Key Points
- The larynx serves to protect the lower airways, facilitates respiration, and plays a key role in phonation. In humans the protective and respiratory functions are compromised in favor of its phonatory function.
- The protective function is entirely reflexive and involuntary, whereas the respiratory and phonatory functions are initiated voluntarily but regulated involuntarily.
- Sensory nerves to the larynx are derived from the internal branch of the superior laryngeal nerve (iSLN) and the recurrent laryngeal nerves (RLNs); both are branches of the vagus nerve. The iSLN and RLN innervate the mucosa above and below the level of the true vocal cord, respectively.
- Water-aerosol inhalation stimulation in partial upper airway obstruction activates water chemoreceptors on the epiglottis and causes reflex respiratory slowing and increase in tidal volume.
- The RLN innervates all intrinsic laryngeal muscles except the cricothyroid muscle, which is innervated by the external division of the SLN (eSLN).
- Abduction of the vocal cords during respiration is brought about by the posterior cricoarytenoid muscle, whereas their adduction involves all the intrinsic muscles, particularly the thyroarytenoid and cricoarytenoid muscles.
- Reflexive glottic closure is achieved by simultaneous adduction of both vocal cords. Anesthesia and sedation impair reflexive vocal cord closure and predispose to aspiration.
- Reflexive glottic closure is inhibited by hypothermia, inspiratory phase, increased arterial pCO2, decreased arterial pO2, and positive intrathoracic pressure. It is facilitated by hyperthermia, expiratory phase, decreased pCO2, increased pO2, and negative intrathoracic pressure.
- Laryngeal denervation leads to vocal dysfunction and aspiration during swallowing.
- Tracheostomy itself leads to centrally mediated impaired reflexes and decannulation (adductor failure and vocal cord fusion).
- False vocal cords, even if denervated, resist air flow from the lower respiratory tract and serve expectorative function. The true vocal cords resist air flow from outside and play a protective role in respiration, thus explaining the difficulty in overcoming laryngospasm by abrupt pressure peaks from above.
Introduction
The larynx serves three important functions in humans. In order of functional priority, they are protective, respiratory, and phonatory. A sound understanding of these functional priorities appears essential to the management of the myriad diseases besetting this complex organ. This review addresses these three categories of function in terms of phylogeny, morphology, and neuromuscular reflexes, followed by a discussion of clinical events that often threaten to disrupt the anatomy and function of this organ. Original experimental data from the Yale Larynx Laboratory are included here to support physiologic performance, which is important to our understanding of clinical behavior.
Phylogeny and Function
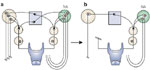
Laryngeal function may be best understood by an appreciation of its origin determined by primitive needs. In this regard, Negus's1 masterful contributions are most illuminating. On an evolutionary scale, as animals migrated from an aquatic to a terrestrial existence, a major change in respiratory requirements became necessary. According to Negus, these accomplishments were reflected in certain contemporary species of fish that developed unique respiratory modifications to allow intermittent sojourns on dry land. Notably, the climbing perch (Anabas scandens) possessed a respiratory diverticulum located above its gills (Figure 1a). The Indian siluroid fish (Saccobranchus) also acquired a long diverticulum leading into an internal air reservoir. These structures, however, contained no valves to prevent the entrance of water when an aquatic existence was resumed.
Figure 1: Structure and function of the larynx viewed phylogenetically (according to Negus)
(Source: Sasaki45 with permission from Lippincott, Williams & Wilkins.)
The most primitive larynx may be found in the bichir lungfish (Polypterus), which inhabits the Nile River. The larynx of this fish consists simply of a muscular sphincter to guard against the entrance of water (Figure 1b). On the other hand, the African lungfish (Protopterus) and Australian lungfish (Neoceratodus) both possess, in addition to sphincteric musculature, discrete muscle fibers that effectively draw the valvular margins apart to produce active dilatation (Figure 1c). The muscular sphincter, therefore, remains contracted when the fish is in the water, but during periods of drought the sphincter is actively opened to allow air to be gulped into the lungs by a swallowing maneuver. This capacity provides obvious advantages of respiratory survival when the supply of water is limited or undependable.
The primitive larynx, therefore, basically functioned as a simple sphincter to protect the lower airway from the intrusion of foreign matter. However, as respiratory requirements in a terrestrial environment grew, the need for adequate ventilation also grew, accompanied by the necessary potential for active dilatation at the primitive sphincter.
To enhance ventilatory flow requirements through the laryngeal aperture, the acquisition of lateral cartilages may be noted in certain amphibians such as the Mexican axolotl (Amblystoma). These lateral cartilages form bars on either side of the glottis to which the dilator muscles insert (Figure 1d). To augment the mechanical advantage of these muscles, a cartilaginous ring (giving origin to the dilator muscles) can be found between the glottis and trachea in other higher vertebrates. Such a configuration is apparent among reptiles such as the alligator (Figure 1e). Therefore, although the lateral cartilages of amphibians share a structural similarity to the arytenoid bodies of humans, the origin of the cartilaginous ring in reptiles can be compared to the cricoid cartilages in other mammals, including humans.
Viewed phylogenetically through Negus's eyes the primary function of the larynx is its use as a sphincter, protecting the lower airway from the intrusion of liquids and food. Its secondary function, supported by the sequential phylogenetic acquisition of the cricoarytenoid complex, centers about its role in respiration governed by active muscular dilatation of the laryngeal aperture. The third function of the larynx, phonation (best observed in mammals), appears to be a late phylogenetic acquisition.
Although the simple primitive larynx of Polypterus possesses the mechanism of airway constriction, thus exhibiting the potential for sound production, phonation using the larynx as a flutter valve is only seen in vertebrates possessing the respiratory requirements of an effective bellows. Such is possible only in the group of vertebrates possessing thoracoabdominal diaphragms, the group we call mammals. Nonetheless, among all mammals, humans alone have acquired the potential for complex sound production using the laryngeal sphincter as a vibratory source. According to Negus, phonation is therefore considered the least significant of the three basic functions ascribed to this complex organ, whose primary role remains that of a guardian to the lower airway.
Structure and Function
The upper airway in adult humans traverses the digestive tract in the region of the pharynx, complicating its sphincteric protection of the lower airway. By sharing a common passageway with the upper digestive system, the larynx is also compromised in its respiratory performance by resultant ventilatory turbulence and, therefore, resistance. Thus, the anatomic configuration in adult humans that benefits phonatory purposes of the larynx simultaneously serves to compromise its sphincteric and respiratory functions. This functional dilemma is resolved at the laryngopharyngeal level by two important organic modifications: structural adaptation and delicate coordination among the three basic laryngeal functions as determined by precisely organized brainstem reflexes.
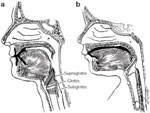
From a structural point of view, protective function of the adult human larynx is admittedly precarious by virtue of its low position in the neck (Figure 2a). Other mammalian species are provided with a relatively high-riding larynx, affording it a close approximation with structures of the posterior nasal cavities. The intranarial position of the larynx, securing a continuous airway from the nose to the bronchi, therefore decreases the risk of pulmonary contamination by swallowed matter. This structural modification is most obvious among certain cetaceans and herbivores but appears to a lesser degree among carnivores that use an elongated epiglottis to effect nasolaryngeal connection during deglutition. In this regard, Negus considers the epiglottis to serve secondarily in an olfactory capacity, ensuring that inspired air enters exclusively through the nose. By a series of anatomic demonstrations in macrosmatic animals, this contention appears very convincing and is supported by later histologic work identifying epiglottic chemoreceptors similar in structure to taste buds of the oral cavity, implying epiglottic participation in chemosensory perception as well.2
Figure 2: The nasolaryngeal relationship.
(Source: Sasaki45 with permission from Lippincott, Williams & Wilkins.)
