Abstract
Hypertension is a frequent and modifiable cardiovascular risk factor with a cyclic relationship with chronic kidney disease (CKD). The diagnosis, treatment, monitoring and control of high blood pressure are all mandatory not only in CKD but also in end-stage renal disease (ESRD). As demonstrated by studies using population and hypertensive patients, white-coat hypertension (WCHT) and masked hypertension (MHT) carry a particular degree of risk. The advantages of ambulatory techniques in the management and prognostic stratification of patients with CKD and ESRD have also been recognized. However, most of the evidence underlines the importance of nocturnal hypertension and neglects WCHT and MHT. The absence of specific reports involving untreated and treated patients hinders the ability to significantly discriminate WCHT from the white-coat effect and MHT from masked uncontrolled hypertension. The heterogeneous definitions that are used add additional difficulty in translating experimental evidence into clinical practice. Reaching a consensus in definitions is mandatory for designing future research. Cross-sectional studies underscore the frequency of misdiagnosis, potentially leading to undertreatment (MHT) and overtreatment (WCHT) in renal disease. The divergent prevalence of WCHT and MHT reported in CKD could be related to the diverse definitions of hypertension and the heterogeneity of the pathologies pooled under the CKD definition. Even in the absence of randomized clinical trials specifically addressing this issue, the scarce longitudinal studies confirm that WCHT carries a risk close to that of sustained normotension, whereas MHT is associated with a risk close or identical to that of sustained hypertension.
Similar content being viewed by others
Introduction
Hypertension is present from the very early stages of chronic kidney disease (CKD), and it is also a major problem in patients reaching end-stage renal disease (ESRD).1, 2, 3, 4, 5 The prevalence, severity and ability to control blood pressure (BP) worsen with the progression of renal disease. Hypertension and CKD are highly prevalent, reaching 20–40% and 10–15% of the general population, respectively,6, 7 and these percentages are projected to increase in the near future.6, 8 Both disorders behave as aggregate risk factors:9 on the one hand, sustained hypertension causes renal impairment and the progression of CKD and, on the other hand, renal disease interferes with BP, from impairing control in the preclinical stages10 to the extreme of being considered a cause of secondary hypertension in established CKD.11 This close relationship is supported by higher rates of hypertension among CKD patients independent of race, sex and age,12, 13, 14, 15, 16 and by a progressive increase in the incidence and prevalence of hypertension from early to advanced stages of CKD.17, 18, 19 However, race, sex and age disparities in the prevalence and rate of BP control have also been described.20
Cardiovascular (CV) disease is one of the main causes of death and nonfatal complications in patients with CKD and ESRD, and these patients have higher rates of CV complications than the general population, mainly because of the major contributions of hypertension.5, 17, 18, 21, 22 The current charts for the stratification of total CV risk are based on BP and also include CKD as a synergistic condition with an inverse relationship between the estimated glomerular filtration rate (eGFR) and CV risk.11 Systolic BP (24-h ambulatory BP) accounts for a major proportion of the explained CV risk in comparison with eGFR (CKD-EPI).3 Furthermore, BP reduction in CKD, independent of drug class, is an effective strategy in preventing the occurrence of cardiovascular events23 and the progression to ESRD.5 For these reasons, the diagnosis, treatment, monitoring and control of high BP are mandatory in CKD.1
The diagnosis and control of hypertension are critically dependent on accurate BP measurements.24 In the 1960s, Sokolow et al.25 observed that ‘a substantial proportion of cases in which casual (office) BPs were considerably elevated, yet hypertensive complications did not develop, as well as cases in which complications occurred although arterial pressures were only moderately increased’ and raised this question: ‘to what extent are the discrepancies between casual BP and clinical course due to the possibility that casual pressures fail to represent the usual BP of the patient?’25 From this pioneering research25, 26 to the present,27, 28, 29, 30, 31, 32 the superiority of out-of-office techniques for BP measurement (ambulatory BP monitoring (ABPM) and self/home BP measurement) over conventional (office) BP measurement has been largely documented and reviewed elsewhere.33, 34 Ambulatory techniques not only result in better diagnostic accuracy but also in more precise prognostic prediction. In the general population, the ABPM thresholds for the diagnosis of hypertension were initially derived from different approaches in cross-sectional studies using the office BP thresholds for reference.35, 36, 37, 38, 39 Ohasama researchers40 and, more recently, IDACO investigators41 provided ABPM thresholds based on a prognostic criterion and the 10-year equivalent risk, respectively. Thus, ABPM is an accurate technique with well-defined thresholds and better performance for risk stratification than office BP. In most cases, both techniques are concordant in identifying normotensives and hypertensives. However, the categories of discrepancy emerging from the cross-classification of ambulatory and office BP measurements also carries a particular degree of risk, as documented in cross-sectional and longitudinal studies.42, 43, 44 White-coat hypertension (WCHT) is defined as an elevated office BP with normal ambulatory BP and should be differentiated from the white-coat effect.45 Masked hypertension (MHT) is defined as normal BP in the office with elevated ambulatory measurement.46 IDACO investigators, based on long-term follow-up of four cohorts and more than 7000 people, recently showed that the risks conferred by WCHT and MHT were intermediate compared with those associated with normotension and sustained hypertension.30 However, the discrepant categories of the cross-classification (WCHT and MHT) are asymmetrical in terms of their associated risks. MHT carries a risk equivalent to sustained hypertension, whereas WCHT carries a risk almost identical to normotension.30 Thus, ambulatory techniques can help refine the diagnosis and risk stratification in the general population as well as in studies of hypertensive patients.47 We will address whether we have evidence that supports the hypothesis that ambulatory techniques improve BP measurement in the diagnosis and risk stratification of patients with chronic renal disease. We will focus on WCHT and MHT.
