Abstract
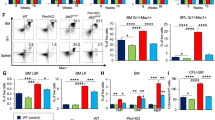
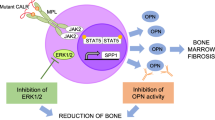
Primary myelofibrosis (PMF) is characterized by bone marrow fibrosis, myeloproliferation, extramedullary hematopoiesis, splenomegaly and leukemic progression. Moreover, the bone marrow and spleens of individuals with PMF contain large numbers of atypical megakaryocytes that are postulated to contribute to fibrosis through the release of cytokines, including transforming growth factor (TGF)-β. Although the Janus kinase inhibitor ruxolitinib provides symptomatic relief, it does not reduce the mutant allele burden or substantially reverse fibrosis. Here we show through pharmacologic and genetic studies that aurora kinase A (AURKA) represents a new therapeutic target in PMF. Treatment with MLN8237, a selective AURKA inhibitor, promoted polyploidization and differentiation of megakaryocytes with PMF-associated mutations and had potent antifibrotic and antitumor activity in vivo in mouse models of PMF. Moreover, heterozygous deletion of Aurka was sufficient to ameliorate fibrosis and other PMF features in vivo. Our data suggest that megakaryocytes drive fibrosis in PMF and that targeting them with AURKA inhibitors has the potential to provide therapeutic benefit.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Gangat, N. et al. DIPSS plus: a refined dynamic international prognostic scoring system for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count and transfusion status. J. Clin. Oncol. 29, 392–397 (2011).
Mascarenhas, J. & Hoffman, R. A comprehensive review and analysis of the effect of ruxolitinib therapy on the survival of patients with myelofibrosis. Blood 121, 4832–4837 (2013).
Ciurea, S.O. et al. Pivotal contributions of megakaryocytes to the biology of idiopathic myelofibrosis. Blood 110, 986–993 (2007).
Vannucchi, A.M. et al. Mutations and prognosis in primary myelofibrosis. Leukemia 27, 1861–1869 (2013).
Abdel-Wahab, O. et al. Unraveling the genetic underpinnings of myeloproliferative neoplasms and understanding their effect on disease course and response to therapy: proceedings from the 6th International Post-ASH Symposium. Am. J. Hematol. 87, 562–568 (2012).
Tibes, R., Bogenberger, J.M., Benson, K.L. & Mesa, R.A. Current outlook on molecular pathogenesis and treatment of myeloproliferative neoplasms. Mol. Diagn. Ther. 16, 269–283 (2012).
Klampfl, T. et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N. Engl. J. Med. 369, 2379–2390 (2013).
Nangalia, J. et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N. Engl. J. Med. 369, 2391–2405 (2013).
Verstovsek, S. et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N. Engl. J. Med. 366, 799–807 (2012).
Harrison, C. et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N. Engl. J. Med. 366, 787–798 (2012).
Tefferi, A. JAK inhibitors for myeloproliferative neoplasms: clarifying facts from myths. Blood 119, 2721–2730 (2012).
Tam, C.S. & Verstovsek, S. Investigational Janus kinase inhibitors. Expert Opin. Investig. Drugs 22, 687–699 (2013).
Harrison, C. et al. Practical management of patients with myelofibrosis receiving ruxolitinib. Expert Rev. Hematol. 6, 511–523 (2013).
Villeval, J.L. et al. High thrombopoietin production by hematopoietic cells induces a fatal myeloproliferative syndrome in mice. Blood 90, 4369–4383 (1997).
Shivdasani, R.A., Fujiwara, Y., McDevitt, M.A. & Orkin, S.H. A lineage-selective knockout establishes the critical role of transcription factor GATA-1 in megakaryocyte growth and platelet development. EMBO J. 16, 3965–3973 (1997).
Vannucchi, A.M. et al. Development of myelofibrosis in mice genetically impaired for GATA-1 expression (GATA-1low mice). Blood 100, 1123–1132 (2002).
