« Prev Next »
Introduction: Who are the "Robust" Australopiths?
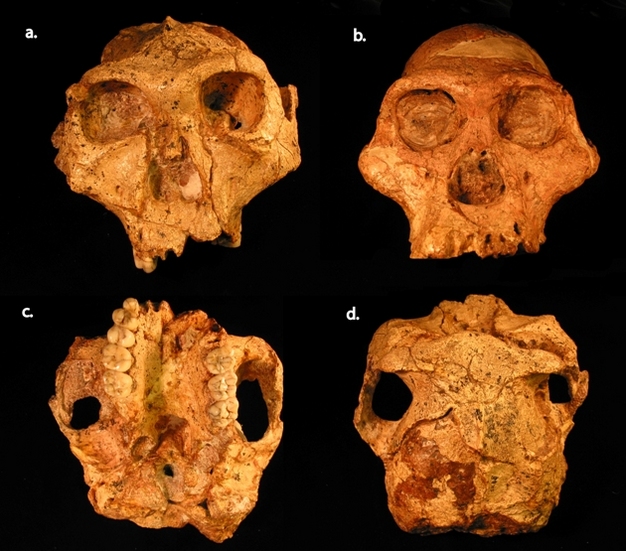
The "robust" australopiths are a group of fossil hominins that existed in East and southern Africa between approximately 2.5 and 1.4 million years ago (Ma). They are referred to here as members of the genus Paranthropus, though considerable disagreement about their proper taxonomy persists (see below). They are characterized by several features of the skull that give them a "robust" appearance when compared to other, more gracile hominins. The most notable of these features are large, thickly enameled, postcanine teeth that were supported by deep and broad mandibular corpora with tall and broad rami (Fig. 1). Other notable features include zygomatic (cheek) bones that were extended both laterally and anteriorly, a face that was more orthognathic (i.e., pulled back towards the rest of the skull) than in other australopiths, and the occasional presence of bony crests on the top and back of the skull, presumably for the attachment of large jaw muscles. Taken together, these traits suggest an animal that could both generate and dissipate high bite forces, and they imply that at least some portion of the Paranthropus diet was particularly difficult to break down1-4.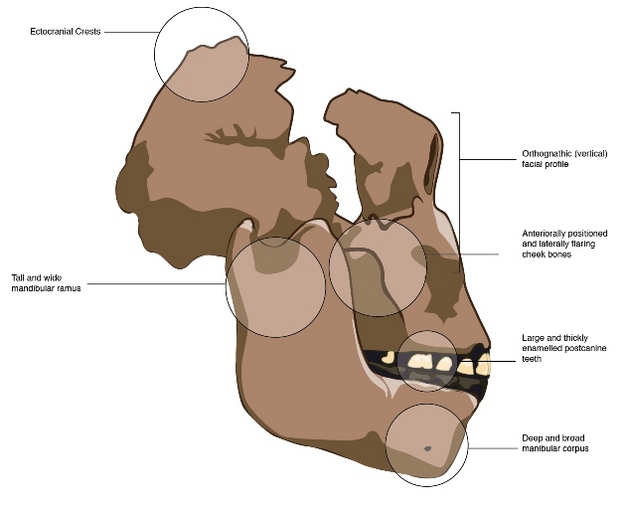
The Fossil Evidence
Southern Africa
A decade later, a second species was added to the genus Paranthropus. This species included fossils recovered by Broom and his colleague John Robinson from Swartkrans Cave, only about 3 miles from Kromdraai (and less than a mile downstream from Sterkfontein)7. Subsequent fieldwork at Swartkrans resulted in the accumulation of a sizeable collection of hominins, most of which (but not all8) were assigned to P. crassidens. Most researchers (e.g.,9-11) have done away with the specific distinction between the Kromdraai and Swartkrans hypodigms and now include specimens from both sites in the species P. robustus (but see12-15). Additional P. robustus specimens have since been discovered at the nearby sites of Drimolen11,16, Gondolin17, and Cooper's Cave18-20.