It is of some interest that the human newborn exhibits similar nasolaryngeal connection by approximation of its epiglottis with the posterior surface of its palate, thus ensuring against aspiration by forming a continuous upper and lower airway (Figure 2b). The observation of obligate nasal breathing in the newborn period may be related to this anatomic configuration, which is lost between 4 and 6 months postnatally.3
In adult humans the characteristic flat, shield-like configuration of the epiglottis serves to direct swallowed food laterally into the pyriform fossae, away from the midline laryngeal aperture (Figure 3). Furthermore, in adult humans, elevation of the larynx toward the nasal cavity during the height of deglutition exaggerates this protective function. Implicit in this maneuver is the role of the aryepiglottic folds, which consist of mucous membrane, connective tissue, and muscle, extending from the epiglottic framework to the arytenoid bodies posteriorly. These lateral folds act as ramparts to the larynx, allowing food to pass on either side of the epiglottis along the gutter produced between each fold and the lateral pharyngeal wall. In this capacity, the cartilages of Santorini and Wrisberg, also called corniculate and cuneiform cartilages, respectively, are contained in the aryepiglottic folds to provide added support and stiffness to these ramparts of the laryngeal aperture. Therefore, from a structural perspective alone, it would appear that the primary role of the supraglottic larynx in adult humans lies in its protection of the lower airway.
Figure 3: Human larynx and pharynx viewed from behind.
Note the role of the epiglottis and aryepiglottic folds in directing swallowed water around the laryngeal inlet into the upper esophagus posteriorly. (Source: Sasaki45 with permission from Lippincott, Williams & Wilkins.)
In the human larynx the ability to perform as an effective valve depends on the unique shelf-like configuration of its superior and inferior folds bilaterally represented (Figure 4). The ventricular folds or false cords, which are located superiorly, act as exit valves, preventing the escape of air from the lower respiratory tract. When positioned by muscular contraction, they seal even more tightly as tracheal pressure is increased from below. This feature of adducted false cords occurs independently of muscle tone, a phenomenon attributable to their unique shape, which is characterized by the down-turned direction of their free margins. Such a configuration is made possible by the lateral ventricles and is exaggerated by the superior extension of the laryngeal saccules. Brunton and Cash,4 working with cadaver larynges, demonstrated that closed ventricular bands (by valvular action alone without muscular contraction) offered a resistance to a pressure head from below, equaling 30 mmHg. When the pressure head was reversed, however, no resistance was offered by the approximately false cords.
Figure 4: Frontal section through the human larynx demonstrating the valvular structure of the false and true cords.
(Source: Sasaki45 with permission from Lippincott, Williams & Wilkins.)
On the other hand, the true cords behaved as a one-way valve in the opposite direction, obstructing the ingress of air. When approximated in the cadaver, the true cords offered little resistance to pressure from below but resisted a pressure head from above exceeding 140 mmHg. Their ability to resist pressure from above was not influenced by closure of the false cords.
The false cords, therefore, prevent the egress of air from the lungs, and the true cords with their up-turned margins are capable of impeding its ingress; these views were confirmed by Lindsay5 on tomographic analysis of the larynx. Therefore, it is not surprising that expectorative functions of the larynx remain unimpaired in bilateral laryngeal paralysis. In this regard, passive closure of the false cords alone appears essential to effective cough production. The valvular component of the true cords, on the other hand, is implicated in the clinical difficulty experienced in overcoming laryngeal spasm by abrupt pressure peaks of positive pressure ventilation that only further serve to protectively seal the true cords. Therefore, from a structural perspective the false cords provide an expectorative function, whereas the true cords assume a protective role in respiration.
Further structural modifications in humans are relevant. The short arytenoid vocal processes in humans effectively increase the relative length of the membranous true cords. Although obviously benefiting the phonatory characteristic of the larynx by maximizing its vibratory surface, these short vocal processes compromise its respiratory capacity. In this regard, Negus calculates the optimum arytenoid length to be 7/10 the length of the true cords, allowing a maximum cross-sectional area at the glottis produced by the pivotal motion of the arytenoid bodies. Such an optimum ratio of arytenoid to vocal cord length is found in the gazelle, with humans possessing a 4:10 ratio instead.
In humans, one must appreciate that both protective and respiratory functions of the larynx have been compromised in favor of its phonatory purposes. This unique compromise is reflected in part by compensatory structural modifications discussed above.
Afferent (Sensory) System
Sensory nerve fibers to the larynx are derived from the internal branch of the superior laryngeal nerve, which ipsilaterally innervates the superior mucosal boundary of the larynx to the level of its true vocal cords. Likewise, below the true cords, ipsilateral sensation is mediated by each recurrent laryngeal nerve. Suzuki and Kirchner,6 however, demonstrated a diamond-shaped area anteriorly in the midline of the subglottic space of the cat that is innervated by both external branches of the superior laryngeal nerve. Afferent impulses from deep muscle receptors and cricothyroid joints also travel cephalad in this nerve branch (Table 1).
The density of sensory innervation appears greatest in the laryngeal inlet, an observation consistent with the concept that the aditus serves as a protective zone for the more distal respiratory system. When nerve staining techniques are used, the laryngeal surface of the epiglottis appears to contain the most compact innervation, whereas the true cords exhibit lesser degrees of sensory density.7 Specifically, the posterior half of the true cords is more heavily furnished with touch receptors than its anterior portion. On the other hand, chemical and thermal sensors appear limited to the supraglottic larynx. In this regard, water chemoreceptors on the epiglottis have been experimentally implicated in the production of prolonged apnea.8 Furthermore, it has been demonstrated that the respiratory response to water-aerosol inhalation for treatment of croup and other upper airway obstruction may be related to the exquisite water sensitivity of these epiglottic receptors. The effect produced consists of respiratory slowing with concurrent increase in tidal volume, certainly an effect beneficial to partial upper airway obstruction. It may be of further interest that this centrally mediated respiratory response appears greater early in life than in adulthood.9 It is generally agreed that sensory components of the superior laryngeal nerve include representation from mucosal touch receptors, epiglottic chemoreceptors, joint receptors, aortic baroreceptors, and stretch receptors from the intrinsic laryngeal muscles.10 Afferent impulses are delivered through ganglion nodosum to the brainstem nucleus tractus solitarius.
Efferent (Motor) Innervation
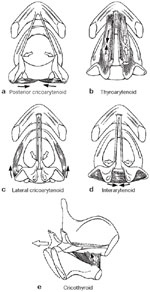
Motor innervation of the larynx is no less complex (Table 2). It is generally agreed that the motor distribution to the intrinsic laryngeal musculature originates in the medullary nucleus ambiguus. Each recurrent laryngeal nerve ipsilaterally innervates all muscles except the cricothyroid muscle, which receives its motor impulses from the external division of its ipsilateral superior laryngeal nerve. The interarytenoid muscles, however, receive bilateral motor innervation from both recurrent laryngeal nerves. The muscle that widens the glottic chink, as takes place in respiration, is solely the posterior cricoarytenoid muscle extending from the posterior aspect of the cricoid plate to the muscular process of the arytenoid (Figure 5a). Exerting a posterolateral pull on the arytenoid body rotates the vocal fold outward, effecting cord abduction on inspiration. On the other hand, vocal fold adduction results from contraction of all other intrinsic musculature, major contributions arising from the action of thyroarytenoid and lateral cricoarytenoid muscles (Figure 5a,c). The interarytenoid muscles serve to close the posterior gap of the glottis (Figure 5d), whereas the cricothyroid muscle adducts and tenses the vocal cord, passively lengthening it by 30%. This is accomplished by shortening the anterior distance between the cricoid and thyroid cartilages, resulting in dorsal and posterior displacement of the posterior cricoid plate. The vocal cord, therefore, undergoes a mechanical stretch displacement, resulting in an increase in the anteroposterior diameter of the laryngeal aperture (Figure 5e).
Figure 5: Laryngoscopic view of the intrinsic muscles responsible for activating vocal cord position.
(Source: Sasaki45 with permission from Lippincott, Williams & Wilkins.)
Briefly, when one cricothyroid muscle is denervated by sectioning the superior laryngeal nerve, the unopposed contraction of the contralateral cricothyroid muscle results in rotation of the posterior glottis toward the inactive side, with foreshortening of the cord on the side of denervation. On the other hand, it is agreed that unilateral recurrent laryngeal nerve injury results in the paramedian position of that cord because the unopposed action of the ipsilateral cricothyroid muscle, innervated by an intact superior laryngeal nerve, produces cord adduction on the side of recurrent laryngeal nerve injury. This physiologic principle replaces Semon's law, which incorrectly asserts that adductor motor components of recurrent laryngeal nerve are more resistant to injury than abductor fibers. Knowledge of this mechanism, in terms of unopposed cricothyroid action, represents the basis of superior laryngeal nerve section advocated to achieve incremental vocal cord lateralization in the treatment of bilateral recurrent laryngeal nerve paralysis in humans.11
Basic functions of the larynx (protective, respiratory, and phonatory) are derived from a complex interrelationship of diverse polysynaptic brainstem reflexes. On the one hand, protective function is entirely reflexive and involuntary, constituting one end of a spectrum that is balanced on the other by respiratory and phonatory performances that may be initiated voluntarily but regulated involuntarily through an array of feedback reflexes.
