Abstract
We rely on movement to explore the environment, for example, by palpating an object. In somatosensory cortex, activity related to movement of digits or whiskers is suppressed, which could facilitate detection of touch. Movement-related suppression is generally assumed to involve corollary discharges. Here we uncovered a thalamocortical mechanism in which cortical fast-spiking interneurons, driven by sensory input, suppress movement-related activity in layer 4 (L4) excitatory neurons. In mice locating objects with their whiskers, neurons in the ventral posteromedial nucleus (VPM) fired in response to touch and whisker movement. Cortical L4 fast-spiking interneurons inherited these responses from VPM. In contrast, L4 excitatory neurons responded mainly to touch. Optogenetic experiments revealed that fast-spiking interneurons reduced movement-related spiking in excitatory neurons, enhancing selectivity for touch-related information during active tactile sensation. These observations suggest a fundamental computation performed by the thalamocortical circuit to accentuate salient tactile information.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Hulliger, M., Nordh, E., Thelin, A.E. & Vallbo, A.B. The responses of afferent fibres from the glabrous skin of the hand during voluntary finger movements in man. J. Physiol. (Lond.) 291, 233–249 (1979).
Moore, J.D., Mercer Lindsay, N., Deschênes, M. & Kleinfeld, D. Vibrissa self-motion and touch are reliably encoded along the same somatosensory pathway from brainstem through thalamus. PLoS Biol. 13, e1002253 (2015).
Campagner, D., Evans, M.H., Bale, M.R., Erskine, A. & Petersen, R.S. Prediction of primary somatosensory neuron activity during active tactile exploration. Elife 5, 5 (2016).
Urbain, N. et al. Whisking-related changes in neuronal firing and membrane potential dynamics in the somatosensory thalamus of awake mice. Cell Rep. 13, 647–656 (2015).
Papakostopoulos, D., Cooper, R. & Crow, H.J. Inhibition of cortical evoked potentials and sensation by self-initiated movement in man. Nature 258, 321–324 (1975).
Chapman, C.E. Active versus passive touch: factors influencing the transmission of somatosensory signals to primary somatosensory cortex. Can. J. Physiol. Pharmacol. 72, 558–570 (1994).
Schneider, D.M., Nelson, A. & Mooney, R. A synaptic and circuit basis for corollary discharge in the auditory cortex. Nature 513, 189–194 (2014).
Hentschke, H., Haiss, F. & Schwarz, C. Central signals rapidly switch tactile processing in rat barrel cortex during whisker movements. Cereb. Cortex 16, 1142–1156 (2006).
Crapse, T.B. & Sommer, M.A. Corollary discharge across the animal kingdom. Nat. Rev. Neurosci. 9, 587–600 (2008).
Chapman, C.E., Jiang, W. & Lamarre, Y. Modulation of lemniscal input during conditioned arm movements in the monkey. Exp. Brain Res. 72, 316–334 (1988).
Lu, S.M. & Lin, R.C.S. Thalamic afferents of the rat barrel cortex: a light- and electron-microscopic study using Phaseolus vulgaris leucoagglutinin as an anterograde tracer. Somatosens. Mot. Res. 10, 1–16 (1993).
Wimmer, V.C., Bruno, R.M., de Kock, C.P., Kuner, T. & Sakmann, B. Dimensions of a projection column and architecture of VPM and POm axons in rat vibrissal cortex. Cereb. Cortex 20, 2265–2276 (2010).
Simons, D.J. Response properties of vibrissa units in rat SI somatosensory neocortex. J. Neurophysiol. 41, 798–820 (1978).
Kawaguchi, Y. & Kubota, Y. Correlation of physiological subgroupings of nonpyramidal cells with parvalbumin- and calbindinD28k-immunoreactive neurons in layer V of rat frontal cortex. J. Neurophysiol. 70, 387–396 (1993).
Porter, J.T., Johnson, C.K. & Agmon, A. Diverse types of interneurons generate thalamus-evoked feedforward inhibition in the mouse barrel cortex. J. Neurosci. 21, 2699–2710 (2001).