East Africa
Not long after the discovery of Paranthropus robustus at Swartkrans, evidence of a similar megadont hominin was discovered by Louis Leakey in East Africa. The first evidence consisted of two teeth found in 1955 at Olduvai Gorge in Tanzania21. However, the taxonomy of these teeth was uncertain until the discovery in 1959 of OH 5, a well-preserved adolescent cranium also from Olduvai Gorge22. Leakey thought the OH 5 cranium was distinct from both Australopithecus and Paranthropus, and consequently he attributed it to a new genus and species, Zinjanthropus boisei. Ultimately, however, Leakey was convinced by his fellow paleoanthropologists that while his fossils likely represented a new species, they were not distinct enough from the specimens at Kromdraai and Swartkrans to warrant the formulation of a separate genus. A few years later, Louis and his wife Mary announced the discovery of the Peninj mandible from the western shore of Lake Natron in Tanzania23. Together with the OH 5 cranium, the nearly complete mandible showed this East African species to be an even more "robust" hominin than its counterparts in southern Africa. Subsequently, the hypodigm of what we now refer to as Paranthropus boisei was augmented by discoveries from the Omo region24 and Konso in Ethiopia25, from Koobi Fora26-28, West Turkana29, and the Baringo basin of Kenya30,31, and from considerably further south in Malema, Malawi32. Some of the geologically oldest of these remains, particularly from the Omo region, were ultimately attributed to Paranthropus aethiopicus, the last Paranthropus taxon to be officially recognized. Initially announced as a separate genus, Paraustralopithecus Arambourg and Coppens, 1968, these fossils have since been subsumed in the genus Paranthropus. However, most scientists retain the specific distinction for the fossils of this area that predate 2.3 Ma based on observed changes in dental and mandibular morphology that appear to have occurred around this time33-37 (but see38,39). Most researchers (e.g.,40,41) also include the cranium KNM-WT 17000 and the mandible KNM-WT 16005 from West Turkana in the P. aethiopicus hypodigm. KNM-WT 17000, more popularly known as the "Black Skull" due to the high concentrations of manganese in the soil that turned the bones a blue-black color upon fossilization, has several traits that link it with Paranthropus such as anteriorly positioned cheek bones and presumably large postcanine teeth (based mostly on tooth root size - the cranium is edentulous except for an associated left P4 41). However, it also has traits that link it with the earlier species Australopithecus afarensis such as increased prognathism and a more posteriorly positioned sagittal crest that merges with the nuchal crest (see Ward and Hammond's Scitable article for a summary of the non-robust australopiths). It thus appears that even if P. boisei and P. aethiopicus are retained as separate species, they likely represent chronospecies of the same lineage (i.e., a single ancestor/descendant anagenetic line). How this lineage is related to P. robustus from southern Africa is a topic of debate (see below). For a list of known Paranthropus sites and associated contextual information, see Table 1.
| Region | Site | Geologic Formation | Estimated Age of Hominins (Myr) | Dating Method | Key Specimens | Species Present |
|
East Africa |
West Turkana, Kenya | Nachukui | 2.5-2.35 | radiometric; marker beds | KNM-WT 17000 (cranium), KNM-WT 16005 (mandible) | P. aethiopicus |
| 2.3-1.6 | Various specimens | P. boisei | ||||
| Koobi Fora, Kenya | Koobi Fora | 2.2-1.88 | radiometric; tephrostratigraphy; fission-track, marker beds | KNM-ER 1500 (partial skeleton) and others | P. boisei | |
| 1.88-1.65 | KNM-ER 406, 407, 732 (all crania) and others | P. boisei | ||||
| 1.65-1.39 | KNM-ER 729, 3230 (both mandibles) and others | P. boisei | ||||
| Omo, Ethiopia | Shungura | 2.6-2.3 | radiometric; marker beds | Omo 18-18 (edentulous mandible; holotype of P. aethiopicus), and others, mostly isolated teeth | P. aethiopicus | |