Neurophysiology of Protective Function
The most basic function of the larynx is to provide sphincteric protection of the lower airway, most efficiently achieved by simultaneous adduction of both vocal cords. Such action serves to close the glottis by involving the activation of both thyroarytenoid muscles, among other groups of adductors. Experimentally, reflexive glottic closure is reliably elicited by stimulating the internal branch of the superior laryngeal nerve (iSLN).
Effect of Anesthesia and the Level of Consciousness
Three categories of protective laryngeal responses have been observed after stimulation of one iSLN. First, an early response involves adduction of the ipsilateral vocal cord with a latency of approximately 10 to 18 msec in anesthetized cats,12 dogs,12 and pigs.13 This short-latency evoked R1 response has also been consistently noted in anesthetized humans.12 A second category of short-latency evoked R1 response involves simultaneous contralateral adduction, also know as the crossed adductor reflex. Although this crossed response with a latency of 10 to 18 msec has been found to be consistently present in anesthetized cats, it is less consistently found in dogs and is rarely observed in anesthetized human subjects.12 Still a third category of adductor response involving a longer-latency reflex, termed R2, has been observed to produce bilateral vocal cord responses, but its presence appears to be most readily noted in awake human subjects; it has a latency of 50 to 80 msec.14
From these observations, the investigators note that prior studies conducted in fully anesthetized dogs, pigs, and humans failed to consistently demonstrate the presence of a crossed R1 or R2 adductor reflex. Because of the absence of the crossed reflex in fully anesthetized humans, the presence of crossed reflexes in awake nonanesthetized human subjects suggests the possibility that the crossed evoked response may be anesthesia-dependent.
In our studies, described below, we found that whereas a contralateral R1 response in humans is supported by central facilitation in the awake state, anesthetic suppression of facilitative mechanisms restricts the response to an ipsilateral one. The anesthesia-dependent loss of the crossed R1 weaken the summated closure response even under conditions of physiologic stimulation.15 Pharmacologic suppression of supramedullary facilitation offers a unified explanation for several interesting and important clinical findings, such as the increase incidence of life-threatening aspiration among sedated patients in an intensive care setting16 and among institutionalized psychiatric patients under heavy psychotropic control, in whom death from aspiration represents a highly significant risk.17 These observations provide the neurophysiologic basis for preventive measures in high-risk patients and could, for example, alter dosing schedules of psychotropic medications, improve respiratory monitoring, or institute lifesaving dietary modifications. These observations may also stimulate the search for alternative agents with pharmacologic properties that preserve or augment facilitation of the glottic closure response without loss of specific psychotropic or sedative properties.
In studies from our laboratory, we examined the crossed adductor reflex in humans by electromyographic (EMG) recordings from the thyroarytenoid muscles under varying levels of anesthesia. We assert that the crossed R1 reflex represents a facilitated response dependent on supramedullary influences that, when abolished by general anesthesia, also abolishes the crossed response. The presence of central facilitation may be an essential component of a bilateral adductor reflex, and its disturbance could result in conversion to a unilateral reflex response overall, resulting in a biomechanically weakened sphincteric closure.15 A precise understanding of this effect may improve the prevention of aspiration in patients at risk while emerging from prolonged sedation16, 18 or while under heavy psychotropic control.17
Five patients undergoing supraglottic laryngectomy were each studied at two different depths of isoflurane anesthesia [0.5 and 1.0 minimal alveolar concentration (MAC)], the order of which was randomly selected as determined by intraoperative requirements. A 1.0 MAC is defined as a 1.5% inhaled isoflurane concentration.19 At 1.0 MAC, periods of electroencephalographic suppression20 are expected to occur, characterized by an increase in delta wave activity,21 whereas at 2.0 MAC, electrical silence is expected to predominate.20, 21 (Patients undergoing supraglottic laryngectomy were selected for this study because the surgical exposure facilitated direct simultaneous access to both iSLNs and to tumor-free vocal cords for precise confirmation of electrode placement within the musculature. In minimizing patient risk, collection of data was limited to a total of 30 minutes per patient, usually at a point after tumor resection when histopathologic frozen section margins were being confirmed.) All five male subjects, ranging in age from 50 to 69 years, gave informed consent before the study. None had received prior chemotherapy or irradiation. At least 20 minutes of equilibration was allowed after each incremental increase of decrease in anesthetic level before evoked responses were recorded.
Arterial oxygen saturation was maintained above 95% at all times. Body temperature was normothermically maintained between 36.4°C and 36.7°C (97.5°F and 98.1°F) with a warming blanket.
An XLTEK NeuroMax provided nerve stimulation and EMG recording capability. Bipolar 200 (milliohm) platinum-iridium stimulating electrodes were applied to the proximal end of each severed iSLN. Monopolar platinum-iridium recording electrodes were inserted into the midportion of the thyroarytenoid muscles bilaterally. Reference electrodes were placed in the ipsilateral strap muscles, and a ground electrode was placed in the sternocleidomastoid muscle. Although simultaneous bilateral iSLN stimulation is perhaps more physiologic, sequential unilateral stimulation was used to allow an accurate analysis of the crossed components of the evoked adductor response that would otherwise be obscured by simultaneous bilateral iSLN stimulation.
With each subject sequentially under varying depths of anesthesia as described earlier, the left iSLN was electrically stimulated with a single rectangular pulse of 0.1 mA/0.1 msec. The stimulation intensity was incrementally increased by 0.1 mA until a consistent EMG response was seen in the thyroarytenoid muscle or until a maximum stimulation intensity of 2.0 mA was attained. An average of three trials was performed in each experimental paradigm.
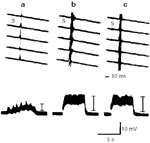
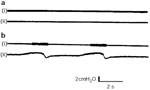
The average latencies of the left ipsilateral and contralateral R1 evoked responses with their standard deviations are summarized in Table 3. The table also summarizes the average latencies obtained when the right iSLN was stimulated. The number of evoked responses compared to the number of stimulus presentations under each level of anesthesia enables the calculated response efficiency achieved within each level of anesthesia. It was noted that ipsilateral evoked R1 responses, organizationally modeled in Figure 6a, occurred at all depths of anesthesia with 100% efficiency (Figure 7a,b). However, the contralateral R1 response, organizationally modeled in Fig. 1B, underwent a dramatic decline with anesthesia levels at 1.0 MAC (Figure 6c). At 1.0 MAC, none of 15 stimulations resulted in contralateral R1 evoked responses (Figure 7c,d). When the percentage of contralateral responses obtained at 0.5 MAC is compared to the percentage of responses obtained at 1.0 MAC, the difference is statistically significant at p <.0001 (Table 3). It should be noted that equivalent stimulus amplitudes were used to obtain the ipsilateral and contralateral responses. No R2 evoked responses were noted.
Figure 6: Organizational models.
a: The ipsilateral R1 reflex. b: The contralateral R1 under light anesthesia. c: Demonstrating loss of contralateral R1 under deep anesthesia. d: The crossed adductor reflex pathway in humans. Lt., left; Rt., right; NA, nucleus ambiguus; NTS, nucleus tractus solitarius; iSLN, internal branch of superior laryngeal nerve; RLN, recurrent laryngeal nerve. (Source: Sasaki CT, Jassin B, Kim YH, Hundal J, Rosenblatt W, Ross DA. Central facilitation of glottic closure reflex. Ann Otol Rhino Laryngol 2003;112 (4): 293-297, with permission from Annals Publishing Co.)
Figure 7: Stimulation of right internal branch of superior laryngeal nerve.