Bruno, R.M. & Simons, D.J. Feedforward mechanisms of excitatory and inhibitory cortical receptive fields. J. Neurosci. 22, 10966–10975 (2002).
Cruikshank, S.J., Lewis, T.J. & Connors, B.W. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat. Neurosci. 10, 462–468 (2007).
Gabernet, L., Jadhav, S.P., Feldman, D.E., Carandini, M. & Scanziani, M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron 48, 315–327 (2005).
Curtis, J.C. & Kleinfeld, D. Phase-to-rate transformations encode touch in cortical neurons of a scanning sensorimotor system. Nat. Neurosci. 12, 492–501 (2009).
O'Connor, D.H. et al. Neural coding during active somatosensation revealed using illusory touch. Nat. Neurosci. 16, 958–965 (2013).
Hires, S.A., Gutnisky, D.A., Yu, J., O'Connor, D.H. & Svoboda, K. Low-noise encoding of active touch by layer 4 in the somatosensory cortex. Elife 4, 4 (2015).
Poulet, J.F., Fernandez, L.M., Crochet, S. & Petersen, C.C. Thalamic control of cortical states. Nat. Neurosci. 15, 370–372 (2012).
Simons, D.J. & Carvell, G.E. Thalamocortical response transformation in the rat vibrissa/barrel system. J. Neurophysiol. 61, 311–330 (1989).
Yu, C., Derdikman, D., Haidarliu, S. & Ahissar, E. Parallel thalamic pathways for whisking and touch signals in the rat. PLoS Biol. 4, e124 (2006).
O'Connor, D.H. et al. Vibrissa-based object localization in head-fixed mice. J. Neurosci. 30, 1947–1967 (2010).
Birdwell, J.A. et al. Biomechanical models for radial distance determination by the rat vibrissal system. J. Neurophysiol. 98, 2439–2455 (2007).
Pammer, L. et al. The mechanical variables underlying object localization along the axis of the whisker. J. Neurosci. 33, 6726–6741 (2013).
Brecht, M. & Sakmann, B. Whisker maps of neuronal subclasses of the rat ventral posterior medial thalamus, identified by whole-cell voltage recording and morphological reconstruction. J. Physiol. (Lond.) 538, 495–515 (2002).
Crochet, S., Poulet, J.F., Kremer, Y. & Petersen, C.C. Synaptic mechanisms underlying sparse coding of active touch. Neuron 69, 1160–1175 (2011).
Bruno, R.M. Synchrony in sensation. Curr. Opin. Neurobiol. 21, 701–708 (2011).
Swadlow, H.A. Influence of VPM afferents on putative inhibitory interneurons in S1 of the awake rabbit: evidence from cross-correlation, microstimulation, and latencies to peripheral sensory stimulation. J. Neurophysiol. 73, 1584–1599 (1995).
Beierlein, M., Gibson, J.R. & Connors, B.W. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J. Neurophysiol. 90, 2987–3000 (2003).
Kinnischtzke, A.K., Simons, D.J. & Fanselow, E.E. Motor cortex broadly engages excitatory and inhibitory neurons in somatosensory barrel cortex. Cereb. Cortex 24, 2237–2248 (2014).
Polack, P.O., Friedman, J. & Golshani, P. Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nat. Neurosci. 16, 1331–1339 (2013).
Zhao, S. et al. Cell type–specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nat. Methods 8, 745–752 (2011).
Halassa, M.M. et al. Selective optical drive of thalamic reticular nucleus generates thalamic bursts and cortical spindles. Nat. Neurosci. 14, 1118–1120 (2011).
Reinhold, K., Lien, A.D. & Scanziani, M. Distinct recurrent versus afferent dynamics in cortical visual processing. Nat. Neurosci. 18, 1789–1797 (2015).
Pinault, D., Bourassa, J. & Deschênes, M. The axonal arborization of single thalamic reticular neurons in the somatosensory thalamus of the rat. Eur. J. Neurosci. 7, 31–40 (1995).
Bureau, I., von Saint Paul, F. & Svoboda, K. Interdigitated paralemniscal and lemniscal pathways in the mouse barrel cortex. PLoS Biol. 4, e382 (2006).