| 2.3-1.2 | Various specimens, mostly teeth | P. boisei | ||||
|
Chesowanja, Kenya |
Chemoigut |
2.0-1.5 |
biostratigraphy; radiometric of capping layer |
CH1 (partial cranium), other fragments |
P. boisei
| |
|
Konso, Ethiopia |
Konso |
1.4 |
radiometric; tephrostratigraphy; marker beds |
KGA 10-525 (skull), and others |
P. boisei
| |
|
Malema, Malawi |
Chiwondo |
1.5 |
biostratigraphy |
RC 911 (maxilla) |
P. boisei
| |
|
Peninj, Tanzania |
Humbu |
1.7-1.3 |
radiometric; magnetostratigraphy |
Peninj mandible |
P. boisei
| |
|
Olduvai Gorge, Tanzania |
Olduvai |
1.9-1.7 |
radiometric; biostratigraphy |
OH 5 (cranium; holotype of P. boisei) |
P. boisei
| |
|
1.7-1.2 |
Various specimens |
P. boisei
| ||||
|
Southern Africa |
Swartkrans, South Africa |
Monte Christo |
1.8-1.5 |
biostratigraphy |
>300 Paranthropus specimens total, mostly isolated dental remains, including SK6 (holotype of P. crassidens) |
P. robustus (P. crassidens) |
|
1.5-1.0 |
P. robustus (P. crassidens) | |||||
|
1.5-1.0 |
P. robustus (P. crassidens) | |||||
|
Kromdraai, South Africa |
Monte Christo |
2.0-1.5 |
biostratigraphy; reversed polarity |
Close to 30 Paranthropus specimens, including TM1517 (skull; holotype of P. robustus) |
P. robustus
| |
| Sterkfontein, South Africa (M5B) |
Monte Christo |
1.4-1.1 |
magnetostratigraphy |
Two specimens: Stw 566 & Stw 569 |
P. robustus
| |
|
Drimolen, South Africa |
Monte Christo |
2.0-1.5 |
Overall faunal assemblage composition; no absoute dates |
>80 hominins, including DNH 7 (nearly complete female skull) and DNH 8 (male mandible) |
P. robustus
| |
|
Gondolin, South Africa |
Eccles |
1.9-1.5 |
biostratigraphy (tentative) |
GDA-2: a very large mandibular M2 |
P. sp. | |
|
Cooper's Cave, South Africa |
Monte Christo |
1.5-1.4 |
radiometric; biostratigraphy |
COB 101 (partial skull) and others, mostly teeth but also postcrania |
P. robustus
| |
|
Table 1: The Paranthropus fossil evidence.
| ||||||
Paranthropus or Australopithecus?
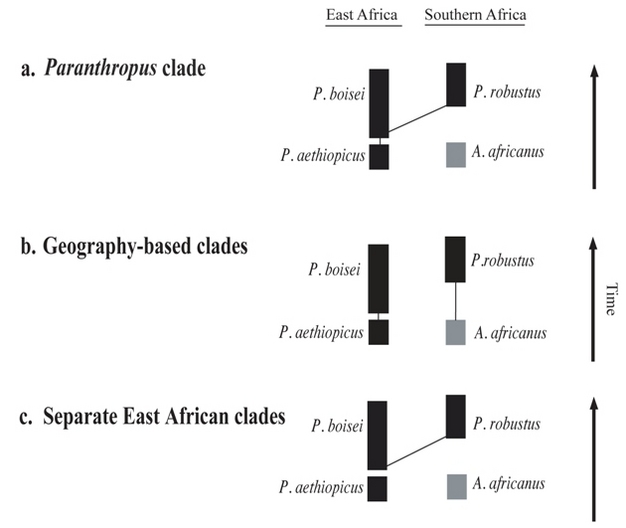
Ever since Broom's 1949 announcement of a new type of hominin from Swartkrans, paleoanthropologists have been debating the taxonomy and phylogeny of the "robust" australopiths 9,10,22,42-47 (Fig. 3). Placing the three commonly recognized species (P. robustus, P. boisei, and P. aethiopicus) in their own genus requires that they are (1) adaptively different from Australopithecus, and (2) monophyletic (i.e., more closely related to each other than to any other species). The current debate largely centers around the latter criterion. Many researchers (e.g.,48) believe that the shared skull morphology of the "robust" australopiths is homoplasious (i.e., independently evolved in two or more of the taxa) and thus place them in the genus Australopithecus by default. Homoplasy does appear to have been prevalent in the evolution of African fauna throughout the course of human evolution49,50, even in the hominins themselves 35,51-54, but several pieces of evidence are nevertheless consistent with Paranthropus monophyly 25,37,55,56. Most telling is the fact that in a thorough cladistic analysis of hominin relationships, Paranthropus monophyly was supported in every instance, even when masticatory characters were excluded56. Therefore, retention of Paranthropus as a distinct genus is warranted until convincing evidence demonstrates otherwise.
Biology and Behavior
Despite the fact that Paranthropus is one of the most well represented hominin groups in the fossil record, there is still much we do not know about the basic biology of this genus. Part of this stems from the fact that we can still not confidently recognize Paranthropus postcrania in the fossil record. It is likely that we already have a reasonable sample of these remains, possibly including such specimens as KNM-ER 150057, OH 3658, and SK 82 and 9759, but we are still unable to unambiguously distinguish them from those of coeval hominins. Solving this problem will allow researchers to provide more reliable estimates of key biological variables such as body size, locomotor habits, and manual dexterity.
