Abstract
There is increasing evidence that multiple chromosomal rearrangements occur in prostate cancer. PTEN loss is considered to be a key event in prostate carcinogenesis but the mechanisms of loss remain to be fully elucidated. We hypothesised that gross rearrangements may exist that cause disruption of the PTEN gene in the absence of genomic deletion. We therefore designed a novel fluorescence in situ hybridisation (FISH) assay with probes overlying regions 3′ and 5′ of PTEN and a third probe overlying the gene. We aimed to identify both genomic deletions and gross rearrangements of PTEN that would be overlooked by previously reported single-probe FISH assays. We proceeded to evaluate a tissue microarray with radical prostatectomy and trans-urethral resection of the prostate specimens from 187 patients. We identified PTEN genomic loss in 45/150 (30%) radical prostatectomy patients and 16/37 (43%) trans-urethral resection of the prostate patients. Importantly, our assay detected novel chromosomal alterations in the PTEN gene (characterised by splitting of FISH signals) in 13 tumours (6.9% of all prostate cancers; 21% of PTEN-lost cancers). All PTEN-rearranged tumours had genomic loss at the other allele and had no expression of PTEN by immunohistochemistry. PTEN-rearranged tumours were significantly more likely to have an underlying ERG rearrangement. Our assay differentiated loss of the probe overlying PTEN in isolation or in combination with either one of or both the probes overlying the 3′ and 5′ regions. This gave an indication of the size of genomic loss and we observed considerable inter-tumoural heterogeneity in the extent of genomic loss in PTEN-lost tumours. In summary, gross rearrangements of the PTEN locus occur in prostate cancer and can be detected by a ‘break-apart’ FISH assay. This observation could explain the absence of PTEN protein expression in a subgroup of tumours previously classified as having heterozygous genomic loss using single-probe traditional FISH assays.
Similar content being viewed by others
Main
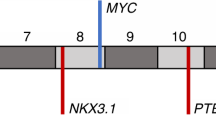
Recent reports of paired-end, massively parallel sequencing of the prostate cancer genome identified novel loss-of-function chromosomal rearrangements1 in addition to the previously described oncogenic chromosomal rearrangements involving ETS and BRAF family genes.2, 3, 4 For example, gross rearrangements of MAGI2 have been described and evaluated using a fluorescence in situ hybridisation (FISH) break-apart assay.1 In this recent paper, PTEN has been described to be altered by chromosomal rearrangement but the alterations described in clinical samples resulted in complete loss of one PTEN probe FISH signal1 (Figure 1a). Gross chromosomal alterations of PTEN without copy number loss have however been reported in breast cancer xenografts with underlying DNA repair defects.5 Loss of PTEN function is common and considered to be an important event in prostate carcinogenesis.6, 7 Multiple mechanisms underlie loss of PTEN function7 with genomic loss occurring in up to 40–50% of primary prostate cancers.8, 9, 10, 11 FISH studies allow the robust evaluation of genomic loss in reasonably sized tumour cohorts and importantly, most have identified an association between genomic PTEN loss and worse clinical outcome.8, 9 However, as most reported studies have utilised single FISH probes over PTEN (Figure 1a), alleles disrupted by gross chromosomal alterations without copy number loss may not have been detected and would have been wrongly classified as PTEN wild type. We therefore sought to develop a FISH assay that would comprehensively identify both copy number loss and gross chromosomal alterations of the PTEN locus; these were then evaluated in a cohort of prostate cancers identified in a PSA-screened population.