Renal disease is a heterogeneous condition from both a pathogenic point of view and a clinical perspective. We will differentiate two conditions of major clinical relevance: the progression through CKD stages and patients with ESRD under renal replacement therapies. We will analyze the usefulness of a diagnosis of WCHT or MHT in these two clinical conditions. Because of the high prevalence of hypertension in CKD and ESRD, most of the studies include a large proportion of patients under antihypertensive treatment (Table 1). In those cases, the terms white-coat effect and masked uncontrolled hypertension would be more appropriate. However, the published data do not discriminate the results by treatment status, generating another limitation in the interpretation of the evidence. For that reason, in this review, we will use the terms WCHT and MHT to represent either untreated or treated patients, but the reader should be familiar with the difference.
WCHT and MHT in CKD
The diagnostic and prognostic superiority of ABPM over office BP measurement in CKD patients has also been documented in cross-sectional and longitudinal studies14, 15, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60 and was also confirmed by a meta-analysis.61 Most of the studies address changes in the circadian pattern of BP, with the analysis centering on nocturnal hypertension or nighttime BP dipping.48, 49, 56, 57, 58, 59, 60 However, some studies also report the prevalence and prognostic relevance of the categories that emerge when office and ambulatory BP measurements are used in combination (Table 1).14, 15, 16, 54, 55, 62, 63, 64, 65, 66, 67, 68, 69 A recent analysis of the Chronic Kidney Disease-Japan Cohort Study (CKD-JCS),70 which includes almost 3000 CKD Asian patients,69 showed that, based solely on office BP, 31.6% of all participants were diagnosed as having hypertension. However, based on 24-h ambulatory BP, the proportion of hypertensive patients rose to 56.9%, with 30.9% of patients having MHT and 5.6% having WCHT (Figure 1). Using a multiple regression approach, the authors analyzed the clinical factors associated with the differences between office and ambulatory BP measurements. Age, diabetes, antihypertensive treatment and lower eGFR (MDRD-4 adjusted for Japanese), but not proteinuria, all significantly contributed to the observed variance between office and ambulatory measurements in the multiple regression analysis.
Two-dimensional scattered plot of office systolic BP and 24-h ambulatory systolic BP in 2977 Japanese patients with chronic kidney disease. The cutoff levels for the diagnosis of hypertension were 140/90 mm Hg for office BP and 130/80 mm Hg for 24-h ambulatory BP (from Iimuro et al.69 with permission). BP, blood pressure; MHT, masked hypertension; SHT, sustained hypertension; SNT, sustained normotension; WCHT, white-coat hypertension.
The prevalence of the concordant categories (sustained normotension (SNT) and sustained hypertension (SHT)), as well as the discrepant categories (WCHT and MHT), is highly variable among studies (Table 1). There is a multitude of potential factors that may contribute to the discrepancies in the prevalence between the categories of the cross-classification in CKD. First, different ABPM parameters were used in the definitions of WCHT and MHT. Even when the classical descriptions of WCHT and MHT were based on daytime BP, in many published studies, it was replaced by either 24-h BP15, 54, 55, 62, 69 or nighttime BP16, 67, 68 (Table 1). Other studies used self/home blood pressure measurement as the out-of-office technique.64, 65 Second, variable and arbitrary cutoffs were used to define hypertension in CKD patients during office and ambulatory measurements. Although most population-based studies used 140/90 mm Hg as the systolic/diastolic cutoff for office BP, most of the studies based on CKD patients used 130/80 mm Hg. This disparity in office BP thresholds between studies results partly from the absence of a consensus on the arbitrary limit to define high BP in CKD patients. Recently, the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines1 established 130/80 mm Hg (systolic and diastolic BP, respectively) as the limit at which antihypertensive drug treatment should be initiated, independent of the presence or absence of proteinuria. Japanese guidelines suggest an even lower cutoff in cases of significant proteinuria.71 However, the National (British) Institute for Health and Clinical Excellence (NICE) guidelines72 recommend a 140/90 mm Hg threshold for CKD in the absence of proteinuria and a 130/80 mm Hg cutoff for proteinuric CKD. Furthermore, the recent joint guidelines of the European Society of Hypertension and European Society of Cardiology (ESH-ESC)11 recommend 140/90 mm Hg as the threshold for CKD patients, independent of proteinuria. The prevalence of MHT in studies that used a stricter definition (clinic <130 mm Hg, ambulatory ⩾130 mm Hg) was lower (5.3%, confidence interval (CI) 3.4–7.2%) compared with studies that used a higher cutoff for office BP measurements (19.8%, CI 16.1–23.6%).61 The opposite was true for WCHT.61 In several published studies, there are also disparities in the thresholds for ABPM parameters even for the 24 h, daytime or nighttime values. Third, the ethnicity, age, associated comorbid conditions and other baseline characteristics of the study participants could be additional sources of variation.73 Fourth, CKD, as defined by eGFR and proteinuria,74 can result from a wide spectrum of pathologic conditions. Some of these conditions are limited to the kidney, whereas others are manifest from systemic diseases (for example, diabetic nephropathy); still others cause severe nephritis that demands the use of steroids, calcineurin inhibitors and other drugs (for example, systemic lupus erythematosus) that could potentially interfere with BP control. Only one study was limited to a well-defined renal disease (IgA nephropathy),62 and all of the remaining studies included variable causes of renal disease. Thus, the heterogeneity of renal disease could be a source of variation that significantly explains the differences between the reported prevalences of WCHT and MHT in CKD. Finally, the design of the study (population vs. patients, hypertensives or CKD) and the proportion of subjects under treatment could be an additional source of variability. A recent publication from the Spanish ABPM Registry15 found 5693 (39.5%) patients with the full definition of CKD74 among a total of 14 382 patients. Among the CKD patients, 3893 (68.4%) patients were in stage 3, and 5152 (90.5%) were in stages 1 to 3. However, some studies either excluded patients with an even moderate decrease in renal function (creatinine >1.5 mg dl−1 equivalent to eGFR (CKD-EPI) ∼40 ml min−1 per 1.73 m2 for 50-year-old men) or did not report specific data on this population.