The various pieces of evidence we do have about Paranthropus lifeways provide a somewhat contradictory picture of how these hominins grew and behaved on the African landscape. Studies of dental growth and development60,61, inner ear morphology62 and brain shape63 all seem to indicate that Paranthropus was more ape-like than initially recognized. Certainly fossils such as KNM-ER 406 and KNM-ER 732 from Koobi Fora seem to indicate that there was a fairly high level of sexual dimorphism, at least in P. boisei64. On the other hand, several lines of circumstantial evidence point to a more "advanced" hominin, including studies suggesting that Paranthropus used, if not made, tools. Stone and bone tools have been found at Swartkrans65-67 and bone tools at Drimolen16,68, two sites where the vast majority of the hominin remains are attributed to P. robustus. Stone tools have also been found in the Oldowan Infill (aka M5B69) of Sterkfontein's Member 5, where approximately half of the specimens are believed to be those of P. robustus70. Paranthropus tool use is also supported by a series of studies that tentatively assigned Swartkrans hand fossils with a modern human-like precision grip to P. robustus65,71-74. Given that our closest living primate relatives use, and occasionally make, tools75-79, it should not be surprising that Paranthropus did so as well. However, stone tool knapping has long been seen as a major behavioral difference between Paranthropus and the larger brained Homo80. Therefore, acceptance of the idea that Paranthropus was using and possibly preparing such tools will require evidence that is more compelling than the loose associations presented to date.
It appears that Paranthropus was one of the first hominin taxa to routinely venture into open grassland areas 18,25,81-83 (but see84) possibly to acquire novel open habitat resources such as termites 67 or plant underground storage organs such as tubers, bulbs, and grass corms 85-88. Biochemical analyses of the diet of P. robustus imply that these hominins were omnivorous 89-93 and possibly even changed their diets seasonally 94. However, recent studies of P. boisei's dental microwear and stable isotope composition paint a different picture — one of a limited, C4-based diet95,96. Going forward, it will be important to determine whether this behavioral variation is ecologically driven, as we see in modern gorilla diets97, or whether it reveals fundamental differences in the adaptive strategies of two independently evolving lineages.
Paranthropus disappears from the fossil record sometime between 1.4 and 0.9 Ma, after a geologic lifespan of just over a million years 18,25,69. The cause(s) of their extinction is a mystery. Early notions that they had become too specialized to cope with changing environmental conditions have been strongly challenged 94,95. Competition with Homo is plausible, but indisputable evidence for either direct or indirect interaction between the two genera has yet to be discovered. The fact that such basic information is still lacking for Paranthropus makes them an exciting hominin group to study, both in their own right and because they provide an important comparative context for the evolution of our own genus, Homo, at a time when we were evolving many of the traits that we now associate with being human (see the early Homo Scitable article by Anton and Laird).
Acknowledgements
Thanks are due to Bernard Wood, who was a coauthor on the papers from which much of this review has been drawn.
References and Recommended Reading
1 Rak, Y. The Australopithecine Face. New York: Academic Press, 1983.
2 Hylander, W. L. Implications of in vivo experiments for interpreting the functional significance of "robust" australopithecine jaws. Evolutionary History of the 'Robust' Australopithecines, 55-83, eds. F. E. Grine. Aldine, New York, 1988.
3 Demes, B. & Creel, N., Bite force, diet, and cranial morphology
of fossil hominids. Journal of Human Evolution 17,
657-670 (1988). doi:10.1016/0047-2484(88)90023-1
4 Constantino, P. et al., Adaptation to hard-object feeding in
sea otters and hominins. Journal of Human Evolution 61, 89-96 (2012). doi:10.1016/j.jhevol.2011.02.009
5 Broom, R. The Pleistocene anthropoid apes of South Africa. Nature
142, 377-379 (1938). doi:10.1038/142377a0
6 Dart, R. A. Australopithecus africanus: the man-ape of South Africa.
Nature 115, 195-199 (1925). doi:10.1038/115195a0
7 Broom, R. Another new type of fossil ape-man. Nature 163, 57 (1949). doi:10.1038/163057a0
8 Broom, R. & Robinson, J. T., A new type of fossil man (from
Swartkrans). Nature 164,
322-323 (1949). doi:10.1038/164322a0
9 Campbell, B. Quantitative taxonomy and human evolution. Classification and Human Evolution, 50-74. eds. S. L. Washburn. Chicago: Aldine Publishing Company, 1963.
10 Tobias, P. V. The Cranium and Maxillary Dentition of Australopithecus (Zinjanthropus) boisei. Olduvai Gorge. Cambridge: Cambridge University Press, 1967.
