Abstract
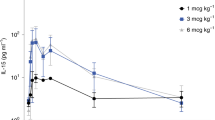
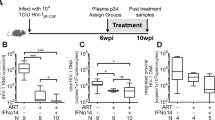
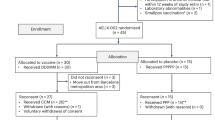
Gene transfer has potential as a once-only treatment that reduces viral load, preserves the immune system and avoids lifetime highly active antiretroviral therapy. This study, which is to our knowledge the first randomized, double-blind, placebo-controlled, phase 2 cell-delivered gene transfer clinical trial, was conducted in 74 HIV-1–infected adults who received a tat-vpr–specific anti-HIV ribozyme (OZ1) or placebo delivered in autologous CD34+ hematopoietic progenitor cells. There were no OZ1-related adverse events. There was no statistically significant difference in viral load between the OZ1 and placebo group at the primary end point (average at weeks 47 and 48), but time-weighted areas under the curve from weeks 40–48 and 40–100 were significantly lower in the OZ1 group. Throughout the 100 weeks, CD4+ lymphocyte counts were higher in the OZ1 group. This study indicates that cell-delivered gene transfer is safe and biologically active in individuals with HIV and can be developed as a conventional therapeutic product.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Taylor, B.S., Sobieszczyk, M.E., McCutchan, F.E. & Hammer, S.M. The challenge of HIV-1 subtype diversity. N. Engl. J. Med. 358–1590–1602 (2008).
Watkins, D.I., Burton, D.R., Kallas, E.G., Moore, J.P. & Koff, W.C. Nonhuman primate models and the failure of the Merck HIV-1 vaccine in humans. Nat. Med. 14, 617–621 (2008).
Baltimore, D. Gene therapy. Intracellular immunization. Nature 335, 395–396 (1988).
Amado, R.G. et al. Anti–human immunodeficiency virus hematopoietic progenitor cell-delivered ribozyme in a phase I study: myeloid and lymphoid reconstitution in human immunodeficiency virus type-1–infected patients. Hum. Gene Ther. 15, 251–262 (2004).
An, D.S. et al. Stable reduction of CCR5 by RNAi through hematopoietic stem cell transplant in non-human primates. Proc. Natl. Acad. Sci. USA 104, 13110–13115 (2007).
Anderson, J. et al. Safety and efficacy of a lentiviral vector containing three anti-HIV genes–CCR5 ribozyme, tat-rev siRNA, and TAR decoy–in SCID-hu mouse-derived T cells. Mol. Ther. 15, 1182–1188 (2007).
Bahner, I. et al. Comparison of trans-dominant inhibitory mutant human immunodeficiency virus type 1 genes expressed by retroviral vectors in human T lymphocytes. J. Virol. 67, 3199–3207 (1993).
Cagnon, L. & Rossi, J. Retroviral delivery and anti-HIV testing of hammerhead ribozymes. Methods Mol. Biol. 74, 451–457 (1997).
Dropulic, B., Elkins, D.A., Rossi, J.J. & Sarver, N. Ribozymes: use as anti-HIV therapeutic molecules. Antisense Res. Dev. 3, 87–94 (1993).
Fanning, G., Amado, R. & Symonds, G. Gene therapy for HIV/AIDS: the potential for a new therapeutic regimen. J. Gene Med. 5, 645–653 (2003).
Kohn, D.B. Gene therapy using hematopoietic stem cells. Curr. Opin. Mol. Ther. 1, 437–442 (1999).
Kohn, D.B. et al. A clinical trial of retroviral-mediated transfer of a rev-responsive element decoy gene into CD34+ cells from the bone marrow of human immunodeficiency virus-1-infected children. Blood 94, 368–371 (1999).
Li, M., Li, H. & Rossi, J.J. RNAi in combination with a ribozyme and TAR decoy for treatment of HIV infection in hematopoietic cell gene therapy. Ann. NY Acad. Sci. 1082, 172–179 (2006).
Lo, A.S., Zhu, Q. & Marasco, W.A. Intracellular antibodies (intrabodies) and their therapeutic potential. Handb. Exp. Pharmacol. 181, 343–373 (2008).
Marasco, W.A., LaVecchio, J. & Winkler, A. Human anti–HIV-1 tat sFv intrabodies for gene therapy of advanced HIV-1-infection and AIDS. J. Immunol. Methods 231, 223–238 (1999).
Mautino, M.R. & Morgan, R.A. Potent inhibition of human immunodeficiency virus type 1 replication by conditionally replicating human immunodeficiency virus-based lentiviral vectors expressing envelope antisense mRNA. Hum. Gene Ther. 11, 2025–2037 (2000).
Morgan, R.A. et al. Preferential survival of CD4+ T lymphocytes engineered with anti-human immunodeficiency virus (HIV) genes in HIV-infected individuals. Hum. Gene Ther. 16, 1065–1074 (2005).
Morris, K.V. & Rossi, J.J. Lentivirus-mediated RNA interference therapy for human immunodeficiency virus type 1 infection. Hum. Gene Ther. 17, 479–486 (2006).