Because of these limitations, the prevalence of WCHT in CKD ranges from 2.3 to 31.7%,14, 64 and the prevalence of MHT in CKD varies from 5.9 to 42.9%.14, 64
Prognostic information associated with WCHT and MHT in CKD patients is scarce. Most reports, even longitudinal studies,14, 66, 69 are based on baseline (cross-sectional) data.
There are very few studies that report data on the prognosis of WCHT and MHT in CKD. Kanno et al.66 evaluated the CKD risk associated with WCHT and MHT as determined by 24-h ABPM in 1023 residents in the general population of Ohasama, Japan. Subjects were categorized using 140/85 mm Hg daytime ABPM and 140/90 mm Hg office BP as thresholds. The odds ratios (ORs) for the prevalence of CKD were calculated using a multiple logistic regression model. Compared with normal BP, the risk of CKD was significantly higher in sustained hypertension (OR, 2.81; 95% CI 1.66–4.75; P=0.0001), MHT (OR, 2.29; 95% CI, 1.45–3.63; P=0.0004) and WCHT (OR, 1.67; 95% CI, 1.03–2.71; P=0.0368). They concluded that WCHT and MHT were significantly associated with CKD. However, data on the prognostic value of these categories were not analyzed. Pogue et al.14 also examined the cross-classification data of a prospective cohort from the AASK study (AASK-CS). MHT was defined by elevated daytime (⩾135/85 mm Hg) or elevated nighttime (⩾120/70 mm Hg) ABPM in those with controlled clinic BP (<140/90 mm Hg). They reported a very high prevalence of MHT. Compared with subjects with controlled clinic BP or WCHT, target organ damage (proteinuria and left ventricular hypertrophy) was more common in subjects with MHT or sustained hypertension when assessed cross-sectionally. They speculated that MHT may account for the disappointing results in the AASK study where, despite excellent in-office BP control, there was still a progression of CKD.
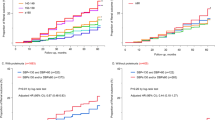
Agarwal et al.51, 52, 53, 56, 75 addressed the issue of the prognostic importance of ABPM in CKD from many perspectives. However, the results of the prognostic relevance of WCHT and MHT are elusive. In a cohort of 217 elderly (67.4±10.9) men (96.3%) with CKD, patients with well-controlled clinic systolic BP measurement did not evolve to ESRD. However, among the patients with poor systolic BP control by clinic measurement, 3/51 (6%) patients with good ambulatory systolic BP (WCHT) reached ESRD, whereas 31/95 (33%) with poor 24-h ambulatory systolic BP (SHT) also reached ESRD (Figure 2).51 This study showed a significant increase in the cumulative risk for progressing to ESRD in patients with sustained hypertension in comparison with patients with WCHT.51 The hazard ratios (HRs) associated with an increase of 1 s.d. in systolic BP with respect to the combined end point ESRD and death or ESRD alone showed the superiority of ambulatory (s.d.: 16.3 mm Hg; HR: 1.88 and 3.04, respectively) vs. office BP (s.d.: 25.6 mm Hg; HR: 1.6 and 2.75, respectively). However, no specific HRs were reported for the categories of the cross-classification.51 From another perspective, it has been widely demonstrated that the risk of cardiovascular complications and death increase proportionally with ambulatory blood pressure. In a recent publication16 of a study using more than 1317 CKD patients, Cha et al.16 demonstrated that 24-h ambulatory BP progressively increases with the categories of SNT to WCHT to MHT to SHT. Thus, WCHT and particularly MHT in CKD patients seem to attain an increased risk; however, accurate prognostic significance is elusive and should be analyzed using standardized definitions in future studies.
Cumulative risk of end-stage renal disease (ESRD) in patients with chronic kidney disease and elevated systolic clinic blood pressure (BP). None of the patients who had well-controlled standardized clinic BP had ESRD in this study. Those patients with poorly controlled standardized clinic BP (⩾130 mm Hg) but well-controlled awake ambulatory BP (<130 mm Hg)—white-coat hypertension (WCHT)—had fewer ESRD events (lower line) compared with those with poorly controlled standardized clinic BP and poorly controlled ambulatory BP (upper line; P<0.05 by log-rank test; from Agarwal and Andersen51 with permission). SHT, sustained hypertension.
