« Prev Next »

One of the major theoretical contributions made by studying primates is an understanding of what influences the nature of complex variation in social structure and organizations. Underlying this is a set of theoretical developments examining why animals live in groups in the first place and why groups are of a particular size. Here, we focus on the question of "what influences the size of groups primates choose to live in?"
Primates are particularly useful taxa to address this question because their group sizes are highly variable, not only within, but also between species. Intraspecific group size for red colobus (Procolobus rufomitratus), for example, varies between 12 and 150 members (Chapman & Chapman 2000a). Interspecific variation ranges from a single individual, as seen in orangutans (Pongo sp.) (van Schaik 1999), to over 800 members in mandrills (Mandrillus sphinx) (Abernethy et al. 2002). Furthermore, within some species, social groups repeatedly divide and re-unite into subgroups of different sizes and combinations over time (e.g., fission-fusion social organization of spider monkeys (Ateles sp.) and chimpanzees (Pan troglodytes), or the multi-level organization of gelada (Theropithecus gelada) and Hamadryas baboons (Papio hamadryas, Aureli et al. 2008). This variation provides the foundation for researchers to develop models to investigate both the ecological and the social drivers of group size.
Grouping is beneficial in several ways. Individuals in larger groups are thought to have a decreased risk of predation (Hamilton 1971), may be better able to find and defend food resources (Cody 1971, Wrangham 1980), and may be protected against conspecific threat, like infanticide by extra-group males (Wrangham 1979). Various researchers have suggested that grouping confers such predictable benefits (Alexander 1974, van Schaik 1983) that differences in group size can be explained by the disadvantages (Wrangham et al. 1993). The most widely accepted potential cost of grouping is thought to be a reduction in foraging efficiency. Being with other individuals with the same dietary requirements means that animals either fight over food (contest competition), or one animal in a group beats another to the food, thus when the second animal comes to an area there is simply no food left (scramble competition, Janson & van Schaik 1988). In both of these situations it is thought that competition over food leads to animals having to travel farther. The logic behind this argument is relatively simple. Animals must forage over an area that can meet their energetic and nutritional requirements. It follows that an increase in group size will increase the area that must be covered to find adequate food supplies. Thus individuals must travel further and expend more energy if they are in a large group, than if they forage in a smaller group. With an increase in the time spent traveling, a point is approached where the energy spent in travel is too costly and smaller groups become advantageous. In this way ecological factors can influence movement patterns and foraging efficiency, thereby constraining the size of groups that can efficiently exploit available food resources. These ideas have been formalized in what has become known as the Ecological Constraints Model (Chapman & Chapman 2000b, Ganas & Robbins 2005, Snaith & Chapman 2007, Teichroeb & Sicotte 2009).
The essential component of the ecological constraints model is that an increase in group size must lead to an increase in within-group feeding competition. It is conceivable that this operates in a slightly different fashion depending on the nature of the resources used by particular species. With frugivorous, and possibly many folivorous primates, that feed in discrete patches — typically trees bearing food items or clumps of trees — additional group members may deplete patches faster and lead to increased day ranges (Chapman 1988, Snaith & Chapman 2005). For more insectivorous species, whose resources may not occur in as discrete patches, continuous travel throughout the canopy in search of insects with additional group members may lead to an increase in the overlap of individual search fields, reducing per capita encounter rates with food and thus increasing the area that must be searched (van Schaik et al. 1983).
So the ecological constraints model suggests that those factors that affect the distance that animals travel should also affect group size. For those animals that typically feed on fruit or leaves that can be depleted, the size of the patch would determine how long a group of a given size could stay and feed. A large group would spend less time in a patch of a given size than a smaller group, because it depletes the patch faster — a large group simply has more mouths to feed. If animals travel between patches once they have depleted them, then the density and distribution of patches will determine the travel costs incurred. When resource patches are at a high density or in a clumped distribution, the distance to the next patch is small, travel costs are low, and animals can therefore form large groups. At such times, any additional cost associated with being a member of a large group, such as the need to visit many patches, can be easily recovered. In contrast, when resource patches occur at low densities, the distance to the next patch is typically large, travel costs are high, and animals cannot afford to rapidly deplete patches, and therefore animals form small groups.
