Key Points
-
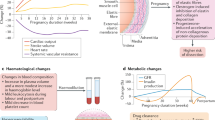
The physiological changes of pregnancy result in changes to the pharmacokinetics of many drugs used for the treatment of cardiovascular disease, often necessitating an increase in dosage
-
Avoiding necessary medication for fear of teratogenicity threatens both mother and fetus, and is often a worse option than accepting the small increase in fetal risk related to the medication
-
Most drugs for cardiovascular disease can be used safely during pregnancy; exceptions include high-dose warfarin during the first trimester, angiotensin-converting-enzyme inhibitors, angiotensin-receptor blockers, amiodarone, and spironolactone
-
Anticoagulation in pregnant women with mechanical heart valves is complex, and these women need expert care in specialized centres throughout their pregnancies
-
Cardiologists should be able to advise obstetricians about the safe use of tocolytic and uterotonic drugs in patients with cardiovascular disease
Abstract
One-third of women with heart disease use medication for the treatment of cardiovascular disease (CVD) during pregnancy. Increased plasma volume, renal clearance, and liver enzyme activity in pregnant women change the pharmacokinetics of these drugs, often resulting in the need for an increased dose. Fetal well-being is a major concern among pregnant women. Fortunately, many drugs used to treat CVD can be used safely during pregnancy, with the exception of high-dose warfarin in the first trimester, angiotensin-converting-enzyme inhibitors, angiotensin-receptor blockers, amiodarone, and spironolactone. A timely and thorough discussion between the cardiologist and the pregnant patient about the potential benefits and adverse effects of medication for CVD is important. Noncompliance with necessary treatment for cardiovascular disorders endangers not only the mother, but also the fetus. This Review is an overview of the pharmacokinetic changes in medications for CVD during pregnancy and the safety of these drugs for the fetus. The implications for maternal treatment are discussed. The Review also includes a short section on the cardiovascular effects of medication used for obstetric indications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Cantwell, R. et al. Saving mothers' lives: reviewing maternal deaths to make motherhood safer: 2006–2008 The eighth report of the confidential enquiries into maternal deaths in the United Kingdom. BJOG 118 (Suppl. 1), 1–203 (2011).
Schutte, J. M. et al. Indirect maternal mortality increases in the Netherlands. Acta Obstet. Gynecol. Scand. 89, 762–768 (2010).
Schutte, J. M. et al. Rise in maternal mortality in the Netherlands. BJOG 117, 399–406 (2010).
Drenthen, W. et al. Predictors of pregnancy complications in women with congenital heart disease. Eur. Heart J. 31, 2124–2132 (2010).
Drenthen, W. et al. Outcome of pregnancy in women with congenital heart disease: a literature review. J. Am. Coll. Cardiol. 49, 2303–2311 (2007).
Roos-Hesselink, J. W. et al. Outcome of pregnancy in patients with structural or ischaemic heart disease: results of a registry of the European Society of Cardiology. Eur. Heart J. 34, 657–665 (2013).
Siu, S. C. et al. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation 104, 515–521 (2001).
Pieper, P. G. et al. Uteroplacental blood flow, cardiac function, and pregnancy outcome in women with congenital heart disease. Circulation 128, 2478–2487 (2013).
Yap, S. C. et al. Comparison of pregnancy outcomes in women with repaired versus unrepaired atrial septal defect. BJOG 116, 1593–1601 (2009).
Drenthen, W. et al. Non-cardiac complications during pregnancy in women with isolated congenital pulmonary valvar stenosis. Heart 92, 1838–1843 (2006).
Vriend, J. W. et al. Outcome of pregnancy in patients after repair of aortic coarctation. Eur. Heart J. 26, 2173–2178 (2005).
Odalovic, M. et al. Predictors of the use of medications before and during pregnancy. Int. J. Clin. Pharm. 35, 408–416 (2013).
Ruys, T. P. et al. Heart failure in pregnant women with cardiac disease: data from the ROPAC. Heart 100, 231–238 (2014).
Ruys, T. P. et al. Cardiac medication during pregnancy, data from the ROPAC. Int. J. Cardiol. 177, 124–128 (2014).
Costantine, M. M. Physiologic and pharmacokinetic changes in pregnancy. Front. Pharmacol. 5, 65 (2014).
Lupattelli, A., Spigset, O. & Nordeng, H. Adherence to medication for chronic disorders during pregnancy: results from a multinational study. Int. J. Clin. Pharm. 36, 145–153 (2014).