WCHT and MHT in ESRD
Hypertension is the second most frequent (incident and prevalent) cause of renal disease that requires renal replacement therapy in the United States and Europe, accounting for almost one-third of new cases.22, 76 Among the total number of patients under renal replacement therapy in 2011 (excluding renal transplantation), ∼92% of patients in the United States and ∼83% of patients in Europe were under hemodialysis therapy.22, 76
It is noteworthy that 60 to 90% of maintenance hemodialysis patients have hypertension.22, 77, 78 Despite the use of multiple medications, high BP in these patients is often poorly controlled.22, 77 There is an elevated prevalence of hypertension in both hemodialysis and peritoneal dialysis; however, it seems more difficult to achieve high BP control in the former.79 In the patients under hemodialysis, BP is dependent on the volume of the extracellular fluid.80, 81 Other factors such as dialysis dose, residual diuresis, increased activity of the sympathetic system and the renin–angiotensin–aldosterone system, the degree of systemic inflammation and arterial stiffness could also play a role.79, 82, 83, 84 Hemodialysis is an intermittent technique with fluctuations (48- to 72-h cycles) in the volume of the extracellular fluid and other parameters.80 One should expect that, in most cases, BP follows these fluctuations, with a progressive increase from postdialysis to the next predialysis measurement. However, different patterns of BP have been described during the interdialytic interval and during the intradialysis procedure even during the same week.85, 86 Thus, BP has a great variability in patients under hemodialytic therapy. In this context, it is unclear which casual BP measurement is the most representative in terms of diagnosis, control and prognosis in patients under hemodialysis. Predialytic,78 postdialytic,78 average or median intradialytic87, 88 BP and the change between pre- and post-dialysis BP have all been considered as potential moments to standardize BP measurements, but no consensus was reached.87 KDOQI guidelines suggest a predialysis BP goal of <140/90 mm Hg and a postdialysis BP goal of <130/80 mm Hg of systolic and diastolic BP, respectively.78
Because ambulatory techniques cover a long period (24-h ABPM) or even the whole interdialytic period (44-h ABPM), it is reasonable to expect a more accurate estimation of the real BP in comparison with the peridialytic casual measurements.89 Compared with peridialytic recordings, interdialytic BP measurements are not only more powerful determinants of target organ damage90, 91 but also stronger predictors of all-cause mortality.92, 93, 94 Thus, an accurate diagnosis and control of hypertension in hemodialysis patients should assess interdialytic ambulatory BP recordings.80
Moreover, the evaluations of the circadian rhythm of BP in hemodialysis patients demonstrate not only a higher prevalence of nocturnal hypertension but also a major prognostic ability of this condition to predict cardiovascular and all-cause mortality.95, 96 However, similar to what we described in CKD, there are few studies that evaluate MHT and WCHT in patients under hemodialysis. Again, the absence of a consensus in definition is closely related to the scarce and heterogeneous reports.97
A recent French study evaluated the prevalence of WCHT and MHT in home BP self-measurement and conventional predialytic measurement of BP in a cohort of hemodialysis patients in two hospitals.98 BP was recorded using the two methods for 1 week. From the 60 patients who were evaluated, 23 (38%) had sustained uncontrolled hypertension, 13 (22%) had MHT, 8 (13%) had WCHT and 16 (27%) had sustained controlled normotension. Based on data from the general population showing that MHT carries a risk of CV events equivalent to sustained hypertension, the authors concluded that the proportion of patients with MHT should alert clinicians because of the poor cardiovascular prognosis associated with MHT.
However, specific prognostic data for WCHT and MHT in hemodialysis patients are elusive and even more so for peritoneal dialysis. Agarwal et al. studied 355 middle-aged (mean 55 years old) patients, mostly black with long-term hemodialysis.97 Using a threshold of 140/80 mm Hg for median midweek dialysis-unit BP and 135/85 mm Hg for 44-h ambulatory BP, the authors defined four categories of BP: SNT, WCHT, MHT and SHT. The prevalence of SNT was 35%, WCHT 15%, MHT 15% and SHT 35%. The rate of death was higher in MHT and SHT in comparison with SNT and WCHT (Figure 3). Unadjusted and multivariate-adjusted analyses showed that the all-cause mortality increases proportionally with the severity of hypertension. Unadjusted HRs from SNT, WCHT, MHT and SHT were 1.00, 1.12, 1.70 and 1.80, respectively (P for trend ⩽0.01). Adjusted HRs were confirmatory: 1.00, 1.30, 1.36 and 1.87, respectively (P for trend ⩽0.02). When a predialysis BP threshold of 140/90 mm Hg was used to classify patients into BP categories, the prevalence of SNT was 24%, WCHT 26%, MHT 4% and SHT 47%, and the HRs for mortality were similar when compared with the median midweek dialysis-unit BP. In agreement with the evidence in the general population,30 a significant relationship between increasing levels of hypertension from SNT to WCHT to MHT to SHT and all-cause mortality have been observed. Even though we will most likely need future confirmatory studies, present evidence97 points to the prognostic significance of MHT and WCHT in patients under hemodialysis.
Kaplan–Meier survival curves for ambulatory systolic blood pressure (BP) and mortality in hemodialysis patients. The numbers below indicate the patients at risk. The mortality of white-coat hypertension (WCHT) was similar to that observed for sustained normotension (SNT). However, the mortality of masked hypertension (MHT) was similar to that of sustained hypertension (SHT). The equality of the survival curves between WCHT and SNT on one hand and MHTN and SHT on the other was tested using the log rank test and found to be significant (P<0.009; from Agarwal et al.97 with permission).
Conclusion
There is convincing evidence supporting the advantages of using ABPM and self/home blood pressure measurement in CKD and ESRD. The prevalence of WCHT (including the white-coat effect) and MHT (including masked uncontrolled hypertension) in CKD and ESRD is higher than in the general population and in studies of hypertensive patients. Cross-sectional and longitudinal studies emphasize that the degree of risk associated with WCHT and MHT in these patients mirrors that of studies involving the general population and hypertensive cohorts. The risk attributed to WCHT is close to that of SNT and the risk of MHT is as high as that of SHT. However, some uncertainty remains regarding the significance of WCHT and MHT in CKD and ESRD that is because of ambiguous definitions and the absence of specific reports on treatment status. Reaching a consensus in the definitions of hypertension in CKD and ESRD and reporting the treatment status are mandatory for designing future research. In the interim, including patients with renal disease in randomized clinical trials and reporting specific results for renal disease by treatment status will help support the present evidence.
References
Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int 2012; 2: 337–414.
Samuels J, Ng D, Flynn JT, Mitsnefes M, Poffenbarger T, Warady BA, Furth S . Ambulatory blood pressure patterns in children with chronic kidney disease. Hypertension 2012; 60: 43–50.
Boggia J, Thijs L, Li Y, Hansen TW, Kikuya M, Bjorklund-Bodegard K, Ohkubo T, Jeppesen J, Torp-Pedersen C, Dolan E, Kuznetsova T, Stolarz-Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Schwedt E, Sandoya E, Kawecka-Jaszcz K, Filipovsky J, Imai Y, Wang J, Ibsen H, O'Brien E, Staessen JA . Risk stratification by 24-hour ambulatory blood pressure and estimated glomerular filtration rate in 5322 subjects from 11 populations. Hypertension 2013; 61: 18–26.