11 Keyser, A. W. The Drimolen skull: the most complete
australopithecine cranium and mandible to date. South African Journal of Science 96, 189-197 (2000). (link)
12 Riesenfield, A. The variability of the temporal
lines, its cause and effects. American Journal of Physical Anthropology 13, 599-620 (1955). doi:10.1002/ajpa.1330130404
13 Howell, F. C. Hominidae. Evolution of African Mammals, 154-248, eds. V. J. Maglio & H. B. S. Cooke Cambridge, Harvard University Press, 1978).
14 Grine, F. New hominid fossils from the Swartkrans
Formation (1979-1986 expeditions): craniodental specimens. American Journal of Physical Anthropology 79, 409-449 (1989). doi:10.1002/ajpa.1330790403
15 Grine, F. E. Description and preliminary analysis of new hominid craniodental fossils from the Swartkrans Formation. Swartkrans: a cave's chronicle of early man Vol. 8, 7-116, eds. C. K. Brain. Swartkrans Museum Monograph Series, 1993.
16 Keyser, A. W. et
al. Drimolen: a new hominid-bearing site in Gauteng,
South Africa. South African Journal of Science 96, 193-197
(2000). (link)
17 Menter, C. G. et
al. First record of hominid teeth from the Plio-Pleistocene site of Gondolin, South
Africa. Journal of Human Evolution 37, 299-307 (1999). doi:10.1006/jhev.1999.0329
18 de Ruiter, D. J. et al., New Australopithecus
robustus fossils and associated U-Pb dates from Cooper's Cave. Journal of Human Evolution 56, 497-513 (2009). doi: 10.1016/j.jhevol.2009.01.009
19 Steininger, C. M., Berger, L. R. & Kuhn, B. F. A partial skull of Paranthropus robustus from Cooper's Cave, South Africa. South African Journal of Science 104, 143-146 (2008). (link)
20 Berger, L. R. et
al. Preliminary results of excavations at the newly investigated Coopers D
deposit, Gauteng, South Africa. South African Journal of Science 99, 276-278 (2003). (link)
21 Leakey, L. S. B., A giant child among the giant animals of Olduvai? A huge fossil milk molar which suggests that Chellean Man in Tanganyika may have been gigantic. The Illustrated London News 232, 1104-1105 (1958).
22 Leakey, L. S. B. A new fossil skull from Olduvai. Nature
184, 491-493 (1959). doi:10.1038/184491a0
23 Leakey, L. S. B. & Leakey, M. D. Recent
discoveries of fossil hominids in Tanganyika:
at Olduvai and near Lake
Natron. Nature 202, 5-7 (1964). doi:10.1038/202005a0
24 Arambourg, C. & Coppens, Y. Decouverte d'un australopithecien nouveau dans les gisements de l'Omo (Ethiopie). South African Journal of Science 64, 58-59 (1968).
25 Suwa, G. et al., The first skull of Australopithecus
boisei. Nature 389,
489-492 (1997). doi:10.1038/39037
26 Leakey, R. E., Mungai, J. M., & Walker, A. C. New
australopithecines from East Rudolf,
Kenya (II). American Journal of Physical Anthropology 36, 235-252
(1972). doi:10.1002/ajpa.1330360212
27 Leakey, M. G. & Leakey, R. E. eds. Koobi Fora Research Project. Volume 1. The Fossil Hominids and an Introduction to their Context, 1968-1974. Oxford: Clarendon Press, 1978.
28 Leakey, R. E. F. New hominid remains and early
artefacts from northern Kenya:
fauna and artefacts from a new Plio-Pleistocene locality near Lake Rudolf in Kenya.
Nature 226, 223-224 (1970). doi:10.1038/226223a0
29 Leakey, R. E. F. & Walker, A. New Australopithecus boisei
specimens from East and West Lake Turkana, Kenya. American Journal of Physical Anthropology
76, 1-24 (1988). doi:10.1002/ajpa.1330760102
30 Carney, J. et
al. Late australopithecine from Baringo
District, Kenya.
Nature 230, 509-514 (1971). doi:10.1038/230509a0
31 Walker,
A. Chesowanja australopithecine. Nature 238, 108-109 (1972). doi:10.1038/238108a0
32 Kullmer, O. et al. The first Paranthropus from the Malawi Rift. Journal of Human Evolution 37, 121-127 (1999).
33 Suwa, G., Evolution of the "robust" australopithecines in the Omo succession: evidence from mandibular premolar morphology Evolutionary History of the "Robust" Australopithecines, 199-222, eds. F. E. Grine. New York: Aldine de gruyte, 1988.
