Abstract
The evolutionary origin of Homo floresiensis, a diminutive hominin species previously known only by skeletal remains from Liang Bua in western Flores, Indonesia, has been intensively debated. It is a matter of controversy whether this primitive form, dated to the Late Pleistocene, evolved from early Asian Homo erectus and represents a unique and striking case of evolutionary reversal in hominin body and brain size within an insular environment1,2,3,4. The alternative hypothesis is that H. floresiensis derived from an older, smaller-brained member of our genus, such as Homo habilis, or perhaps even late Australopithecus, signalling a hitherto undocumented dispersal of hominins from Africa into eastern Asia by two million years ago (2 Ma)5,6. Here we describe hominin fossils excavated in 2014 from an early Middle Pleistocene site (Mata Menge) in the So’a Basin of central Flores. These specimens comprise a mandible fragment and six isolated teeth belonging to at least three small-jawed and small-toothed individuals. Dating to ~0.7 Ma, these fossils now constitute the oldest hominin remains from Flores7. The Mata Menge mandible and teeth are similar in dimensions and morphological characteristics to those of H. floresiensis from Liang Bua. The exception is the mandibular first molar, which retains a more primitive condition. Notably, the Mata Menge mandible and molar are even smaller in size than those of the two existing H. floresiensis individuals from Liang Bua. The Mata Menge fossils are derived compared with Australopithecus and H. habilis, and so tend to support the view that H. floresiensis is a dwarfed descendent of early Asian H. erectus. Our findings suggest that hominins on Flores had acquired extremely small body size and other morphological traits specific to H. floresiensis at an unexpectedly early time.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Brown, P. et al. A new small-bodied hominin from the Late Pleistocene of Flores, Indonesia. Nature 431, 1055–1061 (2004)
Kaifu, Y. et al. Craniofacial morphology of Homo floresiensis: description, taxonomic affinities, and evolutionary implication. J. Hum. Evol. 61, 644–682 (2011)
Kaifu, Y. et al. Unique dental morphology of Homo floresiensis and its evolutionary implications. PLoS ONE 10, e0141614 (2015)
Sutikna, T. et al. Revised stratigraphy and chronology for Homo floresiensis at Liang Bua in Indonesia. Nature 532, 366–369 (2016)
Morwood, M. J. & Jungers, W. L. Conclusions: implications of the Liang Bua excavations for hominin evolution and biogeography. J. Hum. Evol. 57, 640–648 (2009)
Brown, P. & Maeda, T. Liang Bua Homo floresiensis mandibles and mandibular teeth: a contribution to the comparative morphology of a new hominin species. J. Hum. Evol. 57, 571–596 (2009)
Brumm, A. et al. Age and context of the oldest known hominin fossils from Flores. Nature http://dx.doi.org/10.1038/nature17663 (2016)
White, T. D., Johanson, D. C. & Kimbel, W. H. Australopithecus africanus: its phyletic position reconsidered. S. Afr. J. Sci. 77, 445–470 (1981)
Villmoare, B. et al. Early Homo at 2.8 Ma from Ledi-Geraru, Afar, Ethiopia. Science 347, 1352–1355 (2015)
Rosas, A. & Bermudez de Castro, J. M. On the taxonomic affinities of the Dmanisi mandible (Georgia). Am. J. Phys. Anthropol. 107, 145–162 (1998)
Kaifu, Y. et al. Taxonomic affinities and evolutionary history of the Early Pleistocene hominids of Java: dentognathic evidence. Am. J. Phys. Anthropol. 128, 709–726 (2005)
Suwa, G. Serial allocations of isolated mandibuar molars of unknown taxonomic affinities from the Shungra and Usno Formations, Ethiopia, a combined method approach. Hum. Evol . 11, 269–282 (1996)
Kimbel, W. H., Johanson, D. C. & Rak, Y. Systematic assessment of a maxilla of Homo from Hadar, Ethiopia. Am. J. Phys. Anthropol. 103, 235–262 (1997)
Gómez-Robles, A., Martinón-Torres, M., Bermúdez de Castro, J. M., Prado-Simon, L. & Arsuaga, J. L. A geometric morphometric analysis of hominin upper premolars. Shape variation and morphological integration. J. Hum. Evol. 61, 688–702 (2011)