Anderson, G. D. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin. Pharmacokinet. 44, 989–1008 (2005).
Anderson, G. D. & Carr, D. B. Effect of pregnancy on the pharmacokinetics of antihypertensive drugs. Clin. Pharmacokinet. 48, 159–168 (2009).
European Society of Gynecology (ESG) et al. ESC guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur. Heart J. 32, 3147–3197 (2011).
Hytten, F. E. & Paintin, D. B. Increase in plasma volume during normal pregnancy. J. Obstet. Gynaecol. Br. Emp. 70, 402–407 (1963).
Clark, S. L. et al. Central hemodynamic assessment of normal term pregnancy. Am. J. Obstet. Gynecol. 161, 1439–1442 (1989).
Hunter, S. & Robson, S. C. Adaptation of the maternal heart in pregnancy. Br. Heart J. 68, 540–543 (1992).
Sturgiss, S. N., Dunlop, W. & Davison, J. M. Renal haemodynamics and tubular function in human pregnancy. Baillieres Clin. Obstet. Gynaecol. 8, 209–234 (1994).
Dunlop, W. & Davison, J. M. Renal haemodynamics and tubular function in human pregnancy. Baillieres Clin. Obstet. Gynaecol. 1, 769–787 (1987).
Frederiksen, M. C. Physiologic changes in pregnancy and their effect on drug disposition. Semin. Perinatol. 25, 120–123 (2001).
Dunlop, W. Serial changes in renal haemodynamics during normal human pregnancy. Br. J. Obstet. Gynaecol. 88, 1–9 (1981).
Davison, J. M. & Hytten, F. E. Glomerular filtration during and after pregnancy. J. Obstet. Gynaecol. Br. Commonw. 81, 588–595 (1974).
Riva, E. et al. Pharmacokinetics of furosemide in gestosis of pregnancy. Eur. J. Clin. Pharmacol. 14, 361–366 (1978).
Lynch, T. & Price, A. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am. Fam. Physician 76, 391–396 (2007).
Haas, D. M. & D'Alton, M. Pharmacogenetics and other reasons why drugs can fail in pregnancy: higher dose or different drug? Obstet. Gynecol. 120, 1176–1179 (2012).
De Sevo, M. R. Genetic variations in warfarin metabolism: why one size doesn't fit all with some drugs. Nurs. Womens Health 14, 131–136 (2010).
Högstedt, S., Lindberg, B., Peng, D. R., Regårdh, C. G. & Rane, A. Pregnancy-induced increase in metoprolol metabolism. Clin. Pharmacol. Ther. 37, 688–692 (1985).
Prevost, R. R., Akl, S. A., Whybrew, W. D. & Sibai, B. M. Oral nifedipin pharmacokinetics in pregnancy-induced hypertension. Pharmacotherapy 12, 174–177 (1992).
Barbour, L. A., Oja, J. L. & Schultz, L. K. A prospective trial that demonstrates that dalteparin requirements increase in pregnancy to maintain therapeutic levels of anticoagulation. Am. J. Obstet. Gynecol. 191, 1024–1029 (2004).
Lebaudy, C. et al. Changes in enoxaparin pharmacokinetics during pregnancy and implications for antithrombotic therapeutic strategy. Clin. Pharmacol. Ther. 84, 370–377 (2008).
Brehm, K. et al. Mechanical heart valve recipients: anticoagulation in patients with genetic variations of phenprocoumon metabolism. Eur. J. Cardiothorac. Surg. 44, 309–315 (2013).
Lopez-Parra, A. M., Borobia, A. M., Baeza, C., Arroyo-Pardo, E. & Carcas, A. J. A multiplex assay to detect variations in the CYP2C9, VKORC1, CYP4F2 and APOE genes involved in acenocoumarol metabolism. Clin. Biochem. 46, 167–169 (2013).
Wadelius, M. et al. Association of warfarin dose with genes involved in its action and metabolism. Hum. Genet. 121, 23–34 (2007).
Koren, G., Pastuszak, A. & Ito, S. Drugs in pregnancy. N. Engl. J. Med. 338, 1128–1137 (1998).
Rakusan, K. Drugs in pregnancy: Implications for a cardiologist. Exp. Clin. Cardiol. 15, e100–e103 (2010).
Thorpe, P. G. et al. Medications in the first trimester of pregnancy: most common exposures and critical gaps in understanding fetal risk. Pharmacoepidemiol. Drug Saf. 22, 1013–1018 (2013).