34 Suwa, G., White, T. D. & Howell, F. C.
Mandibular postcanine dentition from the Shungura Formation, Ethiopia: crown
morphology, taxonomic allocations and Plio-Pleistocene hominid evolution. American Journal of Physical Anthropology 101, 247-282
(1996). doi:10.1002/(SICI)1096-8644(199610)101:2<247::AID-AJPA9>3.0.CO;2-Z
35 Wood, B. Early hominid species and speciation. Journal of Human Evolution 22, 351-365 (1992). doi:10.1016/0047-2484(92)90065-H
36 Wood, B. A., Wood, C. W. & Konigsberg,
L. W. Paranthropus boisei: an example of evolutionary stasis? American Journal of Physical Anthropology 95, 117-136
(1994). doi:10.1002/ajpa.1330950202
37 Constantino, P. & Wood, B. Paranthropus paleobiology. Zona Arqueologica. Miscelanea en homenaje a Emiliano Aguirre: Paleoantropologia Vol. III., 137-151, Paleoantropologia., 2004.
38 Curnoe, D. Cranial variability in East African
"robust" hominins. Human Evolution 16, 169-198 (2001). doi:10.1007/BF02437411
39 Ramirez-Rozzi, F. Can enamel microstructure be
used to establish the presence of different species of Plio-Pleistocene
hominids from Omo, Ethiopia? Journal of Human Evolution 35, 543-576 (1998). doi:10.1006/jhev.1998.0250
40 Kimbel, W. H., White, T. D. & Johanson, D. C. Implications of KNM-WT 17000 for the evolution of 'robust' Australopithecus in Evolutionary history of the 'robust' australopithecines, 259-268, eds. F. E. Grine. New York: Aldine de Gruyter, 1988.
41 Suwa, G. The premolar of KNM-WT 17000
and relative anterior to posterior dental size. Journal of Human Evolution 18, 795-799 (1989). doi:10.1016/0047-2484(89)90090-0
42 Washburn, S. L. & Patterson, B. Evolutionary
importance of the South African "man-apes". Nature 167, 650-651 (1951). doi:10.1038/167650a0
43 Clark, W. E. L. G. The fossil evidence for human evolution: an introduction to the study of paleoanthropology. Chicago: University of Chicago Press, 1955.
44 Robinson, J. T. The genera and species of the
Australopithecinae. American Journal of Physical Anthropology 12, 181-200 (1954). doi:10.1002/ajpa.1330120216
45 Robinson, J. T. Homo habilis and the
australopithecines. Nature 205,
121-124 (1965). doi:10.1038/205121a0
46 Constantino, P. & Wood, B. The evolution of Zinjanthropus
boisei. Evolutionary Anthropology 16, 49-62 (2007). doi:10.1002/evan.20130
47 Wood, B. & Constantino, P. Paranthropus
boisei: Fifty years of evidence and analysis. American Journal of Physical Anthropology
134, 106-132 (2007). doi:10.1002/ajpa.20732
48 Skelton, R. R. & McHenry, H. M. Evolutionary
relationships among early hominids. Journal of Human Evolution 23, 309-349 (1992). doi:10.1016/0047-2484(92)90070-P
49 Bishop, L. C. Suid paleoecology and habitat preferences at African Pliocene and Pleistocene hominid localities. African biogeography, climate change and human evolution, 216-225, eds. T. G. Bromage & F. Schrenk. New York: Oxford University Press, 1999.
50 Turner, A. & Wood, B. Comparative
palaeontological context for the evolution of the early hominid masticatory
system. Journal of Human Evolution 24,
301-318 (1993). doi:10.1006/jhev.1993.1023
51 Asfaw, B. et al. Australopithecus garhi:
a new species of early hominid from Ethiopia. Science 284, 629-635 (1999). doi:10.1126/science.284.5414.629
52 Collard, M. & Wood, B. Grades among the African early hominids. African biogeography, climate change, and early hominid evolution, 316-327, eds. T. Bromage & F. Schrenk. Oxford University Press, New York, 1999.
53 Elton, S., Bishop, L. C. & Wood, B. Comparative
context of Plio-Pleistocene hominin brain evolution. Journal of Human Evolution 41, 1-27 (2001). doi:10.1006/jhev.2001.0475
54 Leakey, M. G. et al. New hominin genus from
eastern Africa shows diverse middle Pliocene
lineages. Nature 410, 433-440
(2001). doi:10.1038/35068500
55 Wood, B. A. Are 'robust' australopithecines a monophyletic group? in Evolutionary History of the "Robust" Australopithecines, 269-284, eds. F. E. Grine. New York: Aldine de Gruyter, 1988.