(a) FISH probes used in previous publications to detect PTEN gene loss. Probe 1 is a commercially available PTEN-specific probe (Vysis, Downers Grove, IL) previously used.8, 29, 31 Probe 2a is BAC RP11-765C10 and 2b is BAC RP11-959L24 that map to the minimum region of PTEN deletion22 and were used in combination in Reid et al.9 Probe 3 is PAC190P6, covering PTEN exons 3–9 inclusive and mapping to the minimum region of deletion of PTEN and used in Verhagen et al.10 Probe 4 is BAC CTD-2047N14 used to detect PTEN loss in Berger et al.1 Probe 5a is BAC RP11-846G17 mapping to PTEN and probe 5b is BAC RP11-399O19 mapping to the flanking FAS gene. Both probes were used in Sircar et al.21 The direction of gene transcription is indicated with arrows. C10orf59 and MINPP1 are two genes close to PTEN. (b) Novel PTEN FISH assay to detect rearrangements. At the 3′ end, the BAC probe (RP11-210E13, red) immediately flanks PTEN and at the 5′ end, BAC probe (RP11-765C10, green) partially covers PTEN. A further BAC probe over PTEN (CTD-2267G16, yellow) was used to investigate whether PTEN could be lost or rearranged in isolation of flanking regions.
Materials and methods
Tissue Microarrays and Patient Cohort
Tissue microarrays were constructed, as previously described, from tissue obtained at the time of surgery.12 Briefly, one or two 0.6-mm cores were taken from the donor block with a tissue microarrayer (Beecher Instruments, Silver Spring, MD, USA). Sections of 4 μm were cut on a microtome and transferred to glass slides (Menzel–Gläser, Superfrost, Braunschweig). Prostate cancer samples were collected between 6th June 1995 and 4th November 2005 by intended radical prostatectomy from men with histologically confirmed, clinically localised prostate cancer and by trans-urethral resection of the prostate from men with non-localised prostate cancer treated at the Department of Urology, Aarhus University Hospital, Skejby, Denmark. One hundred and ninety-five tumour cores from 150 patients treated by radical prostatectomy for localized prostate cancer and 37 patients treated by trans-urethral resection of the prostate for non-localized prostate cancer were used. Clinicopathological data on all patients was available and is presented in the results per the REporting recommendations for tumour MARKer prognostic studies (REMARK).13 Additionally, high-grade glioma samples were collected from the archives of Kings College Hospital, London. In total, 389 cores from 342 patients were collated in four tissue microarrays. The cases comprised 276 glioblastoma multiforme (WHO grade IV), 17 anaplastic astrocytoma (WHO grade III), and 49 anaplastic oligodendroglioma (WHO grade III). Ethical approval for the collection of the cohorts was obtained from the Ethics Review Committees of the collaborating hospitals. Areas of ‘cancer’ and ‘normal’ were identified on the basis of histopathological examination of haematoxylin and eosin sections that flanked the tissue microarray slice used for FISH and immunohistochemistry studies and for the prostate cancer tissue microarray, p63/AMACR-stained sections were also available. BACs for probes were chosen using the UCSC genome browser (http://genome.ucsc.edu) and were labelled as described previously.14, 15 FISH studies for PTEN followed by rehybridisation using an ERG break-apart assay were then conducted as described previously.9, 15, 16, 17 Tissue microarrays were fluorescently scanned at × 20 magnification on an Ariol SL-50 (Applied Imaging, San Jose, CA, USA) with a 5 × 0.5 μm z-stack, and images were stored and double scored by two operators (AR and GA/SM). FISH signals in a minimum of 200 nuclei were scored in each core, although often FISH signals in >1000 nuclei per core were assessed.
Immunohistochemistry
In all, 4 μm sections were cut and immunostained using a commercially available PTEN antibody (Cell Signaling Technology #9559) and standard heat-induced antigen retrieval methods were used. As all samples were collected at the same institution, variability of tissue fixation was minimised. Samples were, however, collected over a 10-year period, which may impact immunohistochemistry results. PTEN wild-type controls included normal prostate tissue and 22RV-1 xenograft, and PTEN-loss controls included PC3 (prostate cancer cell line—PTEN null) xenografts. For negative control slides, the primary antibody step was omitted and ChromPure rabbit IgG applied instead. Cytoplasmic PTEN staining was scored according to the product of staining intensity on a 0–3 scale multiplied by the percentage of immunoreactive cells in the cancerous areas. We considered any score of ≥0 to represent some degree of positive staining. Cases were analysed only if positive internal controls were present (ie cells that would be expected to stain positive).