Wickersham, I.R. et al. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron 53, 639–647 (2007).
Lefort, S., Tomm, C., Floyd Sarria, J.C. & Petersen, C.C. The excitatory neuronal network of the C2 barrel column in mouse primary somatosensory cortex. Neuron 61, 301–316 (2009).
Hooks, B.M. et al. Laminar analysis of excitatory local circuits in vibrissal motor and sensory cortical areas. PLoS Biol. 9, e1000572 (2011).
Fee, M.S., Mitra, P.P. & Kleinfeld, D. Central versus peripheral determinants of patterned spike activity in rat vibrissa cortex during whisking. J. Neurophysiol. 78, 1144–1149 (1997).
Kremkow, J., Perrinet, L.U., Masson, G.S. & Aertsen, A. Functional consequences of correlated excitatory and inhibitory conductances in cortical networks. J. Comput. Neurosci. 28, 579–594 (2010).
Bruno, R.M. & Sakmann, B. Cortex is driven by weak but synchronously active thalamocortical synapses. Science 312, 1622–1627 (2006).
Wilent, W.B. & Contreras, D. Dynamics of excitation and inhibition underlying stimulus selectivity in rat somatosensory cortex. Nat. Neurosci. 8, 1364–1370 (2005).
Fanselow, E.E. & Nicolelis, M.A. Behavioral modulation of tactile responses in the rat somatosensory system. J. Neurosci. 19, 7603–7616 (1999).
Crochet, S. & Petersen, C.C. Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nat. Neurosci. 9, 608–610 (2006).
Lee, S., Kruglikov, I., Huang, Z.J., Fishell, G. & Rudy, B. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat. Neurosci. 16, 1662–1670 (2013).
Sun, Q.Q., Huguenard, J.R. & Prince, D.A. Barrel cortex microcircuits: thalamocortical feedforward inhibition in spiny stellate cells is mediated by a small number of fast-spiking interneurons. J. Neurosci. 26, 1219–1230 (2006).
Guo, Z.V. et al. Procedures for behavioral experiments in head-fixed mice. PLoS One 9, e88678 (2014).
Clack, N.G. et al. Automated tracking of whiskers in videos of head fixed rodents. PLoS Comput. Biol. 8, e1002591 (2012).
Dörfl, J. The innervation of the mystacial region of the white mouse: a topographical study. J. Anat. 142, 173–184 (1985).
Gradinaru, V. et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell 141, 154–165 (2010).
Hippenmeyer, S. et al. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 3, e159 (2005).
Watabe-Uchida, M., Zhu, L., Ogawa, S.K., Vamanrao, A. & Uchida, N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74, 858–873 (2012).
Ludwig, K.A. et al. Poly(3,4-ethylenedioxythiophene) (PEDOT) polymer coatings facilitate smaller neural recording electrodes. J. Neural Eng. 8, 014001 (2011).
Suter, B.A. et al. Ephus: multipurpose data acquisition software for neuroscience experiments. Front Neural Circuits 4, 100 (2010).
Haidarliu, S. & Ahissar, E. Size gradients of barreloids in the rat thalamus. J. Comp. Neurol. 429, 372–387 (2001).
Hazan, L., Zugaro, M. & Buzsáki, G. Klusters, NeuroScope, NDManager: a free software suite for neurophysiological data processing and visualization. J. Neurosci. Methods 155, 207–216 (2006).
Harris, K.D., Henze, D.A., Csicsvari, J., Hirase, H. & Buzsáki, G. Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J. Neurophysiol. 84, 401–414 (2000).
Rossant, C. et al. Spike sorting for large, dense electrode arrays. Nat. Neurosci. 19, 634–641 (2016).
Ferster, D. & Jagadeesh, B. EPSP-IPSP interactions in cat visual cortex studied with in vivo whole-cell patch recording. J. Neurosci. 12, 1262–1274 (1992).
Margrie, T.W., Brecht, M. & Sakmann, B. In vivo, low-resistance, whole-cell recordings from neurons in the anaesthetized and awake mammalian brain. Pflugers Arch. 444, 491–498 (2002).