Compound muscle action potentials obtained from (a,b) ipsilateral and (c,d) contralateral thyroarytenoid muscles at (a,c) 0.5 minimal alveolar concentration and (b,d) 1.0 minimal alveolar concentration. d: Note loss of evoked contralateral responses at 1.0. (Source: Sasaki CT, Jassin B, Kim YH, Hundal J, Rosenblatt W, Ross DA. Central facilitation of glottic closure reflex. Ann Otol Rhino Laryngol 2003;112 (4): 293-297, with permission from Annals Publishing Co.)
The rationale for the underlying hypothesis is based on the following historical considerations. Prior observations conducted in fully anesthetized dogs, pigs, and humans failed to consistently show a contralateral evoked response. The presence of any crossed R1 or R2 adductor reflexes in the awake state suggests that the contralateral reflex may be anesthesia-dependent. The variable observation by Ludlow et al.14 of crossed R1 responses in two awake human subjects underscores their variable susceptibility to higher-order central control. A pig model reported previously22 supports the notion that a facilitated adductor reflex is likely responsible for a crossed R1 response in the awake state, whereas the sensitivity of the crossed R1 response to pharmacologic sedation implies that relevant facilitatory mechanisms are located central to motor neurons of the nucleus ambiguus. Furthermore, in humans it appears that the latency of the contralateral evoked response is approximately 4 msec longer than the ipsilateral reflex. On the basis of the well-established assumptions that nerve conduction velocity approximates 5 cm/ms,23 that the average length of the neural circuitry from the larynx to the brainstem is 20 cm, and that each synaptic delay is 1.5 msec,23 the data support the following organizational model in humans (Figure 6d): The investigators propose that the iSLN projects to motor neurons of the ipsilateral nucleus ambiguus through at least two synapses: the first, within the ipsilateral nucleus tractus solitarius, and the second, likely within the nucleus ambiguus. However, contralaterally, an increased latency of 4 msec suggests two or three additional interneurons within the reticular formation in a manner supported by Sessle's24 observations. Furthermore, because the pig model,22 as earlier reported, supports but a single interneuron in the reticular formation of the crossed reflex, the more complex neural circuitry of as many as three interneurons in the reticular formation of humans perhaps explains a greater human sensitivity, if not vulnerability, to effects of central facilitation. These results represent the very first demonstration of humans' protective vulnerability, whether from pharmacologic facilitatory suppression or in the normal sleep state when gastric refluxate poses an increased risk of tracheal aspiration.
Effect of Some Other Factors on Reflex Glottic Closure
Reflex glottis closure is facilitated by (1) expiratory phase, (2) decreased arterial partial pressure of carbon dioxide (pCO2), (3) increased arterial partial pressure of oxygen (pO2), (4) negative intrathoracic pressure, and (5) hyperthermia. On the other hand, reflex glottic closure is inhibited by (1) inspiratory phase, (2) increased arterial pCO2, (3) decreased arterial pO2, (4) positive intrathoracic pressure, and (5) hypothermia. These conclusions are based on studies performed in our laboratory as described below.
Studies from Our Laboratory
Effect of respiratory cycle The threshold of the glottic closure reflex was determined with respect to the respiratory phase in the spontaneously breathing subject. In so doing, inspiration and expiration were arbitrarily divided into early and late phases, such that SLN stimulation could be triggered precisely at each of four phases: early and late expiration, and early and late inspiration (Figure 8). Arterial partial pressure of oxygen (pO2) and partial pressure of carbon dioxide (pCO2) were maintained within narrow physiologic limits (pCO2 38 mmHg, pO2 100 mmHg) during this section of experimentation.
Figure 8: Intrathoracic pressure is plotted with respect to time (t) in the spontaneously breathing animal.
Inspiratory and expiratory phases are arbitrarily subdivided into early and late segments. (Source: Ikari T, Sasaki CT. Glottic closure reflex: control mechanisms Ann Otol Rhino Laryngol 1980;89(3): 220-224, with permission from Annals Publishing Co.)
In early inspiration, the mean threshold stimulus measured 0.37 V, 0.1 msec, whereas in late inspiration, mean threshold measured 0.36 V, 0.1 msec. In early expiration, mean threshold measured 0.28 V, 0.1 msec, whereas in late expiration, mean threshold measured 0.29 V, 0.1 msec (Figure 9). In the spontaneously breathing subject, therefore, threshold of reflex glottic closure seemed to increase on inspiration and decrease on expiration. In other words, reflex glottic closure occurred more readily in expiration than inspiration.
Figure 9: Threshold of the adductor reflex is plotted with respect to respiratory phase.
Note the development of a sinusoidal pattern. (Source: Ikari TS, Sasaki CT. Glottic closure reflex: control mechanisms Ann Otol Rhino Laryngol 1980;89(3): 220-224, with permission from Annals Publishing Co.)
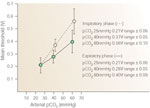
Effect of carbon dioxide The threshold of the glottic closure reflex was determined with respect to precisely controlled variations in arterial pCO2. The mean and range of three separate determinations were obtained in hypocapnia, normocapnia, and hypercapnia. For example, hypocapnia was produced by hyperventilating on room air, normocapnia by administering room air during spontaneous respiration, and hypercapnia by administering 10% CO2 during spontaneous breathing. Arterial blood gas determinations were confirmed in each test situation, such that arterial O2 tension (95 mmHg) remained constant throughout this test period.
In the spontaneously breathing subject, iSLN stimuli were precisely phased-locked to early inspiration and early expiration to avoid variations caused by respiratory phase. During early inspiration, mean threshold of the glottic closure reflex measured 0.21 V at pCO2 of 25 mmHg, 0.37 V at pCO2 of 40 mmHg, and 0.56 V at pCO2 of 60 mmHg. During early expiration, threshold of the glottic closure reflex measured 0.21 V at pCO2 of 25 mmHg, 0.28 V at pCO2 of 40 mmHg, and 0.40 V at pCO2 of 60 mmHg.
A linear response to varying pCO2 was therefore obtained. Thresholds were greater in early inspiration than early expiration. This difference was accentuated in the hypercapneic states (Figure 10).
Figure 10: Threshold of the adductor reflex is plotted with respect to arterial pCO2.
Note the development of a linear relationship. (Source: Ikari T, Sasaki CT. Glottic closure reflex: control mechanisms Ann Otol Rhino Laryngol 1980;89(3): 220-224, with permission from Annals Publishing Co.)
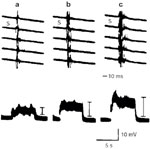
When the right SLN was stimulated by a train of pulses at 8 Hz during early inspiration, 0.60 V, 0.1 msec pulse duration, the right adductor response consisted of a burst of action potentials consistent with laryngeal spasm. This complex motor response was electronically integrated and compared at varying arterial levels of pCO2. Hypocapnia (pCO2 of 25 mmHg) increased the laryngospastic response, whereas hypercapnia greatly reduced it (Figure 11). In other words, both the evoked glottic closure response and laryngospastic response occurred more readily in hypocapnia than hypercapnia.
Figure 11: The increasing pattern of adductor responses (upper) and integrated responses (lower) by 8-Hz stimulation of the superior laryngeal nerve (SLN) (0.6 V, 0.1 msec) under conditions of (a) pCO2 60 mmHg; (b) pCO2 40 mmHg; and (c) pCO2 25 mmHg.
(Source: Ikari T, Sasaki C. Glottic closure reflex: control mechanisms Ann Otol Rhino Laryngol 1980;89(3): 220-224, with permission from Annals Publishing Co.)
Effect of oxygen We determined the threshold of the glottic closure reflex with respect to precisely controlled variations in arterial pO2. The mean and range of three separate determinations were obtained in the spontaneously breathing subject during arterial pO2 of 25 mmHg, 50 mmHg, 90 mmHg, and 150 mmHg, by respectively administering gas mixtures of 5% O2–95% N2, 10% O2–90% N2, room air, and 100% O2 Arterial blood gas determinations were confirmed in each test situation, such that arterial pCO2 (38 mmHg) remained constant throughout this test period.
In the spontaneously breathing subject, iSLN stimuli were phase-locked to early inspiration and early expiration in order to avoid variations caused by the respiratory phase. During early inspiration, the mean thresholds of the glottic closure reflex measured 0.52 V at pO2 of 25 mmHg, 0.36 V at pO2 of 50 mmHg, 0.36 V at 90 mmHg, and 0.36 V at 150 mmHg. During early expiration, threshold of the glottic closure reflex measured 0.38 V at pO2 of 25 mmHg, 0.29 V at pO2 of 50 mmHg, 0.29 V at pO2 of 90 mmHg, and 0.29 V at pO2 of 150 mmHg.