Statistical Analysis
To classify a tissue microarray cancer core as having homozygous PTEN loss, simultaneous lack of both signals of PTEN gene locus-targeted probe and the presence of two signals of chromosome 10 centromeric probe had to occur in ≥10% of nuclei. To classify a core as having heterozygous loss, ≥40% of nuclei had to contain one signal of PTEN gene locus-targeted probe and two signals of chromosome 10 centromeric probes. These cutoffs were established in previous work where FISH signals were counted in normal and cancer nuclei.9 To test significance, the appropriate statistical tool as specified in the text was applied (statistician: DB) and significance was defined as a two-sided P-value of <0.05. Time to biochemical failure was defined as a rise in PSA to >0.2 ng/dl was calculated for patients treated by radical prostatectomy using the Kaplan–Meier method.
Results
Novel Gross Alterations Disrupt the PTEN Locus in Prostate Cancer
We designed and optimised a FISH assay, hereinafter referred to as the ‘break-apart’ assay, to detect alterations at the PTEN gene locus. Two BAC probes were selected that mapped to the PTEN locus. At the 5′ end, a probe (RP11-765C10 labelled with FITC) partially covered PTEN and at the 3′ end, a probe (RP11-210E13 labelled with CY-3) immediately flanked PTEN (Figure 1b). A commercially available, aqua-labelled DNA chromosome 10 centromere probe (chromosome 10, p11.1∼q11.1 Abbott Molecular, Des Plaines) was used to ensure detection of PTEN loss when ploidy was present. Probes were tested on metaphase spreads to ensure that they did not cross-hybridise with any region other than the PTEN locus. Using the ‘break-apart assay’, PTEN loci without ploidy and without loss or rearrangement are visualised in interphase nuclei as adjacent red and green signals. Tissue microarrays from patients having had a radical prostatectomy or trans-urethral resection of the prostate (Table 1) were evaluated with both our single-probe ‘traditional’ PTEN FISH assay (as described previously (Figure 1a))9 and our novel, comprehensive, ‘break-apart’ assay (Figure 1b). Two cancer-containing cores were available from eight patients and one core from the other 179 patients.
As reported previously, we devised cutoffs based on counting of PTEN probes in normal and cancerous prostatic tissue (see Statistical Analysis).9 Using a single probe over PTEN, 105/150 patients treated by radical prostatectomy and 21/37 trans-urethral resection of the prostate cancers had no areas meeting the criteria for PTEN loss and were called ‘normal’. Of the remaining 45 patients treated by radical prostatectomy, 20 had uniform (homogeneous) homozygous loss across the whole core. In keeping with previous reports of tumour heterogeneity in prostate cancer,17, 18, 19 the other 25 tumours had a heterogeneous pattern with areas of both homozygous and heterozygous PTEN loss: 19 heterogeneous tumours were classified as homozygous loss tumours and six as heterozygous (cutoffs defined in Statistical Analysis) (Table 1). Of the remaining 16 patients treated by trans-urethral resection of the prostate, four had homogeneous homozygous loss and two had uniform heterozygous loss. Of the remaining patients, 10 had a heterogeneous pattern: eight classified as homozygous loss and two as heterozygous loss.
Using the ‘break-apart assay’, chromosomal alterations resulting in breakpoints along the PTEN gene and characterised by splitting of FISH signals in the absence of copy number loss were observed in 13 tumours that all had genomic loss at the other allele (6.9% of all prostate cancers and 21% of PTEN-lost cancers) (Figure 2). In this series, we did not detect any cases with splitting of signals in the absence of genomic loss. Using this assay, the operators scored a median of 236 (range: 215–296) nuclei to obtain informative FISH results from 200 nuclei.