Ferster, D. Orientation selectivity of synaptic potentials in neurons of cat primary visual cortex. J. Neurosci. 6, 1284–1301 (1986).
Monier, C., Chavane, F., Baudot, P., Graham, L.J. & Frégnac, Y. Orientation and direction selectivity of synaptic inputs in visual cortical neurons: a diversity of combinations produces spike tuning. Neuron 37, 663–680 (2003).
Muñoz, W., Tremblay, R. & Rudy, B. Channelrhodopsin-assisted patching: in vivo recording of genetically and morphologically identified neurons throughout the brain. Cell Rep. 9, 2304–2316 (2014).
Moore, A.K. & Wehr, M. Parvalbumin-expressing inhibitory interneurons in auditory cortex are well-tuned for frequency. J. Neurosci. 33, 13713–13723 (2013).
Horikawa, K. & Armstrong, W.E. A versatile means of intracellular labeling: injection of biocytin and its detection with avidin conjugates. J. Neurosci. Methods 25, 1–11 (1988).
Hill, D.N., Curtis, J.C., Moore, J.D. & Kleinfeld, D. Primary motor cortex reports efferent control of vibrissa motion on multiple timescales. Neuron 72, 344–356 (2011).
Acknowledgements
We thank A. Agmon, C.E. Chapman, J. Cohen, D. Ferster, Z. Guo, H. Inagaki, N. Li, J. Magee, N. Spruston, D. O'Connor, S. Peron and G. Shepherd for discussions and comments on the manuscript. We thank C. Gerfen for imaging brain slices and cell counting. This work was supported by Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
J.Y. performed cortical recordings and optogenetic experiments. D.A.G. performed chronic silicon probe recordings from the VPM thalamus. J.Y., D.A.G. and K.S. analyzed the data. J.Y., D.A.G., S.A.H. and K.S. interpreted the data. J.Y., D.A.G. and K.S. wrote the paper with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Extracellular recordings in VPM during whisker-based object localization (Extension of Fig. 1).
(a) Electrode track marked with DiI (red). Franklin and Paxino’s mouse atlas for the corresponding coronal plane is shown for reference.
(b) Receptive field mapping in anesthetized mice implanted with movable silicon probes. Example PSTHs before trimming whiskers. The principal whisker was β.
(c) Single unit raster plot for one behavioral session. Early in the session the mouse was anesthetized and whiskers were stimulated passively to map the principal whisker (pink shading). After removal of anesthesia the mouse started to perform the behavioral task. Right, heat of the color corresponds to behavioral performance (running average over 20 trials).
(d) Spike rate and whisking amplitude as a function of trial number within a session.
(e) Spike rate and whisking amplitude co-vary across trials within single behavioral sessions (Pearson correlation coefficient ρ=0.61; P = 10-78; number of trials n=310).
Supplementary Figure 2 VPM neurons recorded during behavior (Extension of Fig. 1).
(a) Left, touch-evoked PSTH for protracting whisker movement. Right, touch-evoked PSTH for retracting whisker movement.
(b) PSTH aligned to whisking onset.
(c) Spike rate as a function of whisking amplitudes.
(d) Spike rate as a function of whisking phase.
(e) Distribution of the goodness-of-fit (r2) of the spike-phase relationship. Dashed line, r2=0.25;
(f) Distribution of the preferred phase and the modulation amplitude (half of the peak-to-trough amplitude) of neurons in which r2 is larger than 0.25.
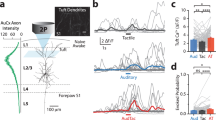
Supplementary Figure 3 L4 excitatory neurons recorded in whole-cell mode (Extension of Fig. 2).
A total of 15 L4 E neurons were labeled with biotin. 7 examples are shown on the right. Others (#2, 5, 12, 13, 18, 24, 29 and 35) were also recovered but were not labeled sufficiently well to show morphology. Scale, 100 μm.
L4 excitatory neurons recorded in loose-seal mode are not shown here but can be found at CRCNS.org (http://doi.org/10.6080/K0T43R01).
