Abstract
Objective:
Neonatal abstinence syndrome (NAS), a postnatal opioid withdrawal syndrome, increased threefold from 2000 to 2009. Since 2009, opioid pain reliever prescriptions and complications increased markedly throughout the United States. Understanding recent changes in NAS and its geographic variability would inform state and local governments in targeting public health responses.
Study design:
We utilized diagnostic and demographic data for hospital discharges from 2009 to 2012 from the Kids’ Inpatient Database and the Nationwide Inpatient Sample. NAS-associated diagnoses were identified utilizing International Classification of Diseases, Ninth Revision, Clinical Modification codes. All analyses were conducted with nationally weighted data. Expenditure data were adjusted to 2012 US dollars. Between-year differences were determined utilizing least squares regression.
Results:
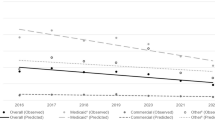
From 2009 to 2012, NAS incidence increased nationally from 3.4 (95% confidence interval (CI): 3.2 to 3.6) to 5.8 (95% CI 5.5 to 6.1) per 1000 hospital births, reaching a total of 21 732 infants with the diagnosis. Aggregate hospital charges for NAS increased from $732 million to $1.5 billion (P<0.001), with 81% attributed to state Medicaid programs in 2012. NAS incidence varied by geographic census division, with the highest incidence rate (per 1000 hospital births) of 16.2 (95% CI 12.4 to 18.9) in the East South Central Division (Kentucky, Tennessee, Mississippi and Alabama) and the lowest in West South Central Division Oklahoma, Texas, Arkansas and Louisiana 2.6 (95% CI 2.3 to 2.9).
Conclusion:
NAS incidence and hospital charges grew substantially during our study period. This costly public health problem merits a public health approach to alleviate harm to women and children. States, particularly, in areas of the country most affected by the syndrome must continue to pursue primary prevention strategies to limit the effects of opioid pain reliever misuse.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
29 July 2015
This article has been corrected since Advance Online Publication and a corrigendum is also printed in this issue.
References
Hudak ML, Tan RC . Neonatal drug withdrawal. Pediatrics 2012; 129 (2): e540–e560.
Kellogg A, Rose CH, Harms RH, Watson WJ . Current trends in narcotic use in pregnancy and neonatal outcomes. Am J Obstet Gynecol 2011; 204 (3): 259 e1–4.
Finnegan LP, Kron RE, Connaughton JF, Emich JP . Assessment and treatment of abstinence in the infant of the drug-dependent mother. Int J Clin Pharmacol Biopharm 1975; 12 (1-2): 19–32.
Patrick SW, Kaplan HC, Passarella M, Davis MM, Lorch SA . Variation in treatment of neonatal abstinence syndrome in US children’s hospitals, 2004-2011. J Perinatol 2014; 34 (11): 867–872.
Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM . Neonatal abstinence syndrome and associated health care expenditures: United States, 2000-2009. JAMA 2012; 307 (18): 1934–1940.
Centers for Disease Control and Prevention (CDC). Vital signs: overdoses of prescription opioid pain relievers — United States, 1999–2008. MMWR Morb Mortal Wkly Rep 2011; 60 (43): 1487–1492.
Epstein RA, Bobo WV, Martin PR, Morrow JA, Wang W, Chandrasekhar R et al. Increasing pregnancy-related use of prescribed opioid analgesics. Ann Epidemiol 2013; 23 (8): 498–503.
Desai RJ, Hernandez-Diaz S, Bateman BT, Huybrechts KF . Increase in prescription opioid use during pregnancy among medicaid-enrolled women. Obstet Gynecol 2014; 123 (5): 997–1002.
Paulozzi LJ, Mack KA, Hockenberry JM . Vital signs: variation among States in prescribing of opioid pain relievers and benzodiazepines – United States, 2012. MMWR Morb Mortal Wkly Rep 2014; 63 (26): 563–568.
Centers for Disease Control and Prevention. Prescription Drug Overdose in the United States: Fact Sheet Atlanta, GA: Centers for Disease Control and Prevention; 2014. Available at http://www.cdc.gov/homeandrecreationalsafety/overdose/facts.html.
HCUP Kids' Inpatient Database. Healthcare Cost and Utilization Project (HCUP) Rockville, MD: Agency for Healthcare Research and Quality; 2000, 2003, 2006, 2009, 2012. Available at http://www.hcup-us.ahrq.gov/kidoverview.jsp.
HCUP Nationwide Inpatient Sample. Healthcare Cost and Utilization Project (HCUP) Rockville, MD: Agency for Healthcare Research and Quality; 2009–2011. Available at http://www.hcup-us.ahrq.gov/nisoverview.jsp.
Patrick SW, Davis MM, Sedman AB, Meddings JA, Hieber S, Lee GM et al. Accuracy of hospital administrative data in reporting central line-associated bloodstream infections in newborns. Pediatrics 2013; 131 (Suppl 1): S75–S80.
Auger KA, Patrick SW, Davis MM . Infant hospitalizations for pertussis before and after Tdap recommendations for adolescents. Pediatrics 2013; 132 (5): e1149–e1155.
Afana M, Brinjikji W, Cloft H, Salka S . Hospitalization costs for acute myocardial infarction patients treated with percutaneous coronary intervention in the United States are substantially higher than medicare payments. Clin Cardiol 2014; 38 (1): 13–19.