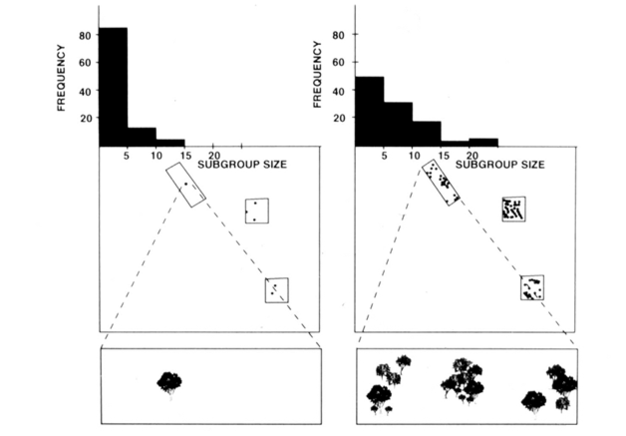
The fission-fusion social organization of spider monkeys, chimpanzees, and a few other primates (Figure 1) offers useful tests of the ecological constraints model because one can attempt to predict temporal changes in subgroup size from direct measures of the size, density, and distribution of food resources (Chapman 1990, Chapman et al. 1995). For spider monkeys and chimpanzees, a multiple regression model was developed to show that the size, density, and distribution of food patches measured each month were a good predictor of subgroup size. It is difficult to visually represent a multiple regression, but the methods that were used for spider monkeys are portrayed in Figure 2. Spider monkeys have a very flexible fission-fusion type of social organization, and in Santa Rosa National Park, Costa Rica, subgroup size can range from 1 to 35 individuals, but on average about 5 individuals are found traveling together. For this population, 50% of the variance in mean monthly subgroup size can be predicted from relatively crude measures of the size, density, and distribution of food patches (Chapman 1990).

In addition to the species mentioned above, the applicability of the ecological constraints model has been generally supported with research coming from a variety of species (Snaith & Chapman 2005) and situations (e.g., general models, Wrangham et al. 1993, and mixed species associations, Chapman & Chapman 2000c). We view that, given the wide-ranging support that this model has received, the time has come that it is reasonable to suggest that species should typically conform to the expectations of the ecological constraints model. This does not mean that all species will conform, and thus it becomes an exciting time for research in this field because investigators can search for exceptions to the model. We suspect that species that do not conform will do so because they have adopted social strategies that run counter to the ecological expectations. This provides a "yardstick" to evaluate the potential importance of various social strategies (i.e., the further one deviates from the expectations of the ecological model, the more important the social selective pressures).
Let us briefly provide two examples from our previous research. Female chimpanzees were more solitary than female spider monkeys, yet they both have similar fission-fusion social organizations. Further, while we could accurately predict the number of males and subadult chimpanzees in a subgroup based on ecological conditions, we were unable to predict the number of female chimpanzees in these subgroups (Chapman et al. 1995). Even when resources were extremely abundant and almost all the males were in one big subgroup, females rarely entered groups, suggesting that the cost of being in a larger subgroup outweighed any benefits, such as predator avoidance. This is unexpected from the perspective that the infants of these females would be the age/sex class most threatened by predation. One testable hypothesis to explain these observations is that the nature of the coalitions in these two species may influence the benefits of group membership. Evidence suggests that, unlike chimpanzees, spider monkey females form coalitions that often operate to allow the members of the coalition exclusive access to food. So spider monkeys, when they join particular individuals, can increase their access to food by excluding others, but for chimpanzee females this is not the case (Chapman et al. 1995).
We identified another interesting deviation, potentially caused by social factors affecting the costs and benefits of being in a group, and this concerns red colobus and black-and-white colobus monkeys (aka. guerezas, Colobus guereza) at Kibale National Park, Uganda. Red colobus form large groups with an average of 65 individuals (25–127 individuals, Snaith et al. 2008), while guerezas live in small groups with an average of 6.5 individuals (4–11 individuals, Harris & Chapman 2007). Despite this difference, there is a great deal of similarity in the plants eaten by the two species. If ecological conditions were responsible for the difference in group size between the two colobine species, one would expect that their diets would differ and the density of food trees would be lower in the home ranges of the guerezas, since they have the smaller group size. However, their diets are very similar; the dietary overlap between a red colobus and a guereza group that had its home range entirely within the home range of the red colobus group was 43.2%, while for the two neighboring groups of red colobus, diet overlapped by only 37.3% (Chapman et al. 2002). It appears that female guereza reproductive success is maximized in small and mid-sized groups, either because larger groups experience higher rates of take-overs and infanticide or more feeding competition (see evidence of scramble competition in the folivorous Trachypithecus phayrei and Semnopithecus sp. [Koenig et al. 1998, Koenig 2000, Koenig & Borries 2006, Borries et al. 2008], but a lack of evidence of guerezas depleting food patches [Tombak et al. 2012]). We have witnessed a number of male take-overs and infant killings in the guerezas (Onderdonk 2000) — see Teichroeb & Sicotte (2008) for an evaluation of theory regarding infanticide in colobines. What is not clear is how the risk of infanticide would limit the size of guereza groups, when they have apparent access to a high density of food that should permit the formation of multi-male groups. In several species, larger female groups are more of a target for immigrating males, and this situation may increase infanticide rates-for example, Semnopithecus sp. (Borries 1997), Theropithecus gelada (Dunbar 1984), Alouatta seniculus (Crockett & Janson 2000), Presbytis thomasi (Steenbeek & van Schaik 2001). However, infanticide is expected to be less frequent if a group contains multiple reproductively active males, because male immigration and subsequent violent take over and infanticide are less likely to occur when the newcomer is faced with several possible sires of future infants (Janson & van Schaik 2000). Guerezas appear to have ample food resources to form larger groups (Tombak et al. 2012), which would decrease the risk of infanticide, yet they do not form large groups. It is possible that the nature of male-male interactions in guerezas makes large groups unstable. This appears to be the case in ursine colobus (Colobus vellerosus), where multi-male groups attract male immigrants and have higher infanticide rates than groups with one strong adult male in his prime (Teichroeb, Wikberg, Badescu, & Sicotte, unpublished data; Figure 3). Thus, even when ecological conditions would permit large groups to form, social conditions preclude their formation (Chapman & Pavelka 2005).