(a) Left, touch-evoked average membrane potential response (black) and PSTH (red) for protracting whisker movement. Right, retracting whisker movement. Neurons (n=37) are sorted by their resting membrane potential.
(b) Average membrane potential response (black) and PSTH (red) aligned to the onset of whisker movement. Shaded areas, mean ± s.e.m.
(c) Average membrane potential (black) and spike rate (red) as a function of amplitude of whisker movement. Vertical lines, mean ± s.e.m. Blue and magenta curves, sigmoid fit.
(d) Average membrane potential (black) and spike rate (red) as a function of the phase of whisker movement. Vertical lines, mean ± s.e.m. Black curves, sinusoidal fit.
(e) Spike waveforms (high-pass filtered >300 Hz).
(f) Distribution of the goodness-of-fit (r2) of the Vm-phase relationship. Dashed line, r2=0.25;
(g) Distribution of the preferred phase and the modulation amplitude (Vm, half of the peak-to-trough amplitude) of neurons in which the r2 is larger than 0.25.
Supplementary Figure 4 Effective reversal potential of touch- versus whisking-induced membrane potential changes for L4 excitatory neurons (Extension of Fig. 2).
We explored the relationship of the change in membrane potential (∆Vm) after an event (touch or whisker movement) as a function of the membrane potential before the event. For each touch event, pre-touch Vm is the mean membrane potential 0 to 5 ms before touch onset. Post-touch Vm is the membrane potential at the time of peak average touch-evoked PSP. ∆Vm is the difference between post-touch and pre-touch Vm. For each transition from non-whisking to whisking epoch, pre-whisking Vm is the mean membrane potential 0 to 200 ms before the onset of whisker movement. Post-whisking Vm is the mean membrane potential 100 to 300 ms after the onset of whisker movement. ∆Vm is the difference between post-touch and pre-touch Vm. The distribution of ∆Vm versus pre-touch or pre-whisking Vm was fit with a linear function. Effective reversal potential is defined as the potential where the fitted lines cross zero.
(a) Schematic illustrating the measurement of pre-touch or pre-whisking Vm (pre-Vm) and ∆Vm.
(b) Relationship of pre-Vm and ∆Vm for touch (blue) and whisking (green) of all neurons. Straight lines are linear fits.
(c) Comparison of effective reversal potentials for touch versus whisking.
Supplementary Figure 5 Membrane potential fluctuations in L4 excitatory neurons (Extension of Fig. 2).
(a) Example membrane potential traces for all 37 L4 excitatory neurons during a trial with touch (blue vertical lines) and one without touch. Green, whisker position. Average membrane potential for each trial is noted on the left. Cell index is the same as in Supplementary Fig. 3.
(b) Power spectra of the membrane potential during periods without (black) and with (red) whisker movement. Shaded areas are the 95% confidence interval.
(c) Average power spectrum across all L4 excitatory neurons.
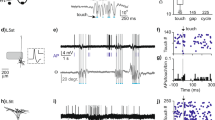
Supplementary Figure 6 Spike waveform analysis of cell-attached recordings to identify FS interneurons (Extension of Fig. 5).
Peak-to-trough ratio plotted against spike duration for 64 cell-attached recordings from VGAT-ChR2-EYFP mice and 4 PV interneurons from PV-IRES-Cre mice. Definition of peak and trough is given in inset.
Filled black symbols, neurons that were not driven by laser stimulation; Open black symbols, neurons that were driven by laser stimulation, thus GABAergic; Open gray symbols, neurons that were driven by laser stimulation in ION-transected mice. Green circles, confirmed PV interneurons, in mice expressing eNpHR in PV+ neurons. Circles, L4; Triangles, L5. The boundary (dashed lines) defines FS interneurons used in our analysis. Arrows pointing to two example spike waveforms shown in inset. The outlier PV interneuron was recorded with a high increase (200 MΩ) in electrode resistance and the recording showed sign of intracellular fluctuations.
Supplementary Figure 7 L4 FS interneurons recorded in whole-cell or cell-attached mode (Extension of Fig. 5).