Koren, G., Clark, S. & Matsui, D. Drugs during pregnancy and lactation: new solutions to serious challenges. Obstet. Gynecol. Int. 2012, 206179 (2012).
Kusters, D. M. et al. Statin use during pregnancy: a systematic review and meta-analysis. Expert Rev. Cardiovasc. Ther. 10, 363–378 (2012).
FDA, HHS. Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling. Final rule. Fed. Regist. 79, 72063–72103 (2014).
US Food and Drug Administration. Pregnancy and lactation labeling final rule [online], (2014).
Balci, A. et al. Pregnancy in women with corrected tetralogy of Fallot: occurrence and predictors of adverse events. Am. Heart J. 161, 307–313 (2011).
Gelson, E. et al. Effect of maternal heart disease on fetal growth. Obstet. Gynecol. 117, 886–891 (2011).
Yakoob, M. Y. et al. The risk of congenital malformations associated with exposure to beta-blockers early in pregnancy: a meta-analysis. Hypertension 62, 375–381 (2013).
Nakhai-Pour, H. R., Rey, E. & Bérard, A. Antihypertensive medication use during pregnancy and the risk of major congenital malformations or small-for-gestational-age newborns. Birth Defects Res. B. Dev. Reprod. Toxicol. 89, 147–154 (2010).
Meidahl Petersen, K. et al. β-Blocker treatment during pregnancy and adverse pregnancy outcomes: a nationwide population-based cohort study. BMJ Open 2, e001185 (2012).
Abalos, E., Duley, L. & Steyn, D. W. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database of Systematic Reviews, Issue 2, Art. No.: CD002252 http://dx.doi.org/10.1002/14651858.CD002252.pub3,.
Ersboll, A. S., Hedegaard, M., Sondergaard, L., Ersboll, M. & Johansen, M. Treatment with oral beta-blockers during pregnancy complicated by maternal heart disease increases the risk of fetal growth restriction. BJOG 121, 618–626 (2014).
Chow, T., Galvin, J. & McGovern, B. Antiarrhythmic drug therapy in pregnancy and lactation. Am. J. Cardiol. 82, 58I–62I (1998).
Crooks, B. N., Deshpande, S. A., Hall, C., Platt, M. P. & Milligan, D. W. Adverse neonatal effects of maternal labetalol treatment. Arch. Dis. Child. Fetal Neonatal Ed. 79, F150–F151 (1998).
Gladstone, G. R., Hordof, A. & Gersony, W. M. Propranolol administration during pregnancy: effects on the fetus. J. Pediatr. 86, 962–964 (1975).
Klarr, J. M., Bhatt-Mehta, V. & Donn, S. M. Neonatal adrenergic blockade following single dose maternal labetalol administration. Am. J. Perinatol. 11, 91–93 (1994).
Kockova, R. et al. Heart rate changes mediate the embryotoxic effect of antiarrhythmic drugs in the chick embryo. Am. J. Physiol. Heart Circ. Physiol. 304, H895–H902 (2013).
Hejnova, L. et al. Adenylyl cyclase signaling in the developing chick heart: the deranging effect of antiarrhythmic drugs. Biomed. Res. Int. 2014, 463123 (2014).
Osol, G. & Mandala, M. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda) 24, 58–71 (2009).
Prefumo, F., Sebire, N. J. & Thilaganathan, B. Decreased endovascular trophoblast invasion in first trimester pregnancies with high-resistance uterine artery Doppler indices. Hum. Reprod. 19, 206–209 (2004).
Baschat, A. A. & Hecher, K. Fetal growth restriction due to placental disease. Semin. Perinatol. 28, 67–80 (2004).
Kampman, M. A. et al. Maternal cardiac function, uteroplacental Doppler flow parameters and pregnancy outcome: a systematic review. Ultrasound Obstet. Gynecol. 46, 21–28 (2015).
Prefumo, F. et al. Maternal cardiovascular function in pregnancies complicated by intrauterine growth restriction. Ultrasound Obstet. Gynecol. 31, 65–71 (2008).
Vasapollo, B., Novelli, G. P. & Valensise, H. Total vascular resistance and left ventricular morphology as screening tools for complications in pregnancy. Hypertension 51, 1020–1026 (2008).