Martinón-Torres, M. et al. Dental remains from Dmanisi (Republic of Georgia): morphological analysis and comparative study. J. Hum. Evol. 55, 249–273 (2008)
Kaifu, Y. et al. Descriptions of the dental remains of Homo floresiensis. Anthropol. Sci. 123, 129–145 (2015)
Tobias, P. V. Olduvai Gorge, 4: The Skulls, Endocasts and Teeth of Homo habilis . Vol. 4 (Cambridge Univ. Press, 1991)
Brumm, A. et al. Early stone technology on Flores and its implications for Homo floresiensis. Nature 441, 624–628 (2006)
van den Bergh, G. D. et al. The Liang Bua faunal remains: a 95k.yr. sequence from Flores, East Indonesia. J. Hum. Evol. 57, 527–537 (2009)
Kaifu, Y. et al. Homo erectus calvaria from Ngawi (Java) and its evolutionary implications. Anthropol. Sci. 123, 161–176 (2015)
Puymerail, L. et al. Structural analysis of the Kresna 11 Homo erectus femoral shaft (Sangiran, Java). J. Hum. Evol. 63, 741–749 (2012)
Ruff, C. B., Puymerail, L., Macchiarelli, R., Sipla, J. & Ciochon, R. L. Structure and composition of the Trinil femora: functional and taxonomic implications. J. Hum. Evol. 80, 147–158 (2015)
Kubo, D., Kono, R. T. & Kaifu, Y. Brain size of Homo floresiensis and its evolutionary implications. Proc. R. Soc. Lond. B 280, 20130338 (2013)
Brumm, A. et al. Hominins on Flores, Indonesia, by one million years ago. Nature 464, 748–752 (2010)
Larick, R. et al. Early Pleistocene 40Ar/39Ar ages for Bapang Formation hominins, Central Jawa, Indonesia. Proc. Natl Acad. Sci. USA 98, 4866–4871 (2001)
Hyodo, M. et al. High-resolution record of the Matuyama–Brunhes transition constrains the age of Javanese Homo erectus in the Sangiran dome, Indonesia. Proc. Natl Acad. Sci. USA 108, 19563–19568 (2011)
Morwood, M. & van Oosterzee, P. The Discovery of the Hobbit (Random House, 2007)
van den Bergh, G. D. et al. Earliest hominin occupation of Sulawesi, Indonesia. Nature 529, 208–211 (2016)
Dennell, R. W., Louys, J., O’Regan, H. J. & Wilkinson, D. M. The origins and persistence of Homo floresiensis on Flores: biogeoraphical and ecological perspectives. Quat. Sci. Rev. 96, 98–107 (2014)
Lister, A. M. Rapid dwarfing of red deer on Jersey in the last interglacial. Nature 342, 539–542 (1989)
Lordkipanidze, D. et al. A complete skull from Dmanisi, Georgia, and the evolutionary biology of early Homo. Science 342, 326–331 (2013)
Wood, B. Fifty years after Homo habilis. Nature 508, 31–33 (2014)
Antón, S. C., Potts, R. & Aiello, L. C. Human evolution. Evolution of early Homo: an integrated biological perspective. Science 345, 1236828 (2014)
Spoor, F. et al. Reconstructed Homo habilis type OH 7 suggests deep-rooted species diversity in early Homo. Nature 519, 83–86 (2015)
Kaifu, Y. Advanced dental reduction in Javanese Homo erectus. Anthropol. Sci. 114, 35–43 (2006)
Zanolli, C. Additional evidence for morpho-dimensional tooth crown variation in a new Indonesian H. erectus sample from the Sangiran Dome (Central Java). PLoS ONE 8, e67233 (2013)
Jacob, T. et al. Pygmoid Australomelanesian Homo sapiens skeletal remains from Liang Bua, Flores: population affinities and pathological abnormalities. Proc. Natl Acad. Sci. USA 103, 13421–13426 (2006)
Suwa, G. A Comparative Analysis of Hominid Dental Remains from the Sungura and Usno Formations, Omo Valley, Ethiopia Ph.D. thesis, University of California, Berkeley (1990)
Wood, B. Koobi Fora Research Project 4: Hominid Cranial Remains . Vol. 4 (Clarendon Press, 1991)
Leakey, M. G. et al. New fossils from Koobi Fora in northern Kenya confirm taxonomic diversity in early Homo. Nature 488, 201–204 (2012)
Weidenreich, F. The dentition of Sinanthropus pekinensis: a comparative odontography of the hominids. Palaeontologia Sinica New Series D 1, 1–180 (1937)
Xing, S., Martinón-Torres, M., Bermúdez de Castro, J. M., Wu, X. & Liu, W. Hominin teeth from the early Late Pleistocene site of Xujiayao, Northern China. Am. J. Phys. Anthropol. 156, 224–240 (2015)
Kuhl, F. P. Elliptic Fourier features of a closed contour. Computer Graphics and Image Processing 18, 236–258 (1982)
Lestrel, P. E., Wolfe, C. A. & Bodt, A. Mandibular shape analysis in fossil hominins: Fourier descriptors in norma lateralis. Homo 64, 247–272 (2013)