Wen, Q. et al. Identification of regulators of polyploidization presents therapeutic targets for treatment of AMKL. Cell 150, 575–589 (2012).
Goldenson, B. & Crispino, J.D. The Aurora kinases in cell cycle and leukemia. Oncogene 34, 537–545 (2015).
Levine, R.L. et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia and myeloid metaplasia with myelofibrosis. Cancer Cell 7, 387–397 (2005).
Stachura, D.L., Chou, S.T. & Weiss, M.J. Early block to erythromegakaryocytic development conferred by loss of transcription factor GATA-1. Blood 107, 87–97 (2006).
Vannucchi, A.M. et al. Abnormalities of GATA-1 in megakaryocytes from patients with idiopathic myelofibrosis. Am. J. Pathol. 167, 849–858 (2005).
Uozumi, K. et al. Establishment and characterization of a new human megakaryoblastic cell line (SET-2) that spontaneously matures to megakaryocytes and produces platelet-like particles. Leukemia 14, 142–152 (2000).
Mullally, A. et al. Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells. Cancer Cell 17, 584–596 (2010).
Goldenson, B., Kirsammer, G., Stankiewicz, M.J., Wen, Q.J. & Crispino, J.D. Aurora kinase A is required for hematopoiesis but is dispensable for mouse megakaryocyte endomitosis and differentiation. Blood 125, 2141–2150 (2015).
den Hollander, J. et al. Aurora kinases A and B are upregulated by Myc and are essential for maintenance of the malignant state. Blood 116, 1498–1505 (2010).
Wernig, G. et al. The Jak2V617F oncogene associated with myeloproliferative diseases requires a functional FERM domain for transformation and for expression of the Myc and Pim proto-oncogenes. Blood 111, 3751–3759 (2008).
Sumi, K., Tago, K., Kasahara, T. & Funakoshi-Tago, M. Aurora kinase A critically contributes to the resistance to anticancer drug cisplatin in JAK2V617F mutant–induced transformed cells. FEBS Lett. 585, 1884–1890 (2011).
Koppikar, P. et al. Efficacy of the JAK2 inhibitor INCB16562 in a mouse model of MPLW515L-induced thrombocytosis and myelofibrosis. Blood 115, 2919–2927 (2010).
Wernig, G. et al. Efficacy of TG101348, a selective JAK2 inhibitor, in treatment of a mouse model of JAK2V617F-induced polycythemia vera. Cancer Cell 13, 311–320 (2008).
Cowley, D.O. et al. Aurora A kinase is essential for bipolar spindle formation and early development. Mol. Cell. Biol. 29, 1059–1071 (2009).
Castro-Malaspina, H. Pathogenesis of myelofibrosis: role of ineffective megakaryopoiesis and megakaryocyte components. Prog. Clin. Biol. Res. 154, 427–454 (1984).
Le Bousse-Kerdilès, M.C. & Martyre, M. C. & French INSERM Research Network on Idiopathic Myeolofibrosis. Involvement of the fibrogenic cytokines, TGF-β and bFGF, in the pathogenesis of idiopathic myelofibrosis. Pathol. Biol. (Paris) 49, 153–157 (2001).
Martyré, M.C., Magdelenat, H., Bryckaert, M.C., Laine-Bidron, C. & Calvo, F. Increased intraplatelet levels of platelet-derived growth factor and transforming growth factor-β in patients with myelofibrosis with myeloid metaplasia. Br. J. Haematol. 77, 80–86 (1991).
Bock, O. et al. Bone morphogenetic proteins are overexpressed in the bone marrow of primary myelofibrosis and are apparently induced by fibrogenic cytokines. Am. J. Pathol. 172, 951–960 (2008).
Hoermann, G. et al. Identification of oncostatin M as a JAK2V617F-dependent amplifier of cytokine production and bone marrow remodeling in myeloproliferative neoplasms. FASEB J. 26, 894–906 (2012).
Chagraoui, H. et al. Prominent role of TGF-β1 in thrombopoietin-induced myelofibrosis in mice. Blood 100, 3495–3503 (2002).