56 Strait, D. S., Grine, F. E. & Moniz, M. A. A
reappraisal of early hominid phylogeny. Journal of Human Evolution 32, 17-82 (1997). doi:10.1006/jhev.1996.0097
57 Grausz, H. M. et al. Associated cranial and postcranial bones of Australopithecus boisei in Evolutionary History of the "Robust" Australopithecines, 127-132, eds. F. E. Grine. New York: Aldine de Gruyter, 1988.
58 Aiello, L. C. et
al. Morphological and taxonomic affinities of the Olduvai ulna (OH 36). American Journal of Physical Anthropology 109, 89-110
(1999). doi:10.1002/(SICI)1096-8644(199905)109:1<89::AID-AJPA8>3.0.CO;2-4
59 Ruff, C. B., McHenry, H. M. & Thackeray, J. F.
Cross-sectional morphology of the SK 82 and 97 proximal femora. American Journal of Physical Anthropology 109, 509-521 (1999). doi:10.1002/(SICI)1096-8644(199908)109:4<509::AID-AJPA7>3.0.CO;2-X
60 Dean, M. C. et
al. Histological reconstruction of dental development and age at death of a
juvenile Paranthropus robustus specimen, SK 63, from Swartkrans, South Africa. American Journal of Physical Anthropology 91,
401-419 (1993). doi:10.1002/ajpa.1330910402
61 Dean, M. C. et al. Growth processes in teeth
distinguish modern humans from Homo erectus and earlier hominins. Nature
414, 628-631 (2001). doi:10.1038/414628a
62 Spoor C. F. The comparative morphology and phylogeny of the human bony labyrinth. Utrecht: Monograph Utrecht University, 1993.
63 Falk, D. et al. Early hominid brain
evolution: a new look at old endocasts. Journal of Human Evolution 38, 695-717 (2000). doi:10.1006/jhev.1999.0378
64 Kimbel, W. H. & White, T. D. Variation, sexual dimorphism and the taxonomy of Australopithecus. Evolutionary history of the 'robust' australopithecines, 175-192 eds. F. E. Grine. New York, Aldine, 1988.
65 Brain, C. K. et al. New evidence of early hominids, their culture and environment from the Swartkrans cave, South Africa. South African Journal of Science 84, 828-835 (1988).
66 Backwell, L. R. & d'Errico, F. Additional evidence on the early hominid bone tools from Swartkrans with reference to spatial distribution of lithic and organic artefacts. South African Journal of Science 99, 259-267 (2003).
67 Backwell, L. R. & d'Errico, F. Evidence of
termite foraging by Swartkrans early hominids. PNAS
98, 1358-1363 (2001). doi:10.1073/pnas.98.4.1358
68 Backwell, L., d'Errico, F. & Wadley, L. Middle
Stone Age bone tools from the Howiesons Poort layers, Sibudu Cave, South
Africa. Journal of Archaeological Science 35, 1566 (2008). doi:10.1016/j.jas.2007.11.006
69 Herries, A. I. R. & Shaw, J. Palaeomagnetic
analysis of the Sterkfontein palaeocave deposits: Implications for the age of
the hominin fossils and stone tool industries. Journal of Human Evolution 60, 523-539 (2011). doi:10.1016/j.jhevol.2010.09.001
70 Kuman, K. & Clarke, R.J. Stratigraphy,
artifact industries and hominid associations for Sterkfontein, Member 5. Journal of Human Evolution 38, 827-847 (2000). doi:10.1006/jhev.1999.0392
71 Susman, R. L. Hand of Paranthropus robustus
from Member 1, Swartkrans: Fossil evidence for tool behaviour. Science 240, 781-784 (1988). doi:10.1126/science.3129783
72 Susman, R. L. New hominid fossils from the
Swartkrans Formation (1979-1986 excavations): postcranial specimens. American Journal of Physical Anthropology 79, 451-474
(1989). doi:10.1002/ajpa.1330790403
73 Susman, R. L. New postcranial remains from Swartkrans and their bearing on the functional morphology and behavior of Paranthropus robustus. Evolutionary History of the 'Robust' Australopithecines, 149-172, eds. F. E. Grine. New York: Aldine de Gruyter, 1988.
74 Susman, R. L. Who made the Oldowan tools? Fossil evidence for tool behavior in Plio-Pleistocene hominids. Journal of Anthropological Research 47, 129-151 (1991).