Unlike the response to variations in pCO2, an exponential response to pO2 was obtained (Figure 12). Thresholds of the glottic closure reflex decreased exponentially as pO2 increased.
Figure 12: Threshold of the adductor reflex is plotted with respect to arterial pO2.
Note the development of an exponential relationship. (Source: Ikari T, Sasaki C. Glottic closure reflex: control mechanisms Ann Otol Rhino Laryngol 1980;89(3): 220-224, with permission from Annals Publishing Co.)
When the right iSLN was stimulated by a train pulses at 8 Hz, 0.60 V, 0.1 msec pulse duration, during early inspiration the right adductor response consisted of a burst of action potentials consistent with laryngospasm. This complex motor response was electronically integrated and compared at varying arterial levels of pO2. Hypoxia sharply decreased the laryngospastic response, whereas hyperoxia had no measurable effect beyond that obtained at pO2 of 90 to 100 mmHg (Figure 13).
Figure 13: The pattern of adductor responses (upper) and integrated responses (lower) by 8-Hz stimulation of SLN (0.6 V, 0.1 msec) under conditions of (a) pO2 25 mmHg; (b) pO2 100 mmHg; and (c) pO2 150 mmHg.
(Source: Ikari T, Sasaki C. Glottic closure reflex: control mechanisms Ann Otol Rhino Laryngol 1980;89(3): 220-224, with permission from Annals Publishing Co.)
Effect of lung inflation and deflation By mechanically ventilating the anesthetized subjects, spontaneous respiration was abolished, thus eliminating the effect of respiratory phase on threshold of the glottic closure response. In addition, chemoreceptor influence was controlled by maintaining arterial pO2 and pCO2 within narrow physiologic limits (pCO2 of 36 mmHg, pO2 of 90 mmHg). During mechanical ventilation, the iSLN was stimulated during lung inflation and deflation. Mean threshold of the glottic closure reflex measured 0.23 V during inflation and 0.17 V during deflation (Figure 14). That is to say, the glottic closure reflex appears to be facilitated during lung deflation and inhibited during inflation.
Figure 14: Threshold of the adductor reflex is plotted with respect to intrathoracic pressure.
Note the development of a linear response. (Source: Ikari T, Sasaki C. Glottic closure reflex: control mechanisms Ann Otol Rhino Laryngol 1980;89(3): 220-224, with permission from Annals Publishing Co.)
Effect of core temperature Is protective glottic closure temperature dependent? Clinical experience would suggest that important relationships may exist.
Thirteen adult dogs and 19 beagle pups were used in this study conducted in the Yale Larynx Laboratory. The canine model was chosen because (1) the central organization of laryngeal reflexes is similar in dogs and humans,12 and (2) thermoregulatory development of the dog is also similar to that of the human.25 Adult dogs were anesthetized with pentobarbital sodium administered intravenously 30 to 35 mg/kg of body weight. Pups received intraperitoneal pentobarbital sodium 25 to 30 mg/kg of body weight. An arterial blood gas catheter and venous catheter for intravenous administration of pentobarbital were placed into the femoral vessels. Subjects were kept at a stage 3, plane 1 depth of anesthesia during data acquisition. A vertical midline neck incision was used to expose the larynx and trachea, and a cannula was inserted through the fourth tracheal ring for unassisted, spontaneous breathing.
The strap muscles were transected at the level of the thyrohyoid membrane to expose the right thyroid ala, which was removed for exposure of the thyroarytenoid (TA) muscle. The iSLN was then identified and divided 2 to 3 mm proximal to its bifurcation. The proximal iSLN was prepared in a mineral oil bath and was attached to a bipolar platinum wire for stimulation. For the purpose of recording, hooked wire electrodes (platinum iridium) were implanted in the TA muscle, the major adductor of the vocal cords.
The iSLN was stimulated with an S-8 stimulator (Grass, West Warwick, RI) at 0.1 to 15 V, 0.1-msec pulse duration. An EMG of the evoked glottic closure reflex was displayed on an oscilloscope 5103N (Tektronics, Beaverton, OR), after passing through a DAM 5A differential preamplifier (WP) and a 5A18N dual trace amplifier (Tektronics). Responses were recorded by a 3960 instrumentation recorder (Hewlett-Packard, Palo Alto, CA). Simultaneously, respiratory rate was monitored on a model 7 polygraph (Grass) via a P23BC manometer transducer (Statham, Los Angeles, CA) linked to a water-filled needle introduced into the thoracic space. Arterial blood gas was analyzed with a PHM71 acid–base analyzer (Radiometer, Copenhagen, Denmark).
The investigators adopted a method of body temperature control, allowing the animals to be warmed with an electric blanket or cooled with an ice water bag. Rectal temperature, measured with an YSI telethermometer, could be varied at will between 34°C and 41°C.
In summary, the right iSLN was stimulated electrically, and reflex muscle responses were recorded from the ipsilateral TA muscle. Each stimulus was phase-locked to the expiratory segment of the respiratory cycle so as to avoid respiratory-linked variations in threshold responses.
Subjects were classified into four groups for comparative investigation of postnasal development: 3-week pups, 6-week pups, 12-week pups, and adult dogs. Rectal temperatures immediately after induction of anesthesia measured 36.8  0.2°C in the 3-week pup, 37.3
0.2°C in the 3-week pup, 37.3  0.4°C in the 6-week pup, 38
0.4°C in the 6-week pup, 38  0.2°C in the 12-week pup, and 38.8
0.2°C in the 12-week pup, and 38.8  0.2°C in adulthood.
0.2°C in adulthood.
Threshold With respect to postnatal development, normothermic thresholds were in agreement with previous reports, in which ipsilateral reflex threshold decreased with age (Table 4). In 3-week pups, thresholds were higher than in other age groups at each body temperature, showing a sigmoid curve with temperature increase. The most rapid change in threshold occurred between 37°C and 39°C (Figure 15a). Six-week pups showed a sigmoid curve similar to that of 3-week pups. The maximum rate of threshold change with respect to temperature appeared to be somewhat less than that shown by the previous age group between 37°C and 39°C (Figure 15b). In 12-week pups, thresholds of laryngeal reflexes were in general markedly lower (Figure 15c). In adult dogs, the thresholds were lower than other age groups tested. In comparing threshold change to temperature, the slope of this function was the lowest of all age groups tested (Figure 15d).
Figure 15: Influence of body temperature on threshold and latency in (a) 3-week-old puppies; (b) 6-week-old puppies; (c) 12-week-old puppies; (d) adult dogs.
(Source: Haraguchi S, Fung RO, Sasaki CT. Effect of hyperthermia on laryngeal closure reflex. Ann Otol Rhino Laryngol 1983;92(1): 24-28, with permission from Annals Publishing Co.)
Thus, greater changes in glottic closure reflex threshold were produced by smaller changes in core body temperature in younger rather than older animals. That is to say, the younger the animal, the more sensitive laryngeal motoneurons were to the change in core body temperature (Figure 16).
Figure 16: Influence of body temperature on threshold in four age groups.
Note the sigmoid relationship in younger age groups. W, weeks. (Source: Haraguchi S, Fung RO, Sasaki CT. Effect of hyperthermia on laryngeal closure reflex. Ann Otol Rhino Laryngol 1983;92(1): 24-28, with permission from Annals Publishing Co.)
Latency The duration between stimulation and the beginning of reflex TA responses was measured oscilloscopically. Laryngeal reflex latency, being the highest at birth, markedly decreased during the first 2 months of postnatal life. In this study the latencies were longer at lower temperatures and shortened as body temperatures increased, showing a linear responses in animals of all age groups (Table 5 and Figures 17 and 18).
Figure 17: Evoked adductor responses elicited by single-shock stimulation of SLN in (a) 3-week-old puppies; (b) 6-week-old puppies; (c) 12-week-old puppies; (d) adult dogs. S, stimulus artifact.
(Source: Haraguchi S, Fung RO, Sasaki CT. Effect of hyperthermia on laryngeal closure reflex. Ann Otol Rhino Laryngol 1983;92(1): 24-28, with permission from Annals Publishing Co.)