Molvi, S. N., Mir, S., Rana, V. S., Jabeen, F. & Malik, A. R. Role of antihypertensive therapy in mild to moderate pregnancy-induced hypertension: a prospective randomized study comparing labetalol with alpha methyldopa. Arch. Gynecol. Obstet. 285, 1553–1562 (2012).
el-Qarmalawi, A. M., Morsy, A. H., al-Fadly, A., Obeid, A. & Hashem, M. Labetalol vs. methyldopa in the treatment of pregnancy-induced hypertension. Int. J. Gynaecol. Obstet. 49, 125–130 (1995).
Al Khaja, K. A., Sequeira, R. P., Alkhaja, A. K. & Damanhori, A. H. Drug treatment of hypertension in pregnancy: a critical review of adult guideline recommendations. J. Hypertens. 32, 454–463 (2014).
van Driel, D. et al. Teratogen update: fetal effects after in utero exposure to coumarins overview of cases, follow-up findings, and pathogenesis. Teratology 66, 127–140 (2002).
Wesseling, J. et al. Coumarins during pregnancy: long-term effects on growth and development of school-age children. Thromb. Haemost. 85, 609–613 (2001).
van Driel, D. et al. In utero exposure to coumarins and cognition at 8 to 14 years old. Pediatrics 107, 123–129 (2001).
Chan, W. S., Anand, S. & Ginsberg, J. S. Anticoagulation of pregnant women with mechanical heart valves: a systematic review of the literature. Arch. Intern. Med. 160, 191–196 (2000).
Geelani, M. A. et al. Anticoagulation in patients with mechanical valves during pregnancy. Asian Cardiovasc. Thorac. Ann. 13, 30–33 (2005).
Akhtar, R. P., Abid, A. R., Zafar, H., Cheema, M. A. & Khan, J. S. Anticoagulation in pregnancy with mechanical heart valves: 10-year experience. Asian Cardiovasc. Thorac. Ann. 15, 497–501 (2007).
Meschengieser, S. S., Fondevila, C. G., Santarelli, M. T. & Lazzari, M. A. Anticoagulation in pregnant women with mechanical heart valve prostheses. Heart 82, 23–26 (1999).
Khamooshi, A. J. et al. Anticoagulation for prosthetic heart valves in pregnancy. Is there an answer? Asian Cardiovasc. Thorac. Ann. 15, 493–496 (2007).
Sadler, L. et al. Pregnancy outcomes and cardiac complications in women with mechanical, bioprosthetic and homograft valves. BJOG 107, 245–253 (2000).
Hassouna, A. & Allam, H. Limited dose warfarin throughout pregnancy in patients with mechanical heart valve prosthesis: a meta-analysis. Interact. Cardiovasc. Thorac. Surg. 18, 797–806 (2014).
Vitale, N. et al. Dose-dependent fetal complications of warfarin in pregnant women with mechanical heart valves. J. Am. Coll. Cardiol. 33, 1637–1641 (1999).
Malik, H. T., Sepehripour, A. H., Shipolini, A. R. & McCormack, D. J. Is there a suitable method of anticoagulation in pregnant patients with mechanical prosthetic heart valves? Interact. Cardiovasc. Thorac. Surg. 15, 484–488 (2012).
Al-Lawati, A. A., Venkitraman, M., Al-Delaime, T. & Valliathu, J. Pregnancy and mechanical heart valves replacement; dilemma of anticoagulation. Eur. J. Cardiothorac. Surg. 22, 223–227 (2002).
Basude, S., Hein, C., Curtis, S. L., Clark, A. & Trinder, J. Low-molecular-weight heparin or warfarin for anticoagulation in pregnant women with mechanical heart valves: what are the risks? A retrospective observational study. BJOG 119, 1008–1013 (2012).
Yinon, Y. et al. Use of low molecular weight heparin in pregnant women with mechanical heart valves. Am. J. Cardiol. 104, 1259–1263 (2009).
Quinn, J. et al. Use of high intensity adjusted dose low molecular weight heparin in women with mechanical heart valves during pregnancy: a single-center experience. Haematologica 94, 1608–1612 (2009).
Xiang, L., Wei, Z. & Cao, Y. Symptoms of an intrauterine hematoma associated with pregnancy complications: a systematic review. PLoS ONE 9, e111676 (2014).
Greer, I. A. & Nelson-Piercy, C. Low-molecular-weight heparins for thromboprophylaxis and treatment of venous thromboembolism in pregnancy: a systematic review of safety and efficacy. Blood 106, 401–407 (2005).
Regitz-Zagrosek, V., Gohlke-Barwolf, C., Iung, B. & Pieper, P. G. Management of cardiovascular diseases during pregnancy. Curr. Probl. Cardiol. 39, 85–151 (2014).