Gross alterations of the PTEN gene. In the picture on the left-hand side, DAPI-stained nuclei are seen with the BAC probe (RP11-210E13) immediately flanking PTEN (red) and the BAC probe (RP11-765C10) partially covering PTEN (green) (described in Figure 1b). A centromere 10 probe in yellow was also used. Two copies of the centromere probe are seen in each nucleus with only one copy of each of the PTEN probes ‘split’ from each other. The probes from the other PTEN allele are completely lost. A cartoon representation of the nuclei with gross PTEN alterations is seen on the right-hand side.
Chromosomal Alterations Involving PTEN Cooperate with Allelic Loss in Prostate Cancer
To confirm that splitting of signals using our ‘break-apart’ FISH assay resulted in loss of PTEN protein, we proceeded to evaluate PTEN status by immunohistochemistry in all tumours acquired by either radical prostatectomy or trans-urethral resection of the prostate. In patients with neither genomic loss nor gross chromosomal alterations of PTEN, 42% of patients were classified as having positive staining of PTEN by immunohistochemistry and 58% were completely negative (Table 2). In some tumours, PTEN status varied across the core—for example, areas with underlying genomic PTEN loss (heterozygous or homozygous as detected by FISH) have been observed adjacent to each other and adjacent to areas of normal PTEN. Therefore, tumours with areas of retained PTEN loci could have positive immunohistochemistry staining in addition to negative staining and we described this as ‘heterogenous’. As homozygous loss was heterogenous in 27 tumours (Table 1) and those cells in a core with retained PTEN loci could result in positive immunohistochemistry staining, we differentiated tumours with heterogenous from those with homogenous loss (Table 2). In fact, 22% of tumours with heterogeneous homozygous genomic loss stained positive by immunohistochemistry and 78% stained negative. In contrast, 5% of tumours with homogeneous homozygous loss stained positive and 95% were negative by immunohistochemistry. All tumours with heterozygous genomic loss and an associated chromosomal alteration were negative by immunohistochemistry. There was no statistically significant association with outcome when tumours with PTEN loss detected by immunohistochemistry were compared with tumours without PTEN loss (data not shown). Interestingly, a core was found that had an area of cancer with heterozygous genomic loss and a chromosomal rearrangement adjacent to an area with normal PTEN FISH; the latter area was positive on immunohistochemistry and the former area was negative (Figure 3).
Tissue microarray cores immunostained with an antibody against PTEN. (a) A cancer core with evidence of strong positive PTEN staining in the cancer glands (indicated with red arrow). The normal glands (indicated with a black arrow) also stain positively but less intensely than the cancer glands. (b) A cancer core with loss of PTEN staining in cancer glands (indicated with a blue arrow) and control endothelial cells staining positively (indicated with a red arrow). (c) One-half of the core has cancer glands, which stain negatively for PTEN (indicated with a blue arrow). FISH with the ‘break-apart’ assay revealed heterozygous genomic loss plus a chromosomal rearrangement in this area. The other half of the cancer glands have a normal PTEN complement (indicated with a red arrow) and FISH revealed two wild-type PTEN copies in this area. Finally, the gland in the middle of the core is a normal gland, which also stains positively (indicated with a black arrow) and retained both PTEN alleles, as expected, by FISH.
Table 3 reports patient demographics and clinicopathological variables for radical prostatectomy tumours with wild-type PTEN FISH, tumours classified as homozygous genomic loss (both heterogeneous and homogeneous) and tumours with heterozygous genomic loss associated with a chromosomal alteration (homozygous loss secondary to a chromosomal alteration).