Wood, B. A. & Abbott, S. A. Analysis of the dental morphology of Plio-Pleistocene hominids. I. Mandibular molars: crown area measurements and morphological traits. J. Anat. 136, 197–219 (1983)
Bailey, S. E. & Lynch, J. M. Diagnostic differences in mandibular P4 shape between Neandertals and anatomically modern humans. Am. J. Phys. Anthropol. 126, 268–277 (2005)
Martinón-Torres, M. et al. Hominin lower second premolar morphology: evolutionary inferences through geometric morphometric analysis. J. Hum. Evol. 50, 523–533 (2006)
Iwata, H. & Ukai, Y. SHAPE: a computer program package for quantitative evaluation of biological shapes based on elliptic Fourier descriptors. J. Hered. 93, 384–385 (2002)
Smith, B. H. Patterns of molar wear in hunter-gathers and agriculturalists. Am. J. Phys. Anthropol. 63, 39–56 (1984)
Hillson, S., Fitzgerald, C. & Flinn, H. Alternative dental measurements: proposals and relationships with other measurements. Am. J. Phys. Anthropol. 126, 413–426 (2005)
Kimbel, W. H., Rak, Y. & Johanson, D. C. The Skull of Australopithecus afarensis (Oxford Univ. Press, 2004)
Kaifu, Y., Aziz, F. & Baba, H. Hominid mandibular remains from Sangiran: 1952–1986 collection. Am. J. Phys. Anthropol. 128, 497–519 (2005)
Chang, C. H. et al. The first archaic Homo from Taiwan. Nature Commun . 6, 6037 (2015)
Acknowledgements
Funding for the So’a Basin project was provided by an Australian Research Council (ARC) Discovery grant (DP1093342) awarded to M.J.M. and A.B., and the project was directed by M.J.M. (2010-2013) and G.D.v.d.B. (2013-2015). The Japan Society for the Promotion of Science provided a grant (No. 24247044) to Y.K. Financial and technical support was provided by the Geological Survey Centre of Indonesia. The Indonesian State Ministry of Research and Technology granted permission to undertake this research, and we thank the successive directors of the Geological Survey Centre, Y. Kusumahbrata, A. Wibowo and A. Pribadi, the Heads of the Geological Agency (R. Sukyiar and Surono), and the successive directors of the Geology Museum in Bandung (S. Baskoro and O. Abdurahman) for facilitating and supporting this research. In addition, we acknowledge support and advice provided by I. Setiadi, D. Pribadi and Suyono. We also thank M. R. Puspaningrum, H. Insani, I. Sutisna, S. Sonjaya, U. P. Wibowo, A. Gunawan, A. M. Saiful, S. Hayes, B. Burhan, E. Sukandar, A. Rahman, A. Rahmadi and E. E. Laksmana for their assistance in the field (2014–2015), and G. Suwa, T. Djubiantono, F. Aziz, T. Jacob, E. Mbua, F. Schrenk, I. Tattersall, K. Mowbray, J. de Vos, P. Mennecier, F. Demeter, Nguyen Kim Thuy, and Nguyen Lan Cuong for access to the specimens in their care.
Author information
Authors and Affiliations
Contributions
G.D.v.d.B. is co-director of the So’a Basin project with A.B. and I.K. M.J.M. (deceased) was also a co-director of the So’a Basin project. The project was initiated by M.J.M. and F.A. G.D.v.d.B. and I.K. undertook initial identification and analyses of the hominin fossils. Y.K. conducted the morphological analysis together with R.T.K. Fieldwork was planned and directed by G.D.v.d.B., I.K., A.B. and E.S.. Y.K., G.D.v.d.B. and A.B. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 CT-based images of the SOA-MM4 mandible and photos of the SOA-MM6 incisor.
a–i, SOA-MM4 mandible. Surface-rendered images of superior (a), lateral (b), inferior (c), lingual (d), anterior (e), and posterior (f) views. Sagittal (h) and horizontal (i). CT sections at the plane indicated by the green (g) and red (h) lines. aMF, a branch of the mandibular foramen; ARR, anterior ramus root; LP, lateral prominence; M1, M1 alveolus; M2, M2 alveolus, M3, M3 alveolus; MC, mandibular canal; Mas, line for the masseter muscle attachment; MHL, mylohyoid line; pbMC, posterior branch of the mandibular canal; SLT, superior lateral torus. j–k, SOA-MM6 mandibular incisor (I1/2) fragments. The crown (j, SOA-MM6a) and a root (k, SOA-MM6b) fragments were used for laser ablation uranium-series dating. The specimen was deposited before at least 0.55 Ma7. Note the bevelled occlusal wear surface (arrow). Scale bar, 5 mm.