Eckardt KU, Coresh J, Devuyst O, Johnson RJ, Kottgen A, Levey AS, Levin A . Evolving importance of kidney disease: from subspecialty to global health burden. Lancet 2013; 382: 158–169.
Robinson BM, Tong L, Zhang J, Wolfe RA, Goodkin DA, Greenwood RN, Kerr PG, Morgenstern H, Li Y, Pisoni RL, Saran R, Tentori F, Akizawa T, Fukuhara S, Port FK . Blood pressure levels and mortality risk among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int 2012; 82: 570–580.
Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J . Global burden of hypertension: analysis of worldwide data. Lancet 2005; 365: 217–223.
Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin AA, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De LD, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA III, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De Leon FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA . Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2095–2128.
Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW . Chronic kidney disease: global dimension and perspectives. Lancet 2013; 382: 260–272.
Barri YM . Hypertension and kidney disease: a deadly connection. Curr Hypertens Rep 2008; 10: 39–45.
Johnson RJ, Herrera-Acosta J, Schreiner GF, Rodriguez-Iturbe B . Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. New Engl J Med 2002; 346: 913–923.
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De BG, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F . 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31: 1281–1357.
Muntner P, Anderson A, Charleston J, Chen Z, Ford V, Makos G, O'Connor A, Perumal K, Rahman M, Steigerwalt S, Teal V, Townsend R, Weir M, Wright JT Jr . Hypertension awareness, treatment, and control in adults with CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 2010; 55: 441–451.
Assadi F . The growing epidemic of hypertension among children and adolescents: a challenging road ahead. Pediatr Cardiol 2012; 33: 1013–1020.
Pogue V, Rahman M, Lipkowitz M, Toto R, Miller E, Faulkner M, Rostand S, Hiremath L, Sika M, Kendrick C, Hu B, Greene T, Appel L, Phillips RA . Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension 2009; 53: 20–27.
Gorostidi M, Sarafidis PA, de la Sierra A, Segura J, de la Cruz JJ, Banegas JR, Ruilope LM . Differences between office and 24-hour blood pressure control in hypertensive patients with CKD: a 5693-patient cross-sectional analysis from Spain. Am J Kidney Dis 2013; 62: 285–294.
Cha RH, Kim S, Ae YS, Ryu DR, Eun OJ, Han SY, Young LE, Ki KD, Kim YS . Association between blood pressure and target organ damage in patients with chronic kidney disease and hypertension: results of the APrODiTe study. Hypertens Res 2013; 37: 172–178.
Annual Report of the United States Renal Data System (USRDS) 2013 Atlas of Chronic Kidney Disease–Volume 1. In: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. http://www.usrds.org/atlas.aspx Visited: 2013..
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY . Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. New Engl J Med 2004; 351: 1296–1305.
Buckalew VM Jr, Berg RL, Wang SR, Porush JG, Rauch S, Schulman G . Prevalence of hypertension in 1,795 subjects with chronic renal disease: the modification of diet in renal disease study baseline cohort. Modification of Diet in Renal Disease Study Group. Am J Kidney Dis 1996; 28: 811–821.
Duru OK, Li S, Jurkovitz C, Bakris G, Brown W, Chen SC, Collins A, Klag M, McCullough PA, McGill J, Narva A, Pergola P, Singh A, Norris K . Race and sex differences in hypertension control in CKD: results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 2008; 51: 192–198.
Di Angelantonio E, Chowdhury R, Sarwar N, Aspelund T, Danesh J, Gudnason V . Chronic kidney disease and risk of major cardiovascular disease and non-vascular mortality: prospective population based cohort study. Br Med J 2010; 341: c4986.
Annual Report of the United States Renal Data System (USRDS) 2013 Atlas of End-Stage Renal Disease–Volume 2. In: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. http://www.usrds.org/atlas.aspx.
Ninomiya T, Perkovic V, Turnbull F, Neal B, Barzi F, Cass A, Baigent C, Chalmers J, Li N, Woodward M, MacMahon S . Blood pressure lowering and major cardiovascular events in people with and without chronic kidney disease: meta-analysis of randomised controlled trials. Br Med J 2013; 347: f5680.
Pickering TG . Principles and techniques of blood pressure measurement. Cardiol Clin 2002; 20: 207–223.
Sokolow M, Werdegar D, Kain HK, Hinman AT . Relationship between level of blood pressure measured casually and by portable recorders and severity of complications in essential hypertension. Circulation 1966; 34: 279–298.
Kain HK, Hinman AT, Sokolow M . Arterial blood pressure measurements with a portable recorder in hypertensive patients. I. Variability and correlation with ‘casual’ pressures. Circulation 1964; 30: 882–892.
Perloff D, Sokolow M, Cowan R . The prognostic value of ambulatory blood pressures. JAMA 1983; 249: 2792–2798.
Perloff D, Sokolow M, Cowan RM, Juster RP . Prognostic value of ambulatory blood pressure measurements: further analyses. J Hypertens 1989; 7 (Suppl 3): S3–S10.
Imai Y, Ohkubo T, Tsuji I, Nagai K, Satoh H, Hisamichi S, Abe K . Prognostic value of ambulatory and home blood pressure measurements in comparison to screening blood pressure measurements: a pilot study in Ohasama. Blood Press Monit 1996; 1 (Suppl 2): S51–S58.
Hansen TW, Kikuya M, Thijs L, Björklund-Bodegård K, Kuznetsova T, Ohkubo T, Richart T, Torp-Pedersen C, Lind L, Jeppesen J, Ibsen H, Imai Y, Staessen JA, on behalf of the IDACO Investigators. Prognostic superiority of daytime ambulatory over conventional blood pressure in four populations: a meta-analysis of 7030 individuals. J Hypertens 2007; 25: 1554–1564.