Bates, S. M. et al. Venous thromboembolism, thrombophilia, antithrombotic therapy, and pregnancy: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest 133, S844–S886 (2008).
Nishimura, R. A. et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Thorac. Cardiovasc. Surg. 148, e1–e132 (2014).
Oran, B., Lee-Parritz, A. & Ansell, J. Low molecular weight heparin for the prophylaxis of thromboembolism in women with prosthetic mechanical heart valves during pregnancy. Thromb. Haemost. 92, 747–751 (2004).
McLintock, C., McCowan, L. M. & North, R. A. Maternal complications and pregnancy outcome in women with mechanical prosthetic heart valves treated with enoxaparin. BJOG 116, 1585–1592 (2009).
Abildgaard, U., Sandset, P. M., Hammerstrom, J., Gjestvang, F. T. & Tveit, A. Management of pregnant women with mechanical heart valve prosthesis: thromboprophylaxis with low molecular weight heparin. Thromb. Res. 124, 262–267 (2009).
Goland, S. et al. Monitoring of anti-Xa in pregnant patients with mechanical prosthetic valves receiving low-molecular-weight heparin: peak or trough levels? J. Cardiovasc. Pharmacol. Ther. 19, 451–456 (2014).
Elkayam, U. & Goland, S. The search for a safe and effective anticoagulation regimen in pregnant women with mechanical prosthetic heart valves. J. Am. Coll. Cardiol. 59, 1116–1118 (2012).
Königsbrügge, O., Langer, M., Hayde, M., Ay, C. & Pabinger, I. Oral anticoagulation with rivaroxaban during pregnancy: a case report. Thromb. Haemost. 112, 1323–1324 (2014).
European Medicines Agency. EPAR summary for the public. Xarelto (rivaroxaban) [online], (2013).
European Medicines Agency. Xarelto. Summary of product characteristics [online], (2014).
Sorensen, H. T., Czeizel, A. E., Rockenbauer, M., Steffensen, F. H. & Olsen, J. The risk of limb deficiencies and other congenital abnormalities in children exposed in utero to calcium channel blockers. Acta Obstet. Gynecol. Scand. 80, 397–401 (2001).
Sorensen, H. T. et al. Pregnancy outcome in women exposed to calcium channel blockers. Reprod. Toxicol. 12, 383–384 (1998).
Davis, R. L. et al. Risks of congenital malformations and perinatal events among infants exposed to calcium channel and beta-blockers during pregnancy. Pharmacoepidemiol. Drug Saf. 20, 138–145 (2011).
Weber-Schoendorfer, C. et al. The safety of calcium channel blockers during pregnancy: a prospective, multicenter, observational study. Reprod. Toxicol. 26, 24–30 (2008).
Bullo, M., Tschumi, S., Bucher, B. S., Bianchetti, M. G. & Simonetti, G. D. Pregnancy outcome following exposure to angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists: a systematic review. Hypertension 60, 444–450 (2012).
Cooper, W. O. et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N. Engl. J. Med. 354, 2443–2451 (2006).
Spaggiari, E. et al. Prognosis and outcome of pregnancies exposed to renin-angiotensin system blockers. Prenat. Diagn. 32, 1071–1076 (2012).
Bartalena, L., Bogazzi, F., Braverman, L. E. & Martino, E. Effects of amiodarone administration during pregnancy on neonatal thyroid function and subsequent neurodevelopment. J. Endocrinol. Invest. 24, 116–130 (2001).
Widerhorn, J., Bhandari, A. K., Bughi, S., Rahimtoola, S. H. & Elkayam, U. Fetal and neonatal adverse effects profile of amiodarone treatment during pregnancy. Am. Heart J. 122, 1162–1166 (1991).
Wagner, X. et al. Coadminsitration of flecainide acetate and sotalol during pregnancy: lack of teratogenic effects, passage across the placenta, and accretion in human breast milk. Am. Heart J. 119, 700–702 (1990).
Godfrey, L. M., Erramouspe, J. & Cleveland, K. W. Teratogenic risk of statins in pregnancy. Ann. Pharmacother. 46, 1419–1424 (2012).
Winterfeld, U. et al. Pregnancy outcome following maternal exposure to statins: a multicentre prospective study. BJOG 120, 463–471 (2013).
Hecker, A., Hasan, S. H., & Neumann, F. Disturbances in sexual differentiation of rat fetuses following spironolactone treatment. Acta Enodcrinol. (Copenh.) 95, 540–545 (1980).