Therefore, within physiologic ranges laryngeal reflex excitability increased with increasing core body temperatures. The younger the subject, the greater the change in reflex thresholds. However, in all age groups, the rate of change was greatest between 37°C and 39°C. These results confirmed that laryngeal excitability was most greatly affected between 37°C and 39°C, and that this effect was greatest in newborns.
This investigation has found that both the latency and threshold of the glottic closure reflex decreases during hyperthermia. Thus, hyperthermia effectively enhances this reflex during its age-related period of hyperexcitability. The effect of temperature on latency may be attributed to changes in axonal conduction and synaptic transmission velocities. Temperature-dependent changes in synaptic transmission are hypothesized as the cause of the observed threshold changes. On the other hand, hypothermia produces quite the opposite effect and may exert considerable influence on altered protective function among hypothermic subjects emerging from general anesthesia or poorly monitored patients in critical care units. A clearer understanding of vocal adductor control may be an essential first step in any therapeutic modification of abnormal glottic closure.
Neurophysiology of Respiratory Function
Negus1 in 1949 noted that the glottis opened a fraction of a second before air was drawn in by the descent of the diaphragm. In 1969, Suzuki and Kirchner26 established phasic vocal cord movement as the direct effect of the medullary respiratory center, thereby opening the way to further understanding of laryngeal respiratory function in terms of a rich historical background in basic respiratory physiology. Therefore, having established that widening of the glottis occurred with rhythmic bursts of activity. Suzuki and Kirchner then demonstrated that, like phrenic activity, this rhythmicity was accentuated by hypercapnia and ventilatory obstruction and depressed by hyperventilation and resultant hypocapnia.
Likewise, as phrenic activity is modified by ventilatory resistance, it would seem reasonable that glottic widening, governed by medullary centers, would be similarly modified. From a purely structural perspective, because the true vocal cord passively act to obstruct the ingress of air to the lungs, active inspiratory abduction by phasic muscular contraction of the posterior cricoarytenoid appears an essential component of successful ventilation. Using EMG techniques on the posterior cricoarytenoid muscle, we showed that phasic inspiratory abduction is, in fact, synchronous with inspiration (Figures 19 and 20).27 Furthermore, the degree of abductor activity appears to vary directly with ventilatory load, disappearing entirely when inspiratory resistance is removed, only to return when resistance to ventilation is reestablished. Because vagotomy abolishes this response, the afferent limb for the reflex regulation of phasic inspiratory abduction lies within the ascending vagus nerve.28 End-organ receptors concerned in this reflex presumably lie within the thorax, although their exact nature and location remain unknown.
Also, pertinent to respiratory laryngeal function is the role of the cricothyroid muscle, known to be a vocal cord adductor and isotonic tensor. We have demonstrated that this muscle contracts phasically with inspiration (Figure 21).29 Although its inspiratory adductor role would appear counterproductive to inspiration by narrowing the glottic aperture, its role in cord lengthening actually enhances the cross-sectional diameter of the glottis by increasing its anteroposterior dimension by 30%. Therefore, it would appear that both posterior cricoarytenoid and cricothyroid muscles are driven by the medullary respiratory center, the level of their activity regulated in eupneic breathing by afferent impulses originating in the chest. Although posterior cricoarytenoid contraction increases the horizontal diameter of the glottic chink, its anteroposterior diameter is increased by phasic inspiratory contraction of cricothyroid muscle (Figure 22).
Figure 21: Cricothyroid EMG (upper tracing) and phrenic EMG (lower tracing).
a: Quiet breathing. b: Expiratory dyspnea. c: Inspiratory dyspnea. The cricothyroid, like the posterior cricoarytenoid, appears to be an inspiratory muscle. (Source: Sasaki45 with permission from Lippincott, Williams and Wilkins.)
Figure 22: Glottic alteration produced by cricothyroid (CT) and posterior cricoarytenoid action (PCA) alone and in combination.
Combined muscle action maximizes the cross-sectional area of the laryngeal aperture. (Source: Sasaki45 with permission from Lippincott, Williams and Wilkins.)
In addition to its inspiratory function, cricothyroid expiratory activity is an equally important consideration. In eupneic states, expiratory flow and duration are principal determinants of respiratory frequency. As others have demonstrated in both animal and human investigations, variations in respiratory rate result primarily from changing the duration of the expiratory phase rather than the inspiratory phase of the respiratory cycle.30, 31
A closer look at expiratory activity of the cricothyroid muscle may provide further insight into its laryngeal influence on respiration. What factors govern cricothyroid activity? And what is its possible role in ventilatory control?
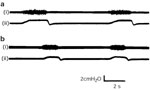
On a mechanical ventilator we observed the following interesting phenomenon. Cricothyroid activity is evoked by positive intratracheal pressure, its contraction synchronized with the phase of the ventilator (Figure 23a). When the respiratory rate is mechanically increased from 20 to 40 per minute, cricothyroid activity appears to track synchronously with this rate change (Figure 23b,c).
Figure 23: Cricothyroid response to mechanical ventilation at rates of (a) 20 per minute, (b) 30 per minute, (c) 40 per minute.
Note the synchronous tracking of cricothyroid to positive-pressure stimuli. (Source: Sasaki45 with permission from Lippincott, Williams and Wilkins.)
When the ventilator is stopped in midexpiration, cricothyroid activity continues as long as positive intratracheal pressure is maintained (Figure 24). When pressure decreases, cricothyroid activity decreases. These results suggest that positive intratracheal pressure is an important determinant of cricothyroid expiratory participation and that expiratory cricothyroid contraction, unlike its inspiratory activity, is essentially a peripherally evoked response.
Figure 24: Duration of positive pressure stimulation determines duration of cricothyroid-evoked activity.
((Source: Sasaki45 with permission from Lippincott, Williams and Wilkins.)
After bilateral vagotomy, cricothyroid tracking is totally abolished, suggesting the importance of vagal afferents in the mediation of this evoked reflex. Rather, vagotomy produces cricothyroid inspiratory predominance by the following mechanism (Figure 25a). Because the vagus is largely inhibitory to the respiratory center, vagotomy releases inspiratory neurons into spontaneous phasic hyperactivity. However, positive pressure stimuli no longer evoke a cricothyroid response following vagotomy (Figure 25b). These data therefore strongly suggest that vagally mediated afferent influences play a direct role in the elicitation of cricothyroid contraction during the expiratory phase of respiration.
Figure 25: a: Vagotomy produces spontaneous inspiratory hyperactivity of cricothyroid motoneurons.
b: Following vagotomy, positive-pressure ventilation no longer triggers cricothyroid activity. Cricothyroid tracking is therefore abolished. (Source: Sasaki45 with permission from Lippincott, Williams and Wilkins.)
Current observation suggests that under normocapnic conditions the rate of intratracheal pressure change appears critical to cricothyroid elicitation. That is to say, a rapid rise in tracheal pressure is more likely to initiate cricothyroid activity than a gradual rise in pressure regardless of the absolute pressure amplitude. The threshold of this cricothyroid-evoked response in normocapnia can then be ascertained with respect to a critical change in pressure with respect to change in time (dp/dt). This critical level of pressure change with respect to time in fact measures approximately 30 cm H2O/sec (Figure 26).
Figure 26: The threshold of cricothyroid elicitation in response to rate of tracheal pressure change measures 30 cmH2O/sec in normocapnia.
Hypercapnia reduces cricothyroid threshold, whereas hypocapnia raises its threshold. (Source: Sasaki45 with permission from Lippincott, Williams and Wilkins.)
The valvular mechanism of laryngeal constriction during expiration is therefore based on the following understanding. During expiration, the cross-sectional area of the laryngeal aperture is reduced by virtue of elastic recoil assuming a cadaveric configuration in midexpiration. However, the rate of recoil, is regulated by active muscular participation.
On the one hand, Remmers and Bartlett31 have indicated that expiratory laryngeal resistance is regulated by continued posterior cricoarytenoid activity well into the expiratory phase of respiration. Such abductor activity in early expiration retards elastic collapse of the glottis, acting in turn to retard the braking mechanism of the larynx. Cricothyroid action in conjunction with this posterior cricoarytenoid activity therefore not only maximizes the effective cross-sectional area of the laryngeal aperture but also provides a greater degree of regulatory potential with respect to the fine control of expiratory resistance. Based on the neural regulation of cricothyroid muscle, its expiratory activity therefore appears to play a significant role in the direct control of the expiratory laryngeal resistance and indirectly in the overall control of respiration itself.
