Open Access A versatile nano display platform from bacterial spore coat proteins
A versatile nano display platform from bacterial spore coat proteins
The densely crosslinked protein coats of bacterial spores are among the most durable static structures in biology. Wu et al.reconstitute the basement layer of a bacterial spore coat on membrane-coated beads, and generate covalently-modified spore-like particles with therapeutic potential.
- I-Lin Wu
- , Kedar Narayan
- & Kumaran S. Ramamurthi
-
Article
| Open Access Structure of a helicase–helicase loader complex reveals insights into the mechanism of bacterial primosome assembly
Structure of a helicase–helicase loader complex reveals insights into the mechanism of bacterial primosome assembly
During the initiation of bacterial DNA replication, loader proteins transfer the hexameric helicase ring onto replication origin DNA. Liu et al.report the crystal structure of a 570-kDa prepriming complex and suggest that the release of loader proteins is associated with the transition of the helicase ring to a spiral configuration.
- Bin Liu
- , William K. Eliason
- & Thomas A. Steitz
-
Article
| Open Access Cell wall elongation mode in Gram-negative bacteria is determined by peptidoglycan architecture
Cell wall elongation mode in Gram-negative bacteria is determined by peptidoglycan architecture
Bacterial cell wall peptidoglycan is essential for viability and shape determination. Using high-resolution microscopy, Foster and colleagues elucidate the peptidoglycan architecture and insertion pattern in Escherichia coliand other Gram-negative bacteria, and propose a new model for cell wall elongation.
- Robert D. Turner
- , Alexander F. Hurd
- & Simon J. Foster

 Activation and self-inactivation mechanisms of the cyclic oligoadenylate-dependent CRISPR ribonuclease Csm6
Activation and self-inactivation mechanisms of the cyclic oligoadenylate-dependent CRISPR ribonuclease Csm6