Glossary
Social organization: Represents the arrangement of individuals in a social group, including the group size, sexual composition (the adult male:adult female sex ratio), and spatiotemporal cohesion (Kappeler & van Schaik 2002).
Social structure: Refers to the patterns of social relationships between individuals in the same group (Kappeler & van Schaik 2002), which is influenced by which sex disperses from the natal group. Usually, a species with female-biased dispersal will show stronger relationships between males compared to females, while a species with male-biased dispersal will show strong bonding between females. If both sexes disperse, there may be strong relationships between the sexes.
Fission-fusion: Refers to a fluid pattern of spatiotemporal cohesion in animals where the larger group splits up into subgroups of varying composition that range separately and may join up together or break apart depending on resource availability.
Patches: Denotes the distribution of food in a discrete, concentrated area, separated by space from other areas with food items. Patches may be represented by a single tree that is producing food items or a cluster of trees of the same species in the same area, all producing food items.
Dietary overlap: Refers to the proportion of species/plant parts that are the same between two animal species and may be represented at different temporal scales (e.g., daily, monthly, or annual overlap in the diet).
References and Recommended Reading
Alexander, R. D. The evolution of social behaviour. Annual Review of Ecology and Systematics 5, 325-382 (1974).
Aureli, F. et al. Fission-fusion dynamics: New frameworks for comparative research. Current Anthropology 49, 627-654 (2008).
Borries, C. Infanticide in seasonally breeding multimale groups of Hanuman langurs (Presbytis entellus) in Ramnagar (South Napal). Behavioral Ecology and Sociobiology 41, 139-150 (1997).
Borries, C. et al. Costs of group size: Lower developmental and reproductive rates in larger groups of leaf monkeys. Behavioural Ecology 19, 1186-1194 (2008).
Chapman, C. A. Patch use and patch depletion by the spider and howling monkeys of Santa Rosa National Park, Costa Rica. Behaviour 105, 99-116 (1988).
Chapman, C. A. Association patterns of spider monkeys: The influence of ecology and sex on social organization. Behavioral Ecology and Sociobiology 26, 409-414 (1990).
Chapman, C. A. & Chapman, L. J. Constraints on group size in red colobus and red-tailed guenons: Examining the generality of the ecological constraints model. International Journal of Primatology 21, 565-585 (2000a).
Chapman, C. A. & Chapman, L. J. "Determinants of group size in primates: The importance of travel costs," in On the Move: How and Why Animals Travel in Groups, eds. S. Boinski & P. A. Garber (Chicago, IL: University of Chicago Press, 2000b) 24-41.
Chapman, C. A. & Chapman, L. J. Interdemic variation in mixed-species association patterns: Common diurnal primates of Kibale National Park, Uganda. Behavioral Ecology and Sociobiology 47, 129-139 (2000c).
Chapman, C. A. & Pavelka, M. S. M. Group size in folivorous primates: Ecological constraints and the possible influence of social factors. Primates 46, 1-9 (2005).
Chapman, C. A., Chapman, L. J. & Gillespie, T. R. Scale issues in the study of primate foraging: Red colobus of Kibale National Park. American Journal of Physical Anthropology 117, 349-363 (2002).
Chapman, C. A., Wrangham, R. W. & Chapman, L. J. Ecological constraints on group size: An analysis of spider monkey and chimpanzee subgroups. Behavioral Ecology and Sociobiology 36, 59-70 (1995).
Cody, M. L. Finch flocks in the Mojave desert. Theoretical Population Biology 2, 141-158 (1971).