A total of 2 L4 FS neurons were labeled with biotin and are shown on the right. Scale, 100 μm.
(a-e) Same as Supplementary Figure 1 for L4 FS interneurons (n=25). Cells 1-7 were recorded in whole-cell mode. Spike waveforms for cell-attached recordings are normalized by the standard deviation (z score).
(f) Light-evoked membrane potential depolarization (FS#2-6) and spikes in VGAT-ChR2-EYFP positive neurons.
(g) Coupling between the spikes of L4 FS interneurons and the whisking phase was either fit with a single sinusoidal function (left, n=18) or a sum of a single sinusoidal function (black) and a sinusoidal function of twice the frequency (purple) (right, n=7) if the fitting quality (r2) was 50% higher than with only a single sinusoidal function. Polar plots summarize the distribution of the preferred phase and the modulation amplitude (in spikes per s). Neurons fit with a sum of two sinusoidal functions are marked with cyan asterisks in d and g.
Supplementary Figure 8 Membrane-potential fluctuations in L4 FS interneurons (Extension of Fig. 5).
(a) Example membrane potential traces for 7 L4 FS interneurons during a trial with touch (blue vertical lines) and one without touch. Green, whisker position. Average membrane potential for each trial is noted on the left. Cell index is the same as in Supplementary Fig. 7.
(b) Power spectra of the membrane potential during periods without (black) and with (red) whisker movement. Shaded areas are the 95% confidence interval.
(c) Average power spectrum across all L4 FS neurons recorded in whole-cell mode (n=7).
Supplementary Figure 9 Membrane-potential recording during VPM photoinhibition (Extension of Fig. 6).
(a) Coronal slice showing the location of optic fiber. Slice was processed with cytochrome c oxidase staining.
(b) Left, diagram of thalamocortical circuit and recording. Right, Vm traces of an example cell in control condition (top) and during thalamus photoinhibition (bottom).
(c) Average Vm across all recorded cells (n=12) at the onset of laser onset. Shaded areas, mean ± s.e.m.
(d) Resting Vm during thalamus photoinhibition versus under control condition. Resting Vm was calculated from Vm outside touch (including both non-whisking and whisking).
(e) Vm variance during thalamus photoinhibition versus under control condition. Vm variance was calculated from Vm outside touch. Vm was high-pass filtered (10 Hz).
(f) Vm hyperpolarization caused by direct cortical GABAergic activation in L4 excitatory neurons (n=7). Shaded areas, mean ± s.e.m.
(g) Diagram of thalamocortical circuit and VGAT-ChR2-assisted tagging of an FS interneuron (spike width at half height, 0.31 ms; spike height, 20-30 mV).
(h) Diagram of thalamocortical circuit and the effect of thalamus photoinhibition on the membrane potential of the FS interneuron in (g). During thalamus photoinhibition, the Vm fluctuations strongly reduced, suggesting a withdrawal of synaptic input. Note whisking did not produce a subthreshold depolarization without thalamic input, indicating that only thalamic input contained whisking-related input.
(i) Spike rate of VPM multiunits as a function of laser power applied through optic fiber. Since the recordings are multi-units and often not in principal barreloid we did not classify spikes according to behavior epochs (non-whisking, whisking and touch). Laser is 100-Hz 5-ms pulse train. Laser power is the average power coming from the fiber tip.
(j) Spike rate of FS interneurons as a function of laser power, separated into different behavioral epochs.
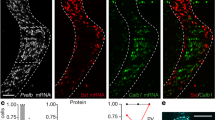
Supplementary Figure 10 Rabies-virus-mediated monosynaptic tracing of input to PV+ interneurons (Extension of Fig. 6).
(a) Schematic illustrating the procedure and time course of virus injection. Mice were PV- IRES-Cre x Ai9. PV+ neurons express tdTomato.
(b) Presynaptic neurons in cortex and in thalamus. Green cells are either presynaptic cells or starter cells. In the cortex, green cells are predominately distributed in L4, suggesting the starter cells are mostly L4 PV neurons, although a small number of L5 PV neurons were likely also starter cells. In thalamus the vast majority of labeled neurons was in VPM, with only a small number in POM. Scale, 200 μm.