There was a significant association between homozygous loss of PTEN by either biallelic genomic deletion or genomic deletion in combination with chromosomal alteration and clinical tumour stage (P=0.023 and 0.005, respectively; χ2 test for trend) but the association with Gleason score was not significant (P=0.36; χ2 test) (Table 3). Complete data for clinical nodal stage was not available. There was no association with median PSA or age (P>0.1; Wilcox t-test). Of the 150 patients treated with radical prostatectomy, 57 had a biochemical recurrence. Eight patients received adjuvant hormones and were not included in the following analysis. For the remaining 141 patients, 5/6 (83%) of tumours with homozygous loss secondary to genomic loss and a rearrangement relapsed; 20/34 (59%) of tumours with biallelic PTEN loss relapsed; and 47/99 (47%) of cases with normal PTEN status had relapsed. The predicted median time to biochemical recurrence was 1114 days (95% CI=695 to unestimatable) for PTEN tumours with homozygous loss secondary to genomic loss and a rearrangement; 1192 days (95% CI=786 to unestimatable) in biallelic PTEN loss; and 1464 days (95% CI=1089 to unestimatable) in PTEN normal cases. There was no statistically significant difference between the three groups in this series (P>0.05).
Footprint of Genomic PTEN Loss Is Variable
We then rehybridised a series of cores using a probe directly over the PTEN gene (CTD-2267G16, yellow), in addition to the two ‘break-apart’ assay probes (RP11-210E13 and RP11-765C10), to establish whether PTEN could be lost or rearranged in isolation of flanking regions (Figure 1b). The probe over PTEN was lost either with the 3′-centromeric probe (red) or with the 5′-telomeric probe (green) but was never lost in isolation. In three cases, the probe directly over PTEN appeared to split, half associating with the red and half with the green probe (Table 4A). In addition to identifying novel rearrangements of PTEN using the ‘break-apart’ FISH assay, we also observed that the extent of PTEN loss varies between patients. In 80% of cases, the pattern of PTEN loss was the same for all alleles (there were cases with ploidy) in each cell. Three patterns of PTEN genomic loss were observed: (1) complete loss of all three probes over PTEN (region of deletion ≥483 kb); (2) loss of the probe 5′ to PTEN (green) and the probe directly over PTEN (yellow) but maintenance of the 3′ probe (red) (region of deletion ∼283 kb); (3) loss of only the 5′ probe (green) with maintenance of the 3′ probe (red) and the probe (yellow) directly over PTEN (region of deletion ∼183 kb) (Table 4B). In the remaining 20% of cases, a heterogeneous different pattern of PTEN loss was observed. For example, we observed complete loss of all three probes in one allele and loss of only the 5′ probe with maintenance of the 3′ probe and the probe directly over PTEN in the other allele in three tumours and similarly, complete loss of all three probes in one allele and loss of the 5′ PTEN probe and the probe directly over PTEN but maintenance of the 3′ probe in the other allele in two tumours.
PTEN Genomic Aberrations Are Associated with Rearrangements of ERG
The distribution of tumours with an ERG rearrangement with respect to PTEN status is shown in Table 5. We observed a significant association between PTEN loss and ERG gene rearrangements both secondary to biallelic genomic loss (P=0.0004; χ2 test) and genomic loss in association with a chromosomal rearrangement (P=0.0187; χ2 test).
PTEN Loss Occurs Commonly in Glioblastoma Multiforme but Gross Chromosomal Alterations Are Rare
We report PTEN rearrangements in prostate cancer and they have also been reported in breast cancer xenografts5 albeit not to date in breast cancer clinical samples. We therefore proceeded to evaluate whether rearrangements occur in glioblastoma, another cancer type with a well-documented high incidence of PTEN loss.20 Heterozygous loss of PTEN was observed in 119/253 (47%) assessable glioblastoma cases, with homozygous deletions seen in a further 10 cases (3.9%). There were no correlations between PTEN loss and clinical outcome. A single case with a split PTEN FISH signal was observed in a 67-year-old female patient with glioblastoma. Heterozygous loss of PTEN was seen in 2/13 anaplastic astrocytoma (15.4%) and 6/41 anaplastic oligodendroglioma (14.6%); there were no homozygous deletions. Therefore, only one case in a series of 307 high-grade glioma samples contained a split PTEN pattern.