Crockett, C. M. & Janson, C. H. "Infanticide in red howlers: Female group size, male membership, and a possible link to folivory," in Infanticide By Males and its Implications, eds. C. P. van Schaik & C. H. Janson (Cambridge, UK: Cambridge University Press, 2000) 75-98.
Dunbar, R. I. M. Reproductive Decisions: An Economic Analysis of Gelada Baboon Social Strategies. Princeton, NJ: Princeton University Press, 1984.
Ganas, J. & Robbins, M. M. Ranging behavior of the mountain gorillas (Gorilla beringei beringei) in Bwindi Impenetrable National Park, Uganda: A test of the ecological constraints model. Behavioral Ecology and Sociobiology 58, 277-288 (2005).
Hamilton, W. D. Geometry of the selfish herd. Journal of Theoretical Biology 31, 295-331 (1971).
Harris, T. & Chapman, C. A. Variation in the diet and ranging behavior of black-and-white colobus monkeys: Implications for theory and conservation. Primates 28, 208-221 (2007).
Janson, C. H. & van Schaik, C. P. "Behavioral ecology of infanticide," in Infanticide By Males and its Implications, eds. C. P. van Schaik & C. H. Janson (Cambridge, UK: Cambridge University Press, 2000) 469-494.
Janson, C. H. & van Schaik, C. P. Recognizing the many faces of primate food competition: Methods. Behaviour 105, 165-186 (1988).
Kappeler, P. M. & van Schaik, C. P. Evolution of primate social systems. International Journal of Primatology 23, 707-740 (2002).
Koenig, A. Competitive regimes in forest-dwelling Hanuman langur females (Semnopithecus entellus). Behavioral Ecology and Sociobiology 48, 93-109 (2000).
Koenig, A. & Borries, C. "The predictive power of socioecological models: A reconsideration of resource characteristics, agonism and dominance hierarchies," in Feeding Ecology in Apes and Other Primates, eds. G. Hohmann, M. M. Robbins & C. Boesch (Cambridge, UK: Cambridge University Press, 2006) 263-284.
Koenig, A. et al. When females should contest for food: Testing hypotheses about resource density, distribution, size and quality with Hanuman langurs (Presbytis entellus). Behavioral Ecology and Sociobiology 42, 225-237 (1998).
Onderdonk, D. A. Infanticide of a newborn black-and-white colobus monkey (Colobus guereza) in Kibale National Park, Uganda. Primates 41, 209-212 (2000).
Snaith, T. V. & Chapman, C. A. Towards an ecological solution to the folivore paradox: Patch depletion as an indicator of within-group scramble competition in red colobus. Behavioral Ecology and Sociobiology 59, 185-190 (2005).
Snaith, T. V. & Chapman, C. A. Primate group size and socioecological models: Do folivores really play by different rules? Evolutionary Anthropology 16, 94-106 (2007).
Snaith, T. V. & Chapman, C. A. Red colobus monkeys display alternative behavioural responses to the costs of scramble competition. Behavioural Ecology 19, 1289-1296 (2008).
Snaith, T. V. et al. Bigger groups have fewer parasites and similar cortisol levels: A multi-group analysis in red colobus monkeys. American Journal of Primatology 70, 1-9 (2008).
Steenbeek, R. & van Schaik, C. P. Competition and group size in Thomas's langurs (Presbytis thomasi): The folivore paradox revisited. Behavioral Ecology and Sociobiology 49, 100-110 (2001).
Teichroeb, J. A. & Sicotte, P. Test of the ecological-constraints model on ursine colobus monkeys (Colobus vellerosus) in Ghana. American Journal of Primatology 71, 49-59 (2009).
Teichroeb, J. A. & Sicotte, P. Infanticide in ursine colobus monkeys (Colobus vellerosus) in Ghana: New cases and a test of the existing hypotheses. Behaviour 145, 727-755 (2008).
Tombak, K. H. et al. Patch depletion behavior differs between sympatric folivorous primates. Primates 53, 57-64 (2012).
van Schaik, C. P. Why are diurnal primates living in groups? Behaviour 87, 120-144 (1983).
van Schaik, C. P. The socioecology of fission-fusion sociality in orangutans. Primates 40, 69-86 (1999).
van Schaik, C. P. et al. The effect of group size on time budgets and social behaviour in wild long-tailed macaques (Macaca fascicularis). Behavioral Ecology and Sociobiology 13, 173-181 (1983).
Wrangham, R. W. On the evolution of ape social systems. Social Science Information 18, 335-368 (1979).
Wrangham, R. W. An ecological model of female-bonded primate groups. Behaviour 75, 262-300 (1980).
Wrangham, R. W., Gittleman, J. L. & Chapman, C. A. Constraints on group size in primates and carnivores: Population density estimates and day-range as assays of exploitation competition. Behavioral Ecology and Sociobiology 32, 199-209 (1993).