(c) Comparison of the number of presynaptic cells found in VPM versus in POM.
Supplementary Figure 11 Membrane-potential fluctuations in L4 excitatory neurons after ION transection (Extension of Fig. 7).
(a) Example membrane potential traces (2 trials) for 10 L4 excitatory neuron after ION transection. Green, whisker position. Average membrane potential for each trial is noted on the left.
(b) Power spectra of the membrane potential during periods without (black) and with (red) whisker movement. Shaded areas are the 95% confidence interval.
(c) Average power spectrum across all L4 excitatory neuron after ION transection (n=10).
Supplementary Figure 12 Spike–phase relationship for L4 FS interneurons after ION transection (Extension of Fig. 7).
(a) Spike rate as a function of phase of whisker movement. Cyan, a sum of a single sinusoidal function and a sinusoidal function of twice the frequency (cf. Supplementary Fig. 7g) was used to fit the data if the goodness-of-fit (r2) is larger than 0.25. Error bars, s.e.m.
(b) Goodness-of-fit of the spike-phase relationship for L4 fast-spiking interneurons in control mice (brown) and in ION-cut mice (black). Arrows mark the median.
Supplementary Figure 13 Example of L4 PV interneuron and L4 excitatory neurons during PV interneuron inactivation (Extension of Fig. 8).
(a) Four L4 PV interneurons recorded. 3 out of 4 are strongly inhibited by eNpHR stimulation. Top row, spike rate during non-whisking and whisking periods. Vertical lines, mean and 95% confidence interval. Bottom row, PSTH of touch responses. Black, control. Orange, eNpHR stimulation.
(b) Same as a for L4 excitatory neurons.
(c) Grand averages of spike rate of L4 FS interneurons and excitatory neurons aligned to the onset of laser stimulation.
Supplementary Figure 14 Suppressing PV interneurons changes behavioral performance (Extension of Fig. 8).
(a) Definition of correct versus incorrect responses of the mouse during behavior.
(b) A behavior session during which PV interneuron inactivation was randomly applied to a small subset of trials. In control condition, mouse performed with a false alarm rate of 20% (Lick during No lick trials). During trials with PV interneuron inactivation, the false alarm rate increased to 61%. Green dots are correct responses and red dots are incorrect responses as defined in (a).
(c) In 8 mice tested, 6 showed a significant decrease of performance during Nolick trials with PV interneuron inactivation (p < 0.05, Chi-squared test, solid lines) and 1 showed a significant increase of performance. In 2 out of 8 mice, there is an increase of performance during Lick trials.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–14 and Supplementary Tables 1 and 2 (PDF 3081 kb)
L4 excitatory neuron #1 during behaviour
Same example neuron as in Fig. 2b, c. Pole movement was controlled by a pneumatic slider (Festo) driven by compressed air. Movement of pole produced sound that can cue the animal to search for the pole. Horizontal lines (blue) after touch onset denote the duration of touches. (AVI 10188 kb)
L4 FS interneuron #1 during behavior.
Same example neuron as in Fig. 4a-e. (AVI 10748 kb)
L4 FS interneuron in mouse after contralateral ION transection.
Same example neuron as in Fig. 7g, h. The procedure of VGAT-ChR2-assisted tagging is illustrated at the end. (AVI 15342 kb)
Rights and permissions
About this article
Cite this article
Yu, J., Gutnisky, D., Hires, S. et al. Layer 4 fast-spiking interneurons filter thalamocortical signals during active somatosensation. Nat Neurosci 19, 1647–1657 (2016). https://doi.org/10.1038/nn.4412
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4412
This article is cited by
-
Neural mechanisms for the localization of unexpected external motion
Nature Communications (2023)
-
Distinguishing externally from saccade-induced motion in visual cortex
Nature (2022)
-
Reduction of corpus callosum activity during whisking leads to interhemispheric decorrelation
Nature Communications (2021)
-
Attractor dynamics gate cortical information flow during decision-making
Nature Neuroscience (2021)
-
Recurrent interactions in local cortical circuits
Nature (2020)