Discussion
We present a novel ‘break-apart’ PTEN FISH assay that detects both gross chromosomal rearrangements and genomic deletion. Previous studies of PTEN with FISH have mostly used single probes overlying the gene, which have not detected rearrangements. Sircar et al21 have used a combination of BAC probes to detect PTEN and two flanking genes (BMPR1A and FAS) (part shown in Figure 1b) but the probe overlying PTEN was again single and rearrangements were not detected with this assay, suggesting that the rearrangements we report in this study occur in a relatively small area around the PTEN locus. In our series, there were no deletions of PTEN involving solely our middle probe (CTD-2267G16) (Figure 1b). This is in keeping with previous studies that reported a minimal region of PTEN loss22 covered by our 5′ probe (RP11-765C10). If this observation is confirmed by ongoing analyses in larger data sets, it could be sufficient to proceed with the three-colour ‘break-apart’ assay using the 3′ (RP11-210E13) and 5′ (RP11-765C10) probes over PTEN together with a reference probe. Our study confirms the presence of loss-of-function gross chromosomal rearrangements in prostate cancer that are similar to recently described rearrangements that involve MAGI2.1 All the chromosomal rearrangements in our series occurred in tumours with genomic loss at the other allele(s). This observation also requires evaluation in a larger series of tumours to investigate whether gross chromosomal rearrangements could occur in the absence of copy number loss. We showed that tumours with heterozygous genomic deletion plus a chromosomal rearrangement had complete loss of expression of PTEN protein. Immunohistochemistry is subject to operator and processing bias and there is no universally established assay for assessing PTEN protein expression. We have developed an immunohistochemistry protocol (described in Materials and methods) that has given robust results. Importantly, there was significant loss of PTEN protein in tumours with homogeneous homozygous PTEN genomic loss, with a ‘false-positive’ rate of 5%. This could be explained by tumour heterogeneity and in fact, tumours that had heterogeneous homozygous loss by FISH (we used the adjacent tissue slice or <5 slices apart for immunohistochemistry and FISH) were more likely to show areas of PTEN immunohistochemistry positivity (Table 3). Immunohistochemistry results can also be influenced by technical challenges such as variable tissue fixation and slide storage before staining. We also here report complete loss of PTEN protein expression in 58% of tumours that were PTEN wild type by FISH, in keeping with previous reports of multiple alternative mechanisms for loss of PTEN protein.7 Assessments with clinical outcome in this series were limited by the duration of follow-up and size of this cohort. Although some reports have described a worse outcome for tumours with homozygous compared with heterozygous genomic PTEN loss,8 we have previously reported no difference between these two groups.9 This could now be explained by tumours with homozygous loss secondary to a chromosomal rearrangement being wrongly classified as having heterozygous PTEN loss using a single-probe assay.
We also report here significant heterogeneity of PTEN loss in primary prostate cancers and to account for this and also sectioning artefacts, we compared FISH probe patterns in nuclei in normal tissue to those in cancers with a range of patterns. We then defined tumours with ≥10% of nuclei showing loss of all PTEN probes as homozygous and tumours with ≥40% of nuclei showing loss of one probe but preservation of the other allele as heterozygous (loss of both probes is less likely to be due to chance than loss of solely one probe, hence the lower proportion of nuclei required to exclude an artefact). The probe over the chromosome 10 centromere also assists in assessing whether a PTEN allele is truly lost as opposed to sectioning or hybridisation artefact. This strict classification may classify cores with a small area of PTEN loss as PTEN normal. Tumours with both homozygous and heterozygous loss were classified as homozygous, recognising the hypothesis that tumour clones with homozygous loss are more aggressive and will outgrow other areas.