Staessen JA, Thijs L, Fagard R, O'Brien ET, Clement D, de Leeuw PW, Mancia G, Nachev C, Palatini P, Parati G, Tuomilehto J, Webster J, for the Systolic Hypertension in Europe Trial Investigators. Predicting cardiovascular risk using conventional vs. ambulatory blood pressure in older patients with systolic hypertension. JAMA 1999; 282: 539–546.
Clement DL, De Buyzere ML, De Bacquer DA, de Leeuw PW, Duprez DA, Fagard RH, Gheeraert PJ, Missault LH, Braun JJ, Six RO, Van der Niepen P, O'Brien E, for the Office versus Ambulatory Pressure Study investigators. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. New Engl J Med 2003; 348: 2407–2415.
Pickering TG, Shimbo D, Haas D . Ambulatory blood-pressure monitoring. New Engl J Med 2006; 354: 2368–2374.
O'Brien E . Twenty-four-hour ambulatory blood pressure measurement in clinical practice and research: a critical reveiw of a technique in need of implementation. J Intern Med 2011; 269: 478–495.
Schettini C, Bianchi M, Nieto F, Sandoya E, Senra H, Hypertension Working Group. Ambulatory blood pressure. Normality and comparison with other measurements. Hypertension Working Group. Hypertension 1999; 34 (Part 2): 818–825.
Staessen J, Fagard R, Lijnen P, Thijs L, Van Hoof R, Amery A . Reference values for ambulatory blood pressure: a meta-analysis. J Hypertens 1990; 8 (Suppl 6): S57–S64.
Staessen J, Bulpitt CJ, Fagard R, Mancia G, O'Brien ET, Thijs L, Vyncke G, Amery A . Reference values for the ambulatory blood pressure and the blood pressure measured at home: a population study. J Hum Hypertens 1991; 5: 355–361.
Staessen J, Bulpitt CJ, Fagard R, Mancia G, O'Brien ET, Thijs L, Vyncke G, Amery A . Reference values for ambulatory blood pressure: a population study. J Hypertens Suppl 1991; 9: S320–S321.
Zhang W, Shi H, Wang R, Yu Y, Wang Z, Zhang L, Wu Z . Reference values for the ambulatory blood pressure: results from a collaborative study. Chin J Cardiol 1995; 10: 325–328.
Ohkubo T, Imai Y, Tsuji I, Nagai K, Ito S, Satoh H, Hisamichi S . Reference values for 24-hour ambulatory blood pressure monitoring based on a prognositic criterion. The Ohasama Study. Hypertension 1998; 32: 255–259.
Kikuya M, Hansen TW, Thijs L, Björklund-Bodegård K, Kuznetsova T, Ohkubo T, Richart T, Torp-Pedersen C, Lind L, Ibsen H, Imai Y, Staessen JA, on behalf of the International Database on ambulatory blood pressure in relation to Cardiovascular Outcome (IDACO) investigators. Diagnostic thresholds for ambulatory blood pressure monitoring based on 10-year cardiovascular risk. Circulation 2007; 115: 2145–2152.
Khattar RS, Senior R, Lahiri A . Cardiovascular outcome in white-coat versus sustained mild hypertension. A 10-year follow-up study. Circulation 1998; 98: 1892–1897.
Gustavsen PH, Hoegholm A, Bang LE, Kristensen KS . White coat hypertension is a cardiovascular risk factor: a 10-year follow-up study. J Hum Hypertens 2003; 17: 811–817.
Ohkubo T, Kikuya K, Metoki H, Asayama K, Obara T, Hashimoto J, Totsune K, Hoshi H, Satoh H, Imai K . Prognosis of ‘masked’ hypertension and ‘white-coat’ hypertension detected by 24-h ambulatory blood pressure monitoring. 10-year follow-up from the Ohasama study. J Am Coll Cardiol 2005; 46: 508–515.
Verdecchia P, Staessen JA, White WB, Imai Y, O'Brien ET . Properly defining white coat hypertension. Eur Heart J 2002; 23: 106–109.
Pickering TG, Davidson K, Gerin W, Schwartz JE . Masked hypertension. Hypertension 2002; 40: 795–796.
O'Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, Clement D, de la Sierra A, de LP, Dolan E, Fagard R, Graves J, Head GA, Imai Y, Kario K, Lurbe E, Mallion JM, Mancia G, Mengden T, Myers M, Ogedegbe G, Ohkubo T, Omboni S, Palatini P, Redon J, Ruilope LM, Shennan A, Staessen JA, vanMontfrans G, Verdecchia P, Waeber B, Wang J, Zanchetti A, Zhang Y . European society of hypertension position paper on ambulatory blood pressure monitoring. J Hypertens 2013; 31: 1731–1768.
Farmer CK, Goldsmith DJ, Quin JD, Dallyn P, Cox J, Kingswood JC, Sharpstone P . Progression of diabetic nephropathy—is diurnal blood pressure rhythm as important as absolute blood pressure level? Nephrol Dial Transplant 1998; 13: 635–639.
Jacob P, Hartung R, Bohlender J, Stein G . Utility of 24-h ambulatory blood pressure measurement in a routine clinical setting of patients with chronic renal disease. J Hum Hypertens 2004; 18: 745–751.
Jacobi J, Rockstroh J, John S, Schreiber M, Schlaich MP, Neumayer HH, Schmieder RE . Prospective analysis of the value of 24-hour ambulatory blood pressure on renal function after kidney transplantation. Transplantation 2000; 70: 819–827.
Agarwal R, Andersen MJ . Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int 2006; 69: 1175–1180.
Agarwal R, Andersen MJ . Blood pressure recordings within and outside the clinic and cardiovascular events in chronic kidney disease. Am J Nephrol 2006; 26: 503–510.
Agarwal R . Hypertension diagnosis and prognosis in chronic kidney disease with out-of-office blood pressure monitoring. Curr Opin Nephrol Hypertens 2006; 15: 309–313.