75 Boesch, C. & Boesch, H. Tool use and tool
making in wild chimpanzees. Folia Primatologica 54, 86-99 (1990). (link)
76 McGrew, W. C. Chimpanzee Material Culture: Implications for Human Evolution. Cambridge: Cambridge University Press, 1992.
77 McGrew, W. C. Culture in nonhuman primates? Annual
Review of Anthropology 27,
301-328 (1998). doi:10.1146/annurev.anthro.27.1.301
78 van Schaik, C. P. et al. Orangutan cultures and the evolution of material culture. Science
299, 102-105 (2003). doi:10.1126/science.1078004
79 Breuer, T., Ndoundou-Hockemba, M. & Fishlock,
V. First observation of tool use in wild gorillas. PLoS Biology 3, e380 (2005). doi:10.1371/journal.pbio.0030380
80 Leakey, L. S. B., Tobias, P.V. & Napier, J. R. A
new species of the genus Homo from Olduvai Gorge.
Nature 202, 7-9 (1964). doi:10.1038/202007a0
81 Reed, K. E. Early hominid evolution and ecological
change through the African Plio-Pleistocene. Journal of Human Evolution 32, 289-322 (1997). doi:10.1006/jhev.1996.0106
82 Sillen, A. et
al. 87Sr/86Sr ratios in modern and fossil food-webs of the Strekfontein Valley: implications for early hominid
habitat preference. Geochimica et Cosmochimica Acta 62, 2463-2473 (1998). doi:10.1016/S0016-7037(98)00182-3
83 Kullmer, O. et al. The Malawi rift: biogeography, ecology, and coexistence of Homo and Paranthropus. Anthropologie (Brno) 37, 221-231 (1999).
84 de Ruiter, D. J., Sponheimer, M. & Lee-Thorp,
J. A. Indications of habitat association of Australopithecus robustus in
the Bloubank Valley, South Africa. Journal of Human Evolution 55, 1015 (2008). doi:10.1016/j.jhevol.2008.06.003
85 Hatley, T. & Kappelman, J. Bears, pigs, and Plio-Pleistocene hominids: a case for the exploitation of belowground food resources. Human Ecology 8, 371-387 (1980).
86 Laden, G. & Wrangham, R. The rise of the
hominids as an adaptive shift in fallback foods: Plant underground storage
organs (USOs) and australopith origins. Journal of Human Evolution 49, 482-498 (2005). doi:10.1016/j.jhevol.2005.05.007
87 Yeakel, J.D. et
al. The isotopic ecology of African mole rats informs hypotheses on the
evolution of human diet. Proceedings of the Royal Society B Biological Sciences 274, 1723-1730 (2007). doi:10.1098/rspb.2007.0330
88 Dominy,
N.J. et
al. Mechanical
properties of plant underground storage organs and implications for dietary
models of early hominins. Evolutionary Biolology 35, 159-175 (2008). doi:10.1007/s11692-008-9026-7
89 Lee-Thorp, J. A., van der Merwe, N. J.
& Brain, C. K. Diet of Australopithecus robustus at Swartkrans from
stable carbon isotopic analysis. Journal of Human Evolution 27, 361-372 (1994). doi:10.1006/jhev.1994.1050
90 Lee-Thorp, J.A., Thackeray, F. & Van de Merwe,
N. The hunters and the hunted revisited. Journal of Human Evolution 39, 565-576 (2000). doi:10.1006/jhev.2000.0436
91 Sillen, A. Strontium-calcium ratios (Sr/Ca) of Australopithecus
robustus and associated fauna from Swartkrans. Journal of Human Evolution 23, 495-516 (1992). doi:10.1016/0047-2484(92)90049-F
92 Sillen, A., Hall, G. & Armstrong, R. Strontium
calcium ratios (Sr/Ca) and strontium isotopic ratios (87Sr/86Sr) of Australopithecus
robustus and Homo sp. in Swartkrans. Journal of Human Evolution 28, 277-285 (1995). doi:10.1006/jhev.1995.1020
93 Sponheimer, M. et al. Hominins, sedges, and
termites: new carbon isotope data from the Sterkfontein valley and Kruger National
Park. Journal of Human Evolution 48, 301-312 (2005). doi:10.1016/j.jhevol.2004.11.008
94 Sponheimer, M. et al. Isotopic evidence for
dietary variability in the early hominin Paranthropus robustus. Science
314, 980-982 (2006). doi:10.1126/science.1133827