Extended Data Figure 2 Linear metric comparisons of the mandibles and permanent teeth.
a–e, Scatter plots of the mandibular corporal dimensions (a, b) and permanent tooth crown diameters (c–e). We identify SOA-MM1 as M1 (e), but there remains a slight possibility that this tooth is M2 (f). Metric data of SOA-MM4: corpus height at M2, 18 mm; corpus height at M2/3, 18.5 mm; corpus width at M2, 12.5 mm; corpus width at M2/3, 13 mm.
Extended Data Figure 3 Mandibular comparisons.
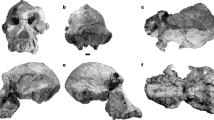
a–m, H. habilis sensu lato: OH 13 (a, late adolescent), OH 37 (b, horizontally flipped image), KNM-ER 1802 (c, late adolescent), KNM-ER 3734 (d, horizontally flipped image), KNM-ER 60000 (e, horizontally flipped image) (photo by F. Spoor, copyright National Museums of Kenya); early Javanese H. erectus, Sangiran 1b (f), Sangiran 9 (g), Sangiran 22 (h), Sb 8103 (i), Sangiran 21 (j); Liang Bua H. floresiensis: LB1 (k), LB6/1 (l, horizontally flipped image; the corpus is distorted); (m) SOA-MM4. Scale bar, 30 mm. Note that the H. habilis mandibles tend to exhibit a thicker corpus, the position of the basal ramus (filled arrow) that is shifted laterally relative to the corpus midline axis, a prominent posterior part of the alveolar prominence (filled triangle), and a wider extramolar sulcus between the anterior ramus root (open arrow) and the molar row. The early Javanese H. erectus sample is variable but includes specimens with weaker expressions in these traits. The Liang Bua H. floresiensis and the SOA-MM4 mandibles share such derived features with early Javanese H. erectus.
Extended Data Figure 4 Comparisons of the hominin mandibles and teeth from So’a Basin (Mata Menge) and H. floresiensis from Liang Bua.
a, SOA-MM4 mandible. b, c, Right lateral and left lateral (horizontally flipped) views of LB1. d, Right lateral view of LB6/1. e, f, The SOA-MM3 and LB1 P3s, respectively. g, SOA-MM1 M1. h–j, Occlusal views of SOA-MM4 (h), LB1 (i), and LB6/1 (j) mandibles. Scale bar, 10 mm.
Extended Data Figure 5 Principal component analyses of the four size-standardized mandibular measurements.
a, Scatter plot of the PC scores. b, Component loading of each PC. PC1 does not distinguish Homo from Au. afarensis, but Au. afarensis and post-habilis Homo are relatively well-separated in PC2. SOA-MM4 belongs to the cluster of Homo in this PC. SOA-MM4 occupies the space in between the two Liang Bua H. floresiensis mandibles, suggesting their shared lateral corporal shape.
Extended Data Figure 6 Metric analyses of mandibular deciduous canines.
a–e, Comparisons of the crown length and breadth (a), and relative crown height (b). Results of the PCA based on size-adjusted five crown diameters (c, d) and the component loadings of each PC (e). ‘Crown size’ = geometric mean of the five crown diameters used. Au. afarensis and H. sapiens are indistinguishable in crown size (d) but they are discriminated from each other by PC1 (P < 0.00002, t-test). SOA-MM7 occupies an intermediate position between Au. afarensis and H. sapiens, suggesting its moderately primitive crown configuration. The other PCs did not discriminate Au. afarensis and H. sapiens.
Supplementary information
Supplementary Information
This file contains Supplementary Notes 1-3. (PDF 89 kb)
Rights and permissions
About this article
Cite this article
van den Bergh, G., Kaifu, Y., Kurniawan, I. et al. Homo floresiensis-like fossils from the early Middle Pleistocene of Flores. Nature 534, 245–248 (2016). https://doi.org/10.1038/nature17999
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature17999
This article is cited by
-
Widespread Denisovan ancestry in Island Southeast Asia but no evidence of substantial super-archaic hominin admixture
Nature Ecology & Evolution (2021)
-
Semiotics and the Origin of Language in the Lower Palaeolithic
Journal of Archaeological Method and Theory (2021)
-
Pleistocene Water Crossings and Adaptive Flexibility Within the Homo Genus
Journal of Archaeological Research (2021)
-
Isotopic evidence for initial coastal colonization and subsequent diversification in the human occupation of Wallacea
Nature Communications (2020)
-
A genotype:phenotype approach to testing taxonomic hypotheses in hominids
The Science of Nature (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.