Current data suggest the following conclusions:
- Laryngeal resistance contributing to expiratory braking is fundamentally produced by elastic recoil of the larynx.
- The rate of recoil producing passive glottis constriction is modified by cricothyroid and posterior cricoarytenoid contraction during expiration.
- In normocapnia, expiratory cricothyroid activity is clearly an evoked pressure-sensitive response.
- Expiratory cricothyroid activity is triggered by a critical rate of elevation in subglottic pressure transmitted to pulmonary vagal receptors, the effect of which may be abolished by vagotomy.
- This critical threshold of dp/dt measures 30 cm H2O/sec in normocapnia.
- The duration of cricothyroid expiratory contraction is principally governed by the presence of positive subglottic pressure and is inhibited by a negative dp/dt.
- The cross-sectional area of the glottis, modified by expansion of the laryngeal aperture in its anteroposterior dimension, is a direct effect of cricothyroid activity. That is to say, the greater the rise in subglottic pressure, the larger the laryngeal aperture in expiration.
- It follows that the larger the expiratory laryngeal lumen, the shorter the expiratory duration, resulting in an increased rate of breathing.
- Because the regulation of expiratory duration is a major determinant of respiratory frequency, the cricothyroid muscle and posterior cricoarytenoid participation represent principal laryngeal effectors of this control.
Neurophysiology of Phonation
The phonatory function of the larynx is probably the least well understood of its three basic functions. Because of advances in investigative technique, many established hypotheses based on animal models have been challenged, owing in large measure to the advent of more sophisticated technology based on human study.32, 33, 34 High-speed cinematography, improved endoscopic techniques with use of laryngeal stroboscope, and direct human EMG measurements made possible by hooked wire electrodes are largely responsible for these newer additions.
It is generally agreed that speech results from the production of a fundamental tone produced at the larynx and is modified by resonating chambers of the upper aerodigestive tract. Intelligible speech, therefore, represents the combined effect of the larynx, tongue, palate, and related structures of the oral vestibule. The production of the fundamental tone is due to the vibration of the vocal folds against each other, generated by the passage of air between them. Vocal cord vibrations may be a passive phenomenon representing the basis of the aerodynamic theory of sound generation. Such a theory finds support in the observation that the completely paralyzed larynx is capable of producing sound, as is the cadaver larynx when subglottic pressure is forcefully increased. Obviously, phonation ceases when a tracheotomy is performed for diversionary purposes.
The aerodynamic theory of sound production therefore replaces the neurochronaxic theory proposed by Husson,35 who incorrectly advanced the notion that the central generation of recurrent laryngeal nerve impulses produced cord vibrations by active contraction of the thyroarytenoid muscles. Each vibration, therefore, represented the result of beat-by-beat impulses through the recurrent laryngeal nerve. This theory, no longer accepted, is of historical interest only.
Although sound production may be considered a passive function, the regulation of its acoustic quality is not a passive phenomenon. Rather, vocal cord shaping and positioning are under active neural regulation.36
The cricothyroid (CT) muscle increases fundamental frequency (F0) by tensing the vocal fold. The vocal fold is stretched, elongated, thinned, and slightly adducted to the paramedian position as the vocal fold is lowered within the larynx. These changes reduce the cross-sectional area of the vocal fold, reducing vibratory mass and increasing F0. Vocalis muscle (Voc), on the other hand, generates the opposite effect as it loosens and thickens the vocal fold. In addition, as it increases glottal resistance, it contributes to vocal intensity as subglottal pressure is increased. Vocal control, therefore, is achieved by the coordinated efforts of respiratory, laryngeal, and articulatory muscles capable of producing great variations of tonal qualities characterizing the human voice.
Tracheotomy and Laryngeal Function
Upper airway obstruction is the most urgent requirement for tracheotomy. This lifesaving surgical procedure, however, may be complicated by postoperative deficiencies in laryngeal function even when no prior laryngeal pathology exists. Decannulation difficulty, resulting from continued airway obstruction originating above the tracheostome, may be due to an array of functional deficiencies produced by the tracheotomy itself, including abductor failure and vocal cord fusion. On the other hand, chronic aspiration, a defect in protective laryngeal function, may occur in response to tracheotomy well before decannulation is even attempted.
The effect of tracheotomy on an otherwise healthy larynx therefore may be considerably more complex than previously appreciated. The following investigational data from our laboratory provide clues to the mechanism of tracheotomy-related laryngeal dysfunction.
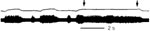
The chronically tracheostomized dog, submitted to neurophysiologic evaluation of protective glottic closure, will develop significant laryngeal incoordination 1 to 8 months after tracheotomy (Figure 27).37 Threshold of the adductor reflex produced by superior laryngeal nerve electrostimulation nearly doubles after prolonged tracheotomy, whereas evoked adductor reflexes undergo wide shifts in their latency. These abnormal changes indicate alteration of both central threshold and conduction time. Furthermore, repetitive superior laryngeal nerve stimulation produces marked attenuation of the adductor reflex, reflected in a weakened closure response after long-term tracheotomy (Figures 28 to 30). Such changes in the behavior of the medullary adductor motoneurons indicate their susceptibility to surgical modifications in lower respiratory function. These changes may help to explain the onset of aspiration owing to a weakened, ill-coordinated closure response resulting from long-term tracheotomy when no laryngotracheal surgical injury can be found.
Figure 27: Thyroarytenoid action potentials elicited by single-shock stimuli applied to the ipsilateral superior laryngeal nerve.
a: Control dogs. b: Chronically tracheostomized dogs (aged 6 to 8 months). Note latency shift in dogs chronically tracheostomized. (Source: Sasaki45 with permission from Lippincott, Williams and Wilkins.)
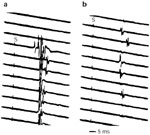
Figure 28: Thyroarytenoid action potentials elicited by repetitive stimulation of ipsilateral superior laryngeal nerve in control dogs.
a: 8 Hz. b: 16 Hz. Note numerous afterdischarges following the primary evoked response. (Source: Sasaki45 with permission from Lippincott, Williams and Wilkins.)
Figure 30: Thyroarytenoid action potentials produced by 16-Hz superior laryngeal stimulation in chronically tracheostomized dogs.
Primary evoked response attenuates within seconds of stimulus onset. a: Onset of stimulus train. b: One to 2 seconds after onset of stimulus train. (Source: Sasaki45 with permission from Lippincott, Williams and Wilkins.)
Respiratory function of the larynx appears even more susceptible to central alterations when the upper airway is bypassed through tracheotomy. When the electromyographic activity of the posterior cricoarytenoid muscle is measured before and after tracheotomy, interesting changes in the level of its activity can be observed.27
During spontaneous nasal breathing when ventilatory resistance is judged to be maximal (Figure 19a), spontaneous phasic posterior cricoarytenoid activity likewise appears to be maximal, corresponding to a negative inspiratory pressure change of 15 mm H2O. Approximately 3 minutes following a change to mouth breathing, posterior cricoarytenoid activity decreases, corresponding to a decrease in intratracheal pressure change (Figure 19b). When spontaneous breathing is then shunted through a tracheotomy (Figure 20a), abductor activity not only gradually diminishes but also completely disappears. When the tracheotomy is partially occluded and breathing in part resumed through the upper air passages, posterior cricoarytenoid activity slowly returns (Figure 20b). A week later, although abductor activity is absent with an open tracheotomy, minimal posterior cricoarytenoid activity can be elicited when the tracheostome is partially occluded to reestablished normal ventilating load (Figure 31).
At 4 weeks, as expected, no abductor activity can be recorded with an open tracheostome (Figure 32a). Repeated partial closure of the tracheostome at this time, however, produces no return in posterior cricoarytenoid activity (Figure 32b). Total stomal closure results in severe laryngeal inspiratory stridor and cyanosis, presumably owing to failure of phasic abductor activity in the posticus muscles.