PTEN is increasingly undergoing evaluation as a biomarker for identifying poor prognosis patients and selecting patients for targeted therapies, including the plethora of new agents targeting the PI3K AKT pathway23 and DNA repair proteins.24, 25 Moreover, the identification of gross chromosomal rearrangements could also give important insights to tumour biology with reports increasingly suggesting that a subset of advanced solid cancer types are characterised by a propensity to acquire chromosomal rearrangements during their development.1 This is not common to all solid tumours as suggested by our observation that PTEN loss in GBM is very rarely associated with a gross chromosomal rearrangement. In preclinical models, loss of PTEN can cooperate with ERG rearrangements to promote prostate cancer progression26, 27 and in fact several studies have now reported an association between ERG rearrangements and PTEN loss identified by FISH.28, 29 We also report here a similar significant association between PTEN loss with a gross chromosomal rearrangement and an ERG gene rearrangement. The previous observation of PTEN chromosomal rearrangements in breast cancer xenografts with an underlying DNA repair defect5 and emerging evidence implicating DNA repair defects in the development of chromosomal rearrangements in prostate cancer1 suggests that demonstration of chromosomal rearrangements in a subset of tumours could be exploited for patient selection for therapeutic agents targeting DNA repair defects.30 Therefore, although chromosomal rearrangements disrupting PTEN are uncommon and may not occur in the absence of genomic deletion at the other allele, complex assays similar to ours that accurately map the PTEN locus should be considered for future PTEN FISH studies.
References
Berger MF, Lawrence MS, Demichelis F, et al. The genomic complexity of primary human prostate cancer. Nature 2011;470:214–220.
Palanisamy N, Ateeq B, Kalyana-Sundaram S, et al. Rearrangements of the RAF kinase pathway in prostate cancer, gastric cancer and melanoma. Nature Med 2010;16:793–798.
Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005;310:644–648.
Tomlins SA, Mehra R, Rhodes DR, et al. TMPRSS2:ETV4 gene fusions define a third molecular subtype of prostate cancer. Cancer Res 2006;66:3396–3400.
Saal LH, Gruvberger-Saal SK, Persson C, et al. Recurrent gross mutations of the PTEN tumor suppressor gene in breast cancers with deficient DSB repair. Nat Genet 2008;40:102–107.
Whang YE, Wu X, Suzuki H, et al. Inactivation of the tumor suppressor PTEN/MMAC1 in advanced human prostate cancer through loss of expression. Proc Natl Acad Sci USA 1998;95:5246–5250.
Salmena L, Carracedo A, Pandolfi PP . Tenets of PTEN tumor suppression. Cell 2008;133:403–414.
Yoshimoto M, Cunha IW, Coudry RA, et al. FISH analysis of 107 prostate cancers shows that PTEN genomic deletion is associated with poor clinical outcome. Br J Cancer 2007;97:678–685.
Reid AH, Attard G, Ambroisine L, et al. Molecular characterisation of ERG, ETV1 and PTEN gene loci identifies patients at low and high risk of death from prostate cancer. Br J Cancer 2010;102:678–684.
Verhagen PC, van Duijn PW, Hermans KG, et al. The PTEN gene in locally progressive prostate cancer is preferentially inactivated by bi-allelic gene deletion. J Pathol 2006;208:699–707.
Taylor BS, Schultz N, Hieronymus H, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010;18:11–22.
Bubendorf L . High-throughput microarray technologies: from genomics to clinics. Eur Urol 2001;40:231–238.
McShane LM, Altman DG, Sauerbrei W, et al. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer 2005;93:387–391.
Lambros MB, Simpson PT, Jones C, et al. Unlocking pathology archives for molecular genetic studies: a reliable method to generate probes for chromogenic and fluorescent in situ hybridization. Lab Invest 2006;86:398–408.
Attard G, Clark J, Ambroisine L, et al. Duplication of the fusion of TMPRSS2 to ERG sequences identifies fatal human prostate cancer. Oncogene 2008;27:253–263.
Attard G, Clark J, Ambroisine L, et al. Heterogeneity and clinical significance of ETV1 translocations in human prostate cancer. Br J Cancer 2008;99:314–320.
Clark J, Attard G, Jhavar S, et al. Complex patterns of ETS gene alteration arise during cancer development in the human prostate. Oncogene 2008;27:1993–2003.
Attard G, Jameson C, Moreira J, et al. Hormone-sensitive prostate cancer: a case of ETS gene fusion heterogeneity. J Clin Pathol 2009;62:373–376.