Gabbai FB, Rahman M, Hu B, Appel LJ, Charleston J, Contreras G, Faulkner ML, Hiremath L, Jamerson KA, Lea JP, Lipkowitz MS, Pogue VA, Rostand SG, Smogorzewski MJ, Wright JT, Greene T, Gassman J, Wang X, Phillips RA . Relationship between ambulatory BP and clinical outcomes in patients with hypertensive CKD. Clin J Am Soc Nephrol 2012; 7: 1770–1776.
Shafi S, Sarac E, Tran H . Ambulatory blood pressure monitoring in patients with chronic kidney disease and resistant hypertension. J Clin Hypertens (Greenwich) 2012; 14: 611–617.
Minutolo R, Agarwal R, Borrelli S, Chiodini P, Bellizzi V, Nappi F, Cianciaruso B, Zamboli P, Conte G, Gabbai FB, De NL . Prognostic role of ambulatory blood pressure measurement in patients with nondialysis chronic kidney disease. Arch Intern Med 2011; 171: 1090–1098.
Timio M, Lolli S, Verdura C, Monarca C, Merante F, Guerrini E . Circadian blood pressure changes in patients with chronic renal insufficiency: a prospective study. Ren Fail 1993; 15: 231–237.
Timio M, Venanzi S, Lolli S, Lippi G, Verdura C, Monarca C, Guerrini E . ‘Non-dipper’ hypertensive patients and progressive renal insufficiency: a 3-year longitudinal study. Clin Nephrol 1995; 43: 382–387.
Mojon A, Ayala DE, Pineiro L, Otero A, Crespo JJ, Moya A, Boveda J, de Lis JP, Fernandez JR, Hermida RC . Comparison of ambulatory blood pressure parameters of hypertensive patients with and without chronic kidney disease. Chronobiol Int 2013; 30: 145–158.
Hermida RC, Smolensky MH, Ayala DE, Fernandez JR, Moya A, Crespo JJ, Mojon A, Rios MT, Fabbian F, Portaluppi F . Abnormalities in chronic kidney disease of ambulatory blood pressure 24 h patterning and normalization by bedtime hypertension chronotherapy. Nephrol Dial Transplant, (e-pub ahead of print 5 September 2013; doi:10.1093/ndt/gft28).
Bangash F, Agarwal R . Masked hypertension and white-coat hypertension in chronic kidney disease: a meta-analysis. Clin J Am Soc Nephrol 2009; 4: 656–664.
Csiky B, Kovacs T, Wagner L, Vass T, Nagy J . Ambulatory blood pressure monitoring and progression in patients with IgA nephropathy. Nephrol Dial Transplant 1999; 14: 86–90.
Agarwal R, Andersen MJ . Prognostic importance of clinic and home blood pressure recordings in patients with chronic kidney disease. Kidney Int 2006; 69: 406–411.
Minutolo R, Borrelli S, Scigliano R, Bellizzi V, Chiodini P, Cianciaruso B, Nappi F, Zamboli P, Conte G, De NL . Prevalence and clinical correlates of white coat hypertension in chronic kidney disease. Nephrol Dial Transplant 2007; 22: 2217–2223.
Terawaki H, Metoki H, Nakayama M, Ohkubo T, Kikuya M, Asayama K, Inoue R, Hoshi H, Ito S, Imai Y . Masked hypertension determined by self-measured blood pressure at home and chronic kidney disease in the Japanese general population: the Ohasama study. Hypertens Res 2008; 31: 2129–2135.
Kanno A, Metoki H, Kikuya M, Terawaki H, Hara A, Hashimoto T, Asayama K, Inoue R, Shishido Y, Nakayama M, Totsune K, Ohkubo T, Imai Y . Usefulness of assessing masked and white-coat hypertension by ambulatory blood pressure monitoring for determining prevalent risk of chronic kidney disease: the Ohasama study. Hypertens Res 2010; 33: 1192–1198.
Hermida RC, Ayala DE, Mojon A, Fernandez JR . Sleep-time blood pressure and the prognostic value of isolated-office and masked hypertension. Am J Hypertens 2012; 25: 297–305.
Rios MT, Dominguez-Sardina M, Ayala DE, Gomara S, Sineiro E, Pousa L, Callejas PA, Fontao MJ, Fernandez JR, Hermida RC . Prevalence and clinical characteristics of isolated-office and true resistant hypertension determined by ambulatory blood pressure monitoring. Chronobiol Int 2013; 30: 207–220.
Iimuro S, Imai E, Watanabe T, Nitta K, Akizawa T, Matsuo S, Makino H, Ohashi Y, Hishida A . Clinical correlates of ambulatory BP monitoring among patients with CKD. Clin J Am Soc Nephrol 2013; 8: 721–730.
Imai E, Matsuo S, Makino H, Watanabe T, Akizawa T, Nitta K, Iimuro S, Ohashi Y, Hishida A . Chronic Kidney Disease Japan Cohort study: baseline characteristics and factors associated with causative diseases and renal function. Clin Exp Nephrol 2010; 14: 558–570.
Ogihara T, Kikuchi K, Matsuoka H, Fujita T, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ito S, Iwao H, Kario K, Kawano Y, Kim-Mitsuyama S, Kimura G, Matsubara H, Matsuura H, Naruse M, Saito I, Shimada K, Shimamoto K, Suzuki H, Takishita S, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Ueshima H, Umemura S, Ishimitsu T, Rakugi H . The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2009). Hypertens Res 2009; 32: 3–107.
National Institute for Health and Care Excellence (2011) Hypertension-Clinical management of primary hypertension in adults. CG127. http://www.nice.org.uk/nicemedia/live/13561/56008/56008.pdf. Accessed 8 December 2013..
Yano Y . Ambulatory blood pressure in chronic kidney disease: do ethnic disparities exist? Hypertens Res 2013; 37: 95–97.
Kidney Disease Improving Global Outcome. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 2013; 3: 1–150.