Kozhimannil KB, Arcaya MC, Subramanian SV . Maternal clinical diagnoses and hospital variation in the risk of cesarean delivery: analyses of a National US hospital discharge database. PLoS Med 2014; 11 (10): e1001745.
International Classification of Diseases Clinical Modification. American Medical Association, 9th Revision, Chicago, IL, USA, 2012; p 360.
US Bureau of Labor Statistics. Consumer Price Index 2014 [cited 15 July 2014]. Available at http://www.bls.gov/cpi/.
Pisati M . Simple thematic mapping. Stata J 2004; 4: 361–378.
National Oceanic and Atmospheric Administration. US States and Territories Silver Spring, MD2014. Available at http://www.nws.noaa.gov/geodata/catalog/national/html/us_state.htm.
Eapen V, Dadds M, Barnett B, Kohlhoff J, Khan F, Radom N et al. Separation anxiety, attachment and inter-personal representations: disentangling the role of oxytocin in the perinatal period. PLoS One 2014; 9 (9): e107745.
Sarkar S, Donn SM . Management of neonatal abstinence syndrome in neonatal intensive care units: a national survey. J Perinatol 2006; 26 (1): 15–17.
Hall ES, Wexelblatt SL, Crowley M, Grow JL, Jasin LR, Klebanoff MA et al. A multicenter cohort study of treatments and hospital outcomes in neonatal abstinence syndrome. Pediatrics 2014; 134 (2): e527–e534.
Agthe AG, Kim GR, Mathias KB, Hendrix CW, Chavez-Valdez R, Jansson L et al. Clonidine as an adjunct therapy to opioids for neonatal abstinence syndrome: a randomized, controlled trial. Pediatrics 2009; 123 (5): e849–e856.
Jansson LM . ABM clinical protocol #21: guidelines for breastfeeding and the drug-dependent woman. Breastfeed Med 2009; 4 (4): 225–228.
Wachman EM, Hayes MJ, Brown MS, Paul J, Harvey-Wilkes K, Terrin N et al. Association of OPRM1 and COMT single-nucleotide polymorphisms with hospital length of stay and treatment of neonatal abstinence syndrome. JAMA 2013; 309 (17): 1821–1827.
Jansson LM, Velez M, Harrow C . Methadone maintenance and lactation: a review of the literature and current management guidelines. J Hum Lact 2004; 20 (1): 62–71.
Abrahams RR, Kelly SA, Payne S, Thiessen PN, Mackintosh J, Janssen PA . Rooming-in compared with standard care for newborns of mothers using methadone or heroin. Can Fam Physician 2007; 53 (10): 1722–1730.
Hunseler C, Bruckle M, Roth B, Kribs A . Neonatal opiate withdrawal and rooming-in: a retrospective analysis of a single center experience. Klin Padiatr 2013; 225 (5): 247–251.
Saiki T, Lee S, Hannam S, Greenough A . Neonatal abstinence syndrome–postnatal ward versus neonatal unit management. Eur J Pediatr 2010; 169 (1): 95–98.
ACOG Committee on Health Care for Underserved Women, American Society of Addiction Medicine. ACOG Committee Opinion No. 524: Opioid abuse, dependence, and addiction in pregnancy. Obstetrics and Gynecology (New York 1953) 2012; 119 (5): 1070–1076.
Fullerton CA, Kim M, Thomas CP, Lyman DR, Montejano LB, Dougherty RH et al. Medication-assisted treatment with methadone: assessing the evidence. Psychiatr Serv 2014; 65 (2): 146–157.
TennCare. Neonatal Abstinence Syndrome among Tenncare enrollees Provisional 2012 data Nashville, TN2013 [cited 27 October 2014]. Available at http://www.tn.gov/tenncare/forms/TennCareNASData2012.pdf.
PDMP Assist. PDMP program status 2014 [cited 18 June 2014]. Available at http://www.pdmpassist.org/pdf/PDMPProgramStatus2014.pdf.
Peirce GL, Smith MJ, Abate MA, Halverson J . Doctor and pharmacy shopping for controlled substances. Med Care 2012; 50 (6): 494–500.
Burns L, Mattick RP . Using population data to examine the prevalence and correlates of neonatal abstinence syndrome. Drug Alcohol Rev 2007; 26 (5): 487–492.
Acknowledgements
The authors acknowledge Kelly Patrick for her contributions to this manuscript. This study was supported by CTSA award KL2TR000446 from the National Center for Advancing Translational Sciences and by the National Institute on Drug Abuse through the award 1K23DA038720-01 (Dr Patrick).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Disclaimer
The sponsor had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript or the decision to submit.
Rights and permissions
About this article
Cite this article
Patrick, S., Davis, M., Lehmann, C. et al. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J Perinatol 35, 650–655 (2015). https://doi.org/10.1038/jp.2015.36
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2015.36
This article is cited by
-
Pragmatic, randomized, blinded trial to shorten pharmacologic treatment of newborns with neonatal opioid withdrawal syndrome (NOWS)
Trials (2023)
-
Characteristics and outcomes of neonatal opioid withdrawal syndrome in preterm infants: a retrospective cohort study in the current era
Journal of Perinatology (2023)
-
Association between pharmacologic treatment and hospital utilization at birth among neonatal opioid withdrawal syndrome mother-infant dyads
Journal of Perinatology (2023)
-
Evaluation of Administrative Data for Identifying Maternal Opioid Use at Delivery in Florida
Maternal and Child Health Journal (2023)
-
Morphine versus methadone for neonatal opioid withdrawal syndrome: a randomized controlled pilot study
BMC Pediatrics (2022)