Rossi, J.J. The application of ribozymes to HIV infection. Curr. Opin. Mol. Ther. 1, 316–322 (1999).
Strayer, D.S. et al. Current status of gene therapy strategies to treat HIV/AIDS. Mol. Ther. 11, 823–842 (2005).
Taylor, J.A. et al. Foamy virus vectors expressing anti-HIV transgenes efficiently block HIV-1 replication. Mol. Ther. 16, 46–51 (2008).
von Laer, D., Hasselmann, S. & Hasselmann, K. Gene therapy for HIV infection: what does it need to make it work? J. Gene Med. 8, 658–667 (2006).
Zahn, R.C. et al. Efficient entry inhibition of human and nonhuman primate immunodeficiency virus by cell surface-expressed gp41-derived peptides. Gene Ther. 15, 1210–1222 (2008).
Sarver, N. et al. Ribozymes as potential anti–HIV-1 therapeutic agents. Science 247, 1222–1225 (1990).
Schambach, A. et al. Towards hematopoietic stem cell–mediated protection against infection with human immunodeficiency virus. Gene Ther. 13, 1037–1047 (2006).
Rossi, J.J., June, C.H. & Kohn, D.B. Genetic therapies against HIV. Nat. Biotechnol. 25, 1444–1454 (2007).
Macpherson, J.L., Ely, J.A., Sun, L.Q. & Symonds, G.P. Ribozymes in gene therapy of HIV-1. Front. Biosci. 4, D497–D505 (1999).
Levine, B.L. et al. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc. Natl. Acad. Sci. USA 103, 17372–17377 (2006).
Macpherson, J.L. et al. Long-term survival and concomitant gene expression of ribozyme-transduced CD4+ T lymphocytes in HIV-infected patients. J. Gene Med. 7, 552–564 (2005).
Podsakoff, G.M. et al. Selective survival of peripheral blood lymphocytes in children with HIV-1 following delivery of an anti-HIV gene to bone marrow CD34(+) cells. Mol. Ther. 12, 77–86 (2005).
Sun, L.Q., Ely, J.A., Gerlach, W. & Symonds, G. Anti-HIV ribozymes. Mol. Biotechnol. 7, 241–251 (1997).
Sun, L.Q., Wang, L., Gerlach, W.L. & Symonds, G. Target sequence-specific inhibition of HIV-1 replication by ribozymes directed to tat RNA. Nucleic Acids Res. 23, 2909–2913 (1995).
Sun, L.Q. et al. Resistance to human immunodeficiency virus type 1 infection conferred by transduction of human peripheral blood lymphocytes with ribozyme, antisense, or polymeric trans-activation response element constructs. Proc. Natl. Acad. Sci. USA 92, 7272–7276 (1995).
Rossi, J.J. Ribozyme therapy for HIV infection. Adv. Drug Deliv. Rev. 44, 71–78 (2000).
Wang, L. et al. Preclinical characterization of an anti-tat ribozyme for therapeutic application. Hum. Gene Ther. 9, 1283–1291 (1998).
Center for Biologics Evaluation and Research. Guidance for Industry: Gene Therapy Clinical Trials—Observing Subjects for Delayed Adverse Events. (US Department of Health and Human Services, Rockville, Maryland, 2006).
Center for Biologics Evaluation and Research. Guidance for Industry: Supplemental Guidances on Testing for Replication Competent Retrovirus in Retroviral Vector Based Gene Therapy Products and During Follow-up of Patients in Clinical Trials Using Retroviral Vectors. (US Department of Health and Human Services, Rockville, Maryland, 2006).
Cavallaro, A.M. et al. Three to six year follow-up of normal donors who received recombinant human granulocyte colony-stimulating factor. Bone Marrow Transplant. 25, 85–89 (2000).
Nicolini, F.E. et al. Long-term persistent lymphopenia in hematopoietic stem cell donors after donation for donor lymphocyte infusion. Exp. Hematol. 32, 1033–1039 (2004).
Novotny, J. et al. Sustained decrease of peripheral lymphocytes after allogeneic blood stem cell aphereses. Br. J. Haematol. 100, 695–697 (1998).
Malech, H.L. et al. Prolonged production of NADPH oxidase–corrected granulocytes after gene therapy of chronic granulomatous disease. Proc. Natl. Acad. Sci. USA 94, 12133–12138 (1997).
Aiuti, A. et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science 296, 2410–2413 (2002).
Hacein-Bey-Abina, S. et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 302, 415–419 (2003).
Ott, M.G. et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1–EVI1, PRDM16 or SETBP1. Nat. Med. 12, 401–409 (2006).
The Strategies for Management of Antiretroviral Therapy (SMART) Study Group. CD4+ count-guided interruption of antiretroviral treatment. N. Engl. J. Med. 355, 2283–2296 (2006).
Schmidt, M. et al. High-resolution insertion-site analysis by linear amplification-mediated PCR (LAM-PCR). Nat. Methods 4, 1051–1057 (2007).
