Abstract
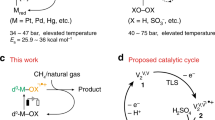
Natural gas contains large volumes of light alkanes, and its abundant reserves make it an appealing feedstock for value-added chemicals and fuels. However, selectively activating the C–H bonds in these useful hydrocarbons is one of the greatest challenges in catalysis. Here we report an attractive oxybromination method for the one-step functionalization of methane under mild conditions that integrates gas-phase alkane bromination with heterogeneously catalysed HBr oxidation, a step that is usually executed separately. Catalyst-design strategies to provide optimal synergy between these two processes are discussed. Among many investigated material families, vanadium phosphate (VPO) is identified as the best oxybromination catalyst, as it provides selectivity for CH3Br up to 95% and stable operation for over 100 hours on stream. The outstanding performance of VPO is rationalized by its high activity in HBr oxidation and low propensity for methane and bromomethane oxidation. Data on the oxybromination of ethane and propane over VPO suggest that the reaction network for higher alkanes is more complex.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
McFarland, E. Unconventional chemistry for unconventional natural gas. Science 338, 340–342 (2012).
Horn, R. & Schlögl, R. Methane activation by heterogeneous catalysis. Catal. Lett. 145, 23–39 (2015).
Hammer, G. et al. in Ullmann's Encyclopedia of Industrial Chemistry Vol. 23 (eds Bellussi, G. et al.) 739–792 (Wiley-VCH, 2012).
Zhou, X. P. et al. Integrated process for synthesizing alcohols and ethers from alkanes. US patent 6472572 B1 (2002).
Zhou, X.-P. et al. An integrated process for partial oxidation of alkanes. Chem. Commun. 2294–2295 (2003).
Lorkovic, I. M. et al. C1 coupling via bromine activation and tandem catalytic condensation and neutralization over CaO/zeolite composites. Chem. Commun. 566–567 (2004).
Breed, A. et al. Natural gas conversion to liquid fuels in a zone reactor. Catal. Today 106, 301–304 (2005).
Svelle, S. et al. The methyl halide to hydrocarbon reaction over H-SAPO-34. J. Catal. 241, 243–254 (2006).
Osterwalder, N. & Stark, W. J. Direct coupling of bromine-mediated methane activation and carbon-deposit gasification. ChemPhysChem 8, 297–303 (2007).
Ding, K., Metiu, H. & Stucky, G. D. Interplay between bromine and iodine in oxidative dehydrogenation. ChemCatChem 5, 1906–1910 (2013).
Olah, G. A. & Molnár, Á. Hydrocarbon Chemistry Ch. 10.2 (John Wiley & Sons, Inc., 2003).
Olah, G. A., Goeppert, A. & Prakash, G. K. S. Beyond Oil and Gas: The Methanol Economy Ch. 12.3.4 (Wiley-VCH, 2009).
Moser, M., Rodríguez-García, L., Amrute, A. P. & Pérez-Ramírez, J. Catalytic bromine recovery: an enabling technology for emerging alkane functionalization processes. ChemCatChem 5, 3520–3523 (2013).
Moser, M., Czekaj, I., López, N. & Pérez-Ramírez, J. The virtue of defects: stable bromine production by catalytic oxidation of hydrogen bromide on titanium oxide. Angew. Chem. Int. Ed. 53, 8628–8633 (2014).
Wang, K. X., Xu, H. F., Li, W. S. & Zhou, X. P. Acetic acid synthesis from methane by non-synthesis gas process. J. Mol. Catal. A 225, 65–69 (2005).
Liu, Z. et al. Higher hydrocarbons from methane condensation mediated by HBr. J. Mol. Catal. A 273, 14–20 (2007).
Yang, F., Liu, Z., Li, W. S., Wu, T. H. & Zhou, X. P. The oxidative bromination of methane over Rh/SiO2 catalyst. Catal. Lett. 124, 226–232 (2008).
Lin, R. et al. Efficient and stable silica-supported iron phosphate catalysts for oxidative bromination of methane. J. Catal. 272, 65–73 (2010).
He, J. et al. Transformation of methane to propylene: a two-step reaction route catalyzed by modified CeO2 nanocrystals and zeolites. Angew. Chem. Int. Ed. 51, 2438–2442 (2012).
Gorin, E. Hydrocarbon conversion process. US patent 2407828 (1946).
Albonetti, S., Cavani, F. & Trifirò, F. Key aspects of catalyst design for the selective oxidation of paraffins. Catal. Rev. Sci. Eng. 38, 413–438 (1996).
Schlögl, R. in Modern Heterogeneous Oxidation Catalysis: Design, Reactions and Characterization (eds Schlögl, R. & Mizuno, N.) 1–42 (Wiley-VCH, 2009).
Carroll, R. T., De Witt, E. J., Falls, C. & Trapasso, L. E. Oxychlorination of lower alkanes. US patent 3173962 (1965).
Gadewar, S. et al. Continuous process for converting natural gas to liquid hydrocarbons. US patent 20100096588 A1 (2010).
Ding, K. et al. Hydrodebromination and oligomerization of dibromomethane. ACS Catal. 2, 479–486 (2012).