Mehra R, Han B, Tomlins SA, et al. Heterogeneity of TMPRSS2 gene rearrangements in multifocal prostate adenocarcinoma: molecular evidence for an independent group of diseases. Cancer Res 2007;67:7991–7995.
Ohgaki H, Kleihues P . Genetic pathways to primary and secondary glioblastoma. Am J Pathol 2007;170:1445–1453.
Sircar K, Yoshimoto M, Monzon FA, et al. PTEN genomic deletion is associated with p-Akt and AR signalling in poorer outcome, hormone refractory prostate cancer. J Pathol 2009;218:505–513.
Hermans KG, van Alewijk DC, Veltman JA, et al. Loss of a small region around the PTEN locus is a major chromosome 10 alteration in prostate cancer xenografts and cell lines. Genes Chromosomes Cancer 2004;39:171–184.
Sarker D, Reid AH, Yap TA, et al. Targeting the PI3K/AKT pathway for the treatment of prostate cancer. Clin Cancer Res 2009;15:4799–4805.
Mendes-Pereira AM, Martin SA, Brough R, et al. Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors. EMBO Mol Med 2009;1:315–322.
Dedes KJ, Wetterskog D, Mendes-Pereira AM, et al. PTEN deficiency in endometrioid endometrial adenocarcinomas predicts sensitivity to PARP inhibitors. Sci Transl Med 2010;2:53ra75.
Carver BS, Tran J, Gopalan A, et al. Aberrant ERG expression cooperates with loss of PTEN to promote cancer progression in the prostate. Nat Genet 2009;41:619–624.
King JC, Xu J, Wongvipat J, et al. Cooperativity of TMPRSS2-ERG with PI3-kinase pathway activation in prostate oncogenesis. Nat Genet 2009;41:524–526.
Han B, Mehra R, Lonigro RJ, et al. Fluorescence in situ hybridization study shows association of PTEN deletion with ERG rearrangement during prostate cancer progression. Mod Pathol 2009;22:1083–1093.
Yoshimoto M, Joshua AM, Cunha IW, et al. Absence of TMPRSS2:ERG fusions and PTEN losses in prostate cancer is associated with a favorable outcome. Mod Pathol 2008;21:1451–1460.
Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 2009;361:123–134.
Yoshimoto M, Cutz JC, Nuin PA, et al. Interphase FISH analysis of PTEN in histologic sections shows genomic deletions in 68% of primary prostate cancer and 23% of high-grade prostatic intra-epithelial neoplasias. Cancer Genet Cytogenet 2006;169:128–137.
Acknowledgements
We acknowledge funding from the following organisations ‘Cancer Research UK’, ‘The Prostate Cancer Foundation‘, ‘The Grand Charity of Freemasons’, ‘The Prostate Cancer Charity’, ‘The Bob Champion Cancer Trust’, and ‘The RoseTrees Trust’ and Alexa Jury and Sergey Popov for constructing and characterising the high-grade glioma series.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Several of the authors are employed by The Institute of Cancer Research that has a commercial interest in the development of PI3K inhibitors.
Rights and permissions
About this article
Cite this article
Reid, A., Attard, G., Brewer, D. et al. Novel, gross chromosomal alterations involving PTEN cooperate with allelic loss in prostate cancer. Mod Pathol 25, 902–910 (2012). https://doi.org/10.1038/modpathol.2011.207
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2011.207
Keywords
This article is cited by
-
Clinical implications of PTEN loss in prostate cancer
Nature Reviews Urology (2018)
-
Biomarkers for the Management of Castration-Resistant Prostate Cancer: We Are Not There Yet
Targeted Oncology (2017)
-
Integrated analysis of the genomic instability of PTEN in clinically insignificant and significant prostate cancer
Modern Pathology (2016)
-
Image-based computational quantification and visualization of genetic alterations and tumour heterogeneity
Scientific Reports (2016)
-
PTEN loss in circulating tumour cells correlates with PTEN loss in fresh tumour tissue from castration-resistant prostate cancer patients
British Journal of Cancer (2015)