The following findings help to explain the difficulty in tracheotomy decannulation when laryngeal abductor activity is temporarily lost. They may also provide insight into the posttracheotomy mechanism of fused vocal cords traumatized by previous endotracheal intubation. Such injury could lead to fusion of the denuded cordal surfaces apposed in the absence of phasic inspiratory abduction. In summary, the following findings are demonstrated:
- Phasic abductor activity diminishes as ventilatory load decreases.
- The elimination of all measurable ventilatory load results in complete cessation of laryngeal abductor activity within 3 to 5 minutes.
- The loss of abductor activity persists for as long as airway load remains severely reduced.
- Phasic abductor activity can be reestablished in 3 to 5 minutes by gradually increasing ventilatory resistance.
- The longer the duration of reduced resistance, the more difficulty is encountered in reestablishing abductor function once it is lost.
Therefore, although the effects of tracheotomy on the lower respiratory trach have been studied in great detail, little previous effort has been addressed to its influence on the upper respiratory system. It is apparent that tracheotomy bears a greater effect on upper respiratory function than previously appreciated.
Laryngeal Paralysis: Effects of Selective Laryngeal Denervation
The clinical effects of neurologic injuries to the larynx continue to intrigue and mystify laryngologists. Isolated recurrent nerve paralysis variably affects both voice and swallow functions. However, superior laryngeal nerve injuries produce even greater variability ranging from mild hoarseness to severe dysphagia, whereas combined superior and recurrent laryngeal nerve injuries seem to extract the highest price in vocal and swallow function in the collective experience of most clinicians.
In general, animal and human models of laryngeal denervation or anesthesia fail to completely clarify our clinical uncertainties,38, 39, 40 despite the general recognition that some neurologic injuries are capable of profoundly disrupting swallow function, resulting in life-threatening aspiration and pulmonary sepsis.41, 42 Furthermore, remedial surgery results in partial restoration of swallow function without our complete understanding of its basic mechanisms.
The following observations to improve our understanding of laryngeal swallow function measure neither upstream neurophysiologic mechanisms of neural coordination nor indirect effects of neural injury on vocal cord positioning. Rather, they describe direct measures of the closing force generated between the vocal cords in response to protective reflex glottic closure.43 The final product of laryngeal sphincter function, its glottic closing force, would appear to be a highly relevant component of protection against aspiration.
As noted, reflex glottic closure constitutes the primary mechanism for prevention of intra- and postdeglutative aspiration.44 Laryngeal paralysis, therefore, exerts a considerable impact on deglutition and yet little is understood regarding the biomechanical effects of selective denervation on laryngeal protective function. In our laboratory investigators measured the glottic closing force (GCF) (Figure 33) in each of six male, 40-kg Yorkshire pigs after selective unilateral (1) iSLN section, (2) recurrent laryngeal nerve (RLN) section, and (3) combined iSLN-RLN sections as both right and left iSLNs were simultaneously stimulated to evoke the glottic closure response.
Figure 33: Placement of pressure transducer.
Sensor tip (a) is secured at the midportion of the membranous vocal folds by suturing it to the thyroid cartilage with 3-0 Dexon (b). Vocal folds are fully exposed by anterior traction of the thyroid cartilage (c). (Source: Sasaki CT et al.43 with permission from Annals Publishing Co. )
The mean GCFs recorded in each protocol together with their standard deviations are shown in Table 6. The mean control GCF was 276.57 mmHg. UnilateraliSLN section reduced the mean GCF to 149.25 mmHg (range 133.36–162.96 mmHg). The additional sectioning of RLN creating conditions akin to high vagal paralysis, reduced the mean GCF further to 62.54 mmHg (38.67–76.54 mmHg). Finally, selective RLN section reduced the mean GCF to 58.45 mmHg (40.37–72.42 mmHg).
Assuming that control GCF represents the closing force under physiologically normal conditions, unilateral iSLN paralysis reduces GCF to 54.14% of control, whereas RLN and combined iSLN-RLN paralysis reduces it to 23.39% and 22.67% of control, respectively (Table 7).
Several observations are noteworthy. First, the reduction of glottic closing force to 54.14% of control when superior laryngeal nerve is unilaterally sectioned would not have been predicted by clinical observations alone. Few would have predicted that section of a predominantly afferent, that is sensory nerve, would or could affect the force of glottic closure.
In fact, most clinicians have observed that when a unilateral superior laryngeal nerve injury has occurred, stimulation of the contralateral intact superior laryngeal nerve continues to generate a bilateral glottic closure response resulting in a clinical observation belying the lost contribution of total active motor units involved in an apparently intact glottic closure reflex.
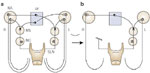
Indeed, biomechanical changes observed in this investigation support what is already known neurophysiologically. Each superior laryngeal nerve projects to its ipsilateral and contralateral nucleus ambiguus via the ipsilateral nucleus tractus solitarius, resulting in the combined recruitment of the motor neurons not only ipsilaterally, but also contralaterally (Figure 34).
Figure 34: Organizational model of the glottic closure reflex pathway demonstrating the effect of a unilateral SLN section.
(Source: Sasaki CT et al.43 with permission from Annals Publishing Co. )
In this organizational model injury to one iSLN takes out almost half of the active motor neurons (Figure 34). The 50% reduction in total motor units when one iSLN is lost supports the reduction of glottic closing force observed in the present investigation and is consistent with altered protective glottic closure following conservation laryngectomy or other forms resulting in isolated superior laryngeal nerve section or injury. Again, unilateral sensory denervation contributes to reflex glottic incompetence by a biomechanism other than a simple sensory field deficit that may reduce perception of bolus localization, volume, or consistency. Rather, unilateral superior laryngeal nerve injury actually reduces the force of reflex glottic closure to 54.14% of control.
This, in fact, may be the more dominant mechanism of laryngeal aspiration described but previously unexplained in both animal and human models of selective anesthesia or denervation. Of course, bilateral superior laryngeal nerve denervation totally abolishes reflex glottic closure, a consequence well known to all head and neck surgeons who routinely perform open supraglottic laryngectomy.
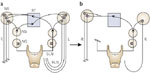
On the other hand, isolated recurrent laryngeal nerve section totally removes participation of one vocal cord in the generation of reflex glottic closure, not surprisingly resulting in a marked reduction of glottic closing force to 24% of control (Figure 35), corresponding to a clinical incidence of aspiration in 38% of patients with unilateral vocal cord movement impairment. Perhaps more interesting is the observation that glottic closing force generated by combined superior and recurrent nerve section measuring 23% of control is nearly equivalent to, if only modestly less than, a closing force generated by unilateral recurrent laryngeal nerve section, measuring 24% of control. This observation in a sense allows us to further explore the organizational configuration of each motor nucleus in the following way. If motor neurons of the nucleus ambiguus associated with the crossed reflex comprise an equal or greater number of motor units separate from those of the ipsilateral reflex, the total motor units participating in reflex vocal cord adduction would be markedly reduced when superior laryngeal and recurrent nerves are both unilaterally sectioned compared to recurrent nerve section alone (Figure 36). In this organizational diagram three remaining motor units are effectively reduced to one.
Figure 35: Organizational model demonstrating the effect of converting a unilateral recurrent laryngeal nerve (RLN) section (a) to a combined unilateral RLN-superior laryngeal nerve (SLN) section (b) when motor neurons involved ipsilaterally are exceeded by those contralaterally.
NA, nucleus ambiguus; NG, nodose ganglion; NS, nucleus solitarius; RF, reticular formation; L, left; R, right. (Source: Sasaki CT et al.43 with permission from Annals Publishing Co. )
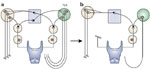
In fact, we observed the contrary. Glottic closing force from combined iSLN-RLN section is minimally less than that of the isolated RLN section alone, strongly suggesting an organizational configuration in which motor units functionally involved ipsilaterally likely outnumber those contralaterally (Figure 37). In this organizational model, three motor units are effectively reduced to two or more.
Conclusion
The present investigation underscores the profound biomechanical effects exerted by isolated or combined neurologic lesions while providing insight into the functional organizational configuration of a complex yet important protective brainstem reflex.
As our understanding of laryngeal function grows, it is hoped that our management of its dysfunction will be better understood in terms consistent with sound physiologic principles. The impact of Negus's1 masterful work over 50 years ago establishes the foundation of our present knowledge and provides us with the necessary guidance for future investigation—hence this selective review of a subject that should be of interest to focused inquiry within a greater clinical context.