Bruns, I. et al. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat. Med. 20, 1315–1320 (2014).
Zhao, M.P.J. et al. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat. Med. 20, 1321–1326 (2014).
Schepers, K. et al. Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell Stem Cell 13, 285–299 (2013).
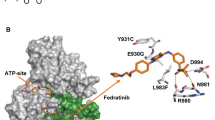
Dodson, C.A., Yeoh, S., Haq, T. & Bayliss, R. A kinetic test characterizes kinase intramolecular and intermolecular autophosphorylation mechanisms. Sci. Signal. 6, ra54 (2013).
Chou, T.C. & Talalay, P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 22, 27–55 (1984).
Kühn, R., Schwenk, F., Aguet, M. & Rajewsky, K. Inducible gene targeting in mice. Science 269, 1427–1429 (1995).
de Boer, J. et al. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur. J. Immunol. 33, 314–325 (2003).
Akashi, K., Traver, D., Miyamoto, T. & Weissman, I.L. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404, 193–197 (2000).
Acknowledgements
We thank A. Stern, J. Licht, Z. Huang and members of the Crispino lab for helpful discussions, and T. Van Dyke (National Cancer Institute) for the generous gift of Aurka-floxed mice. We also thank Z. Huang (Wuhan University) for providing the CALR T1 and CALR T2 plasmids, G. Gilliland (Fred Hutchinson Cancer Research Center) for the MPLW515L construct, M. Weiss (St. Jude Children's Research Hospital) for the G1ME cells and T. Arima (Kagoshima University) for the SET-2 cells. This work was supported by US National Institutes of Health (NIH) grant no. HL112792 (J.D.C.), a Leukemia and Lymphoma Society Translational Research Project grant (J.D.C.), the Samuel Waxman Cancer Research Foundation (J.D.C.), a Dixon Young Investigator Award from Northwestern Memorial Foundation (Q.J.W.), the Northwestern University Clinical and Translational Sciences Institute (Q.J.W.) and American Cancer Society grant no. 278808 (Q.J.W.). The project was also supported by the National Center for Research Resources (NCRR), the National Center for Advancing Translational Sciences (NCATS) and the NIH through grant no. TL1R000108 (B.G.).
Author information
Authors and Affiliations
Contributions
Q.J.W., Q.Y., B.G., S.M., L.J.B., R.S., L.G. and P.K. performed experiments, interpreted data and contributed to the writing of the manuscript. T.L., A.P., B.S. and A.T. provided patient specimens, interpreted data and contributed to the writing of the manuscript. S.G. and R.K.S. analyzed pathology, interpreted data and contributed to the writing of the manuscript. O.A.-W., A.M., R.L.L. and J.D.C. designed experiments, interpreted data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–15 and Supplementary Tables 1–3 (PDF 2374 kb)
Rights and permissions
About this article
Cite this article
Jeremy Wen, Q., Yang, Q., Goldenson, B. et al. Targeting megakaryocytic-induced fibrosis in myeloproliferative neoplasms by AURKA inhibition. Nat Med 21, 1473–1480 (2015). https://doi.org/10.1038/nm.3995
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3995
This article is cited by
-
Myelofibrosis-type megakaryocyte dysplasia (MTMD) as a distinct category of BCR::ABL-negative myeloproliferative neoplasms. Challenges and perspectives
Leukemia (2023)
-
SRSF2-P95H decreases JAK/STAT signaling in hematopoietic cells and delays myelofibrosis development in mice
Leukemia (2023)
-
Cytarabine-induced differentiation of AML cells depends on Chk1 activation and shares the mechanism with inhibitors of DHODH and pyrimidine synthesis
Scientific Reports (2022)
-
Cabozantinib promotes erythroid differentiation in K562 erythroleukemia cells through global changes in gene expression and JNK activation
Cancer Gene Therapy (2022)
-
Inhibition of interleukin-1β reduces myelofibrosis and osteosclerosis in mice with JAK2-V617F driven myeloproliferative neoplasm
Nature Communications (2022)