Martin, D. & Duprez, D. Mobility of surface species on oxides. 1. Isotopic exchange of 18O2 with 16O of SiO2, Al2O3, ZrO2, MgO, CeO2, and CeO2–Al2O3. Activation by noble metals. Correlation with oxide basicity. J. Phys. Chem. 100, 9429–9438 (1996).
Ferretto, L. & Glisenti, A. Surface acidity and basicity of a rutile powder. Chem. Mater. 15, 1181–1188 (2003).
Hevia, M. A. G., Amrute, A. P., Schmidt, T. & Pérez-Ramírez, J. Transient mechanistic study of the gas-phase HCl oxidation to Cl2 on bulk and supported RuO2 catalysts. J. Catal. 276, 141–151 (2010).
Chen, C.-Y. & Pignatello, J. J. Catalytic oxidation for elimination of methyl bromide fumigation emissions using ceria-based catalysts. Appl. Catal. B 142–143, 785–794 (2013).
Ai, M. & Ohdan, K. Oxidation by iron phosphate catalyst. J. Mol. Catal. A 159, 19–24 (2000).
Bautista, F. M. et al. Effect of precipitation medium on surface acidity and catalytic performance of chromium orthophosphates in cyclohexene skeletal isomerization and cumene conversion. J. Mater. Chem. 3, 975–978 (1993).
Busca, G., Cavani, F., Centi, G. & Trifirò, F. Nature and mechanism of formation of vanadyl pyrophosphate: active phase in n-butane selective oxidation. J. Catal. 99, 400–414 (1986).
Hutchings, G. J., Desmartin-Chomel, A., Olier, R. & Volta, J.-C. Role of the product in the transformation of a catalyst to its active state. Nature 368, 41–45 (1994).
Coulston, G. W. et al. The kinetic significance of V5+ in n-butane oxidation catalyzed by vanadium phosphates. Science 275, 191–193 (1997).
Mahdavi, V. & Hasheminasab, H. R. Vanadium phosphorous oxide catalyst promoted by cobalt doping for mild oxidation of benzyl alcohol to benzaldehyde in the liquid phase. Appl. Catal. A 482, 189–197 (2014).
Aït-Lachgar, K. et al. Selective oxidation of n-butane to maleic anhydride on vanadyl pyrophosphate. II. Characterization of the oxygen-treated catalyst by electrical conductivity, Raman, XPS, and NMR spectroscopic techniques. J. Catal. 177, 224–230 (1998).
Frey, J. et al. Vanadium phosphates on mesoporous supports: model catalysts for solid-state NMR studies of the selective oxidation of n-butane. Solid State Nucl. Magn. Reson. 35, 130–137 (2009).
Hutchings, G. J. Vanadium phosphate: a new look at the active components of catalysts for the oxidation of butane to maleic anhydride. J. Mater. Chem. 14, 3385–3395 (2004).
Kistiakowsky, G. B. & Van Artsdalen, E. R. Bromination of hydrocarbons. I. Photochemical and thermal bromination of methane and methyl bromide. Carbon–hydrogen bond strength in methane. J. Chem. Phys. 12, 469–478 (1944).
Luo, Y.-R. in Comprehensive Handbook of Chemical Bond Energies Ch. 3.1, 4.1 (Taylor & Francis Group, 2007).
Cavani, F., Ballarini, N. & Cericola, A. Oxidative dehydrogenation of ethane and propane: how far from commercial implementation. Catal. Today 127, 113–131 (2007).
Tsang, W. The stability of alkyl radicals. J. Am. Chem. Soc. 107, 2872–2880 (1985).
Acknowledgements
The authors acknowledge financial support from the Swiss National Science Foundation (project no. 200021-156107). The Scientific Center for Optical and Electron Microscopy (ScopeM) at ETH Zurich is thanked for providing access to the facility. Z. Guo is acknowledged for assistance with catalyst preparation. The authors are grateful to F. Krumeich for HRTEM analyses and R. Verel for 31P MAS NMR analyses.
Author information
Authors and Affiliations
Contributions
J.P.-R. conceived and coordinated all the stages of this research. V.P. prepared the catalyst samples, performed the catalytic tests and characterized materials by XRD and H2-TPR, and G.Z. and M.M. conducted part of the catalytic tests. A.P.A. assisted in the HRTEM and solid-state NMR characterization. V.P., M.M., A.P.A. and J.P.-R. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 2464 kb)
Rights and permissions
About this article
Cite this article
Paunović, V., Zichittella, G., Moser, M. et al. Catalyst design for natural-gas upgrading through oxybromination chemistry. Nature Chem 8, 803–809 (2016). https://doi.org/10.1038/nchem.2522
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2522
This article is cited by
-
Direct conversion of methane with O2 at room temperature over edge-rich MoS2
Nature Catalysis (2023)
-
Sustainable methane utilization technology via photocatalytic halogenation with alkali halides
Nature Communications (2023)
-
Mg/Ca modified hierarchical porous ZSM-5 zeolite for light-olefins production from chloromethane
Journal of Porous Materials (2023)
-
Water enables mild oxidation of methane to methanol on gold single-atom catalysts
Nature Communications (2021)
-
Facile construction of non-crystalline ZrO2 as an active yet durable catalyst for methane oxychlorination
Journal of Sol-Gel Science and Technology (2019)