Agarwal R . Ambulatory blood pressure and cardiovascular events in chronic kidney disease. Semin Nephrol 2007; 27: 538–543.
ERA-EDTA Registry. Annual Report 2011, Academic Medical Center, Department of Medical Informatics: Amsterdam, The Netherlands 2013.
Agarwal R, Nissenson AR, Batlle D, Coyne DW, Trout JR, Warnock DG . Prevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United States. Am J Med 2003; 115: 291–297.
National Kidney Foundation. K/DOQI Clinical Practice Guidelines for Cardiovascular Disease in Dialysis Patients. Guideline 12: Blood Pressure. Am J Kidney Dis 2005; 45: S1–S153.
Bianchi G . Hypertension in chronic renal failure and end-stage renal disease patients treated with haemodialysis or peritoneal dialysis. Nephrol Dial Transplant 2000; 15 (Suppl 5): 105–110.
Agarwal R . Epidemiology of interdialytic ambulatory hypertension and the role of volume excess. Am J Nephrol 2011; 34: 381–390.
Katzarski KS, Charra B, Luik AJ, Nisell J, Divino Filho JC, Leypoldt JK, Leunissen KM, Laurent G, Bergstrom J . Fluid state and blood pressure control in patients treated with long and short haemodialysis. Nephrol Dial Transplant 1999; 14: 369–375.
Rubinger D, Backenroth R, Sapoznikov D . Sympathetic activation and baroreflex function during intradialytic hypertensive episodes. PLoS ONE 2012; 7: e36943.
Horl MP, Horl WH . Hemodialysis-associated hypertension: pathophysiology and therapy. Am J Kidney Dis 2002; 39: 227–244.
Van Buren PN, Inrig JK . Hypertension and hemodialysis: pathophysiology and outcomes in adult and pediatric populations. Pediatr Nephrol 2012; 27: 339–350.
Kuipers J, Usvyat LA, Oosterhuis JK, Dasselaar JJ, de Jong PE, Westerhuis R, Sands JJ, Wang Y, Kotanko P, Franssen CF . Variability of predialytic, intradialytic, and postdialytic blood pressures in the course of a week: a study of Dutch and US maintenance hemodialysis patients. Am J Kidney Dis 2013; 62: 779–788.
Van Buren PN, Toto R, Inrig JK . Interdialytic ambulatory blood pressure in patients with intradialytic hypertension. Curr Opin Nephrol Hypertens 2012; 21: 15–23.
Sinha AD, Agarwal R . Peridialytic, intradialytic, and interdialytic blood pressure measurement in hemodialysis patients. Am J Kidney Dis 2009; 54: 788–791.
Agarwal R, Light RP . Median intradialytic blood pressure can track changes evoked by probing dry-weight. Clin J Am Soc Nephrol 2010; 5: 897–904.
Mitra S, Chandna SM, Farrington K . What is hypertension in chronic haemodialysis? The role of interdialytic blood pressure monitoring. Nephrol Dial Transplant 1999; 14: 2915–2921.
Erturk S, Ertug AE, Ates K, Duman N, Aslan SM, Nergisoglu G, Diker E, Erol C, Karatan O, Erbay B . Relationship of ambulatory blood pressure monitoring data to echocardiographic findings in haemodialysis patients. Nephrol Dial Transplant 1996; 11: 2050–2054.
Agarwal R, Brim NJ, Mahenthiran J, Andersen MJ, Saha C . Out-of-hemodialysis-unit blood pressure is a superior determinant of left ventricular hypertrophy. Hypertension 2006; 47: 62–68.
Alborzi P, Patel N, Agarwal R . Home blood pressures are of greater prognostic value than hemodialysis unit recordings. Clin J Am Soc Nephrol 2007; 2: 1228–1234.
Agarwal R . Blood pressure and mortality among hemodialysis patients. Hypertension 2010; 55: 762–768.
Tripepi G, Fagugli RM, Dattolo P, Parlongo G, Mallamaci F, Buoncristiani U, Zoccali C . Prognostic value of 24-hour ambulatory blood pressure monitoring and of night/day ratio in nondiabetic, cardiovascular events-free hemodialysis patients. Kidney Int 2005; 68: 1294–1302.
Liu M, Takahashi H, Morita Y, Maruyama S, Mizuno M, Yuzawa Y, Watanabe M, Toriyama T, Kawahara H, Matsuo S . Non-dipping is a potent predictor of cardiovascular mortality and is associated with autonomic dysfunction in haemodialysis patients. Nephrol Dial Transplant 2003; 18: 563–569.
Amar J, Vernier I, Rossignol E, Bongard V, Arnaud C, Conte JJ, Salvador M, Chamontin B . Nocturnal blood pressure and 24-hour pulse pressure are potent indicators of mortality in hemodialysis patients. Kidney Int 2000; 57: 2485–2491.
Agarwal R, Sinha AD, Light RP . Toward a definition of masked hypertension and white-coat hypertension among hemodialysis patients. Clin J Am Soc Nephrol 2011; 6: 2003–2008.
Duval-Sabatier A, Brunet P, Seck SM, Haddad F, Giaime P, Ramananarivo P, Bouchouareb D, Berland Y . [Experiment of self blood pressure measurement at home in haemodialysis patients in a hospital unit]. Nephrol Ther 2011; 7: 544–548.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boggia, J., Silvariño, R., Luzardo, L. et al. Significance of white-coat and masked hypertension in chronic kidney disease and end-stage renal disease. Hypertens Res 37, 882–889 (2014). https://doi.org/10.1038/hr.2014.82
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2014.82
Keywords
This article is cited by
-
Blood pressure phenotype reproducibility in CKD outpatients: a clinical practice report
Internal and Emergency Medicine (2020)
-
Masked hypertension and its associated cardiovascular risk in young individuals: the African-PREDICT study
Hypertension Research (2016)