Acknowledgements
The following clinicians, research nurses and coordinators contributed substantially to the trial: S. Agrawal, R. Amado, C. Anderson, P. Cain, R. Cordova, G. Dolan, C. Farthing, E. Glutzer, M. Gonzalez, M. Harbour, N. Hyland, A. Lebel, S. Lundy, K. Macrae, S. Miller, J. Norris, L. O'Donnell, L. Pearce, J. Perram, V. Rees, R. Richardson, J. Sarangapany, D. Slamowitz, R. Vale, G. Vasquez, B. Williams and A. Zolopa. We also acknowledge the contribution of the leukapheresis collection and cell processing staff, W. Quan for assistance with quality assurance and A. Miller of the Biologics Group for assistance with regulatory aspects. S. Margrie had a key role in the development of the statistical analysis plan and in the statistical analyses. C. Ang and A. King assisted with the preparation of the manuscript. We acknowledge W. Gerlach, D. Wade, J. Peacock, R. de Feyter and L. Farrell for their vital role during the early stages of the development of OZ1. We sincerely thank the participants who enrolled in this intensive study and the members of the Data Safety Monitoring Board. The University of California–Los Angeles Center for AIDS Research and University of California–Los Angeles General Clinical Research Center were supported by US National Institutes of Health grants AI28697 and M01-RR00865.
Author information
Authors and Affiliations
Contributions
R.T.M., T.C.M., A.C. and D.A.C. were involved in the design and clinical investigator leadership of the trial; J.A.Z., M.A.W. and J.L.M. were laboratory directors of cell manufacturing at their respective clinical sites; R.T.M., T.C.M., A.C., D.A.C., C.W., M.B., J.L., S.B., L.T., B.A., H.K., R.F., R.M. and D.E.S. were clinical investigators involved in the planning and conduct of the study, R.G., D.M. and J.M.M. provided advice about the design and ongoing conduct of the study; M.L. designed and reviewed the statistical analysis; C.v.K. set up, supervised and advised on the integration analysis; J.A.E. and S.M. Patino were the global clinical trial managers; A.E.K. was the project manager and lead clinical research associate; P.W. was head of quality assurance cell manufacturing and esoteric tests; A.V.T., M.H. and C.F. managed the central laboratory testing for esoteric tests; J.L.M., global product scientist, was responsible for the design and conduct of the cell and gene manufacturing protocols; G.P.S., global product leader, provided the interface between the manufacturing and sponsor clinical trial teams and overall strategic direction; L.A.E., global medical leader, provided oversight of the protocol, safety monitoring, regulatory reporting, data analysis and the sponsor clinical trial team; S.M. Pond, the global trial director, had oversight of the entire program; R.T.M., T.C.M., A.C., J.L.M., G.P.S., L.A.E., S.M. Pond and D.A.C. were the major contributors to writing of the manuscript; and all authors reviewed the raw data and the drafts and final manuscript.
Corresponding author
Ethics declarations
Competing interests
R.T.M. and T.C.M. are investigators who were contracted by Johnson & Johnson Research (JJR) to perform the clinical trial. This included payments for visits with subjects, equipment and salaries as part of the contracts with their institutions. D.A.C., C.W., M.B., J.L., S.B., L.T., B.A., H.K., R.F., R.M. and D.E.S. are investigators who were contracted by JJR to perform the clinical trial. A.C., J.A.Z. and M.A.W. are employees of the institutions where the clinical trial was conducted and acted as advisers during the conduct of the clinical trial. R.G., D.M., M.L. and J.M. are advisers who were contracted by JJR for their expertise during the conduct of the study. C.v.K. was responsible for one of the contract laboratories that was contracted by JJR during the conduct of the study. J.A.E., S.M. Patino, A.E.K., P.W., M.H., C.F. and L.A.E. are or were employees of JJR. J.L.M., G.P.S., S.M. Pond and A.V.T. are employees of JJR who hold shares in Johnson & Johnson in excess of $10,000.
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–4, Supplementary Tables 1 and and Supplementary Methods (PDF 646 kb)
Rights and permissions
About this article
Cite this article
Mitsuyasu, R., Merigan, T., Carr, A. et al. Phase 2 gene therapy trial of an anti-HIV ribozyme in autologous CD34+ cells. Nat Med 15, 285–292 (2009). https://doi.org/10.1038/nm.1932
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.1932
This article is cited by
-
HLA-mismatched allogeneic adoptive immune therapy in severely immunosuppressed AIDS patients
Signal Transduction and Targeted Therapy (2021)
-
An insight on promising strategies hoping to cure HIV-1 infection by targeting Rev protein—short review
Pharmacological Reports (2021)
-
Re-engineering 10–23 core DNA- and MNAzymes for applications at standard room temperature
Analytical and Bioanalytical Chemistry (2019)
-
Gene therapy research in Asia
Gene Therapy (2017)
-
Stem cell transplantation in strategies for curing HIV/AIDS
AIDS Research and Therapy (2016)