Potondi, A. Congenital rhabdomyoma of the heart and intrauterine digitalis poisoning. J. Forensic Sci. 11, 81–88 (1966).
Sherman, J. L. & Locke, R. V. Transplacental neonatal digitalis intoxication. Am. J. Cardiol. 6, 834–837 (1960).
Kozer, E. et al. Aspirin consumption during the first trimester of pregnancy and congenital anomalies: a meta-analysis. Am. J. Obstet. Gynecol. 187, 1623–1630 (2002).
Werler, M. M., Sheehan, J. E. & Mitchell, A. A. Maternal medication use and risks of gastroschisis and small intestinal atresia. Am. J. Epidemiol. 155, 26–31 (2002).
Perkin, R. M., Levin, D. L. & Clark, R. Serum salicylate levels and right-to-left ductus shunts in newborn infants with persistent pulmonary hypertension. J. Pediatr. 96, 721–726 (1980).
Alano, M. A., Ngougmna, E., Ostrea, E. M. Jr & Konduri, G. G. Analysis of nonsteroidal antiinflammatory drugs in meconium and its relation to persistent pulmonary hypertension of the newborn. Pediatrics 107, 519–523 (2001).
James, A. H., Brancazio, L. R. & Price, T. Aspirin and reproductive outcomes. Obstet. Gynecol. Surv. 63, 49–57 (2008).
Norgard, B., Puho, E., Czeizel, A. E., Skriver, M. V. & Sorensen, H. T. Aspirin use during early pregnancy and the risk of congenital abnormalities: a population-based case-control study. Am. J. Obstet. Gynecol. 192, 922–923 (2005).
Kozer, E. et al. Effects of aspirin consumption during pregnancy on pregnancy outcomes: meta-analysis. Birth Defects Res. B. Dev. Reprod. Toxicol. 68, 70–84 (2003).
Al-Aqeedi, R. F. & Al-Nabti, A. D. Drug-eluting stent implantation for acute myocardial infarction during pregnancy with use of glycoprotein IIb/IIIa inhibitor, aspirin and clopidogrel. J. Invasive Cardiol. 20, E146–E149 (2008).
Hubinont, C. & Debieve, F. Prevention of preterm labour: 2011 update on tocolysis. J. Pregnancy 2011, 941057 (2011).
Turnbull, J. & Bell, R. Obstetric anaesthesia and peripartum management. Best Pract. Res. Clin. Obstet. Gynaecol. 28, 593–605 (2014).
Svanstrom, M. C. et al. Signs of myocardial ischaemia after injection of oxytocin: a randomized double-blind comparison of oxytocin and methylergometrine during Caesarean section. Br. J. Anaesth. 100, 683–689 (2008).
Jonsson, M., Hanson, U., Lidell, C. & Nordén-Lindeberg, S. ST depression at caesarean section and the relation to oxytocin dose. A randomised controlled trial. BJOG 117, 76–83 (2010).
Mousa, H. A., McKinley, C. A. & Thong, J. Acute postpartum myocardial infarction after ergometrine administration in a woman with familial hypercholesterolaemia. BJOG 107, 939–940 (2000).
Tsui, B. C., Stewart, B., Fitzmaurice, A. & Williams, R. Cardiac arrest and myocardial infarction induced by postpartum intravenous ergonovine administration. Anesthesiology 94, 363–364 (2001).
Dunlop, W. & Davison, J. M. Renal haemodynamics and tubular function in human pregnancy. Baillieres Clin. Obstet. Gynaecol. 1, 769–787 (1987).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Pieper, P. Use of medication for cardiovascular disease during pregnancy. Nat Rev Cardiol 12, 718–729 (2015). https://doi.org/10.1038/nrcardio.2015.172
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2015.172
This article is cited by
-
Disease features and management of cardiomyopathies in women
Heart Failure Reviews (2024)
-
Contemporary Management of Cardiomyopathy and Heart Failure in Pregnancy
Cardiology and Therapy (2024)
-
Pregnant and lactating women should be included in clinical trials for cardiovascular disease
Nature Medicine (2023)
-
Early pregnancy stage 1 hypertension and high mean arterial pressure increased risk of adverse pregnancy outcomes in Shanghai, China
Journal of Human Hypertension (2022)
-
Women Living with Familial Hypercholesterolemia: Challenges and Considerations Surrounding Their Care
Current Atherosclerosis Reports (2020)