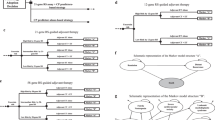
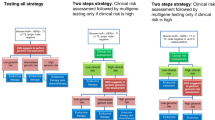
Abstract
We aimed to investigate the cost-effectiveness of a 2000-gene-expression profiling (GEP) test to help identify the primary tumor site when clinicopathological diagnostic evaluation was inconclusive in patients with cancer of unknown primary (CUP). We built a decision-analytic-model to project the lifetime clinical and economic consequences of different clinical management strategies for CUP. The model was parameterized using follow-up data from the Manitoba Cancer Registry, cost data from Manitoba Health administrative databases and secondary sources. The 2000-GEP-based strategy compared to current clinical practice resulted in an incremental cost-effectiveness ratio (ICER) of $44,151 per quality-adjusted life years (QALY) gained. The total annual-budget impact was $36.2 million per year. A value-of-information analysis revealed that the expected value of perfect information about the test’s clinical impact was $4.2 million per year. The 2000-GEP test should be considered for adoption in CUP. Field evaluations of the test are associated with a large societal benefit.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Canadian Cancer Society’s Advisory Committee on Cancer Statistics Canadian Cancer Statistics 2014. ON: Canadian Cancer Society: Toronto, 2014.
BC Cancer Agency. Cancer Management Guidelines http://www.bccancer.bc.ca/HPI/ Cancer Management Guidelines/ default.htm. Accessed August 14, 2014.
Greco FA, Oien K, Erlander M, Osborne R, Varadhachary G, Bridgewater J et al. Cancer of unknown primary: progress in the search for improved and rapid diagnosis leading toward superior patient outcomes. Ann Oncol 2012; 23: 298–304.
Dumur CI, Lyons-Weiler M, Sciulli C, Garrett CT, Schrijver I, Holley TK et al. Interlaboratory performance of a microarray-based gene expression test to determine tissue of origin in poorly differentiated and undifferentiated cancers. J Mol Diagn 2008; 10: 67–77.
Morris GJ, Greco FA, Hainsworth JD, Engstrom PF, Scialla S, Jordan WE 3rd et al. Cancer of unknown primary site. Semin Oncol 2010; 37: 71–79.
Greco FA . Therapy of adenocarcinoma of unknown primary: are we making progress? J Natl Compr Canc Netw 2008; 6: 1061–1067.
Abbruzzese JL, Abbruzzese MC, Lenzi R, Hess KR, Raber MN . Analysis of a diagnostic strategy for patients with suspected tumors of unknown origin. J Clin Oncol 1995; 13: 2094–2103.
Hainsworth JD, Rubin MS, Spigel DR, Boccia RV, Raby S, Quinn R et al. Molecular gene expression profiling to predict the tissue of origin and direct site-specific therapy in patients with carcinoma of unknown primary site: a prospective trial of the Sarah Cannon research institute. J Clin Oncol 2013; 31: 217–223.
Li X, Quigg RJ, Zhou J, Gu W, Nagesh Rao P, Reed EF . Clinical utility of microarrays: current status, existing challenges and future outlook. Curr Genomics 2008; 9: 466–474.
Hemminki K, Liu H, Heminki A, Sundquist J . Power and limits of modern cancer diagnostics: cancer of unknown primary. Ann Oncol 2012; 23: 760–764.
Varadhachary GR, Talantov D, Raber MN, Meng C, Hess KR, Jatkoe T et al. Molecular profiling of carcinoma of unknown primary and correlation with clinical evaluation. J Clin Oncol 2008; 26: 4442–4448.
Bloom G, Yang IV, Boulware D, Kwong KY, Coppola D, Eschrich S et al. Multi-platform, multi-site, microarray-based human tumor classification. Am J Pathol 2004; 164: 9–16.
Bridgewater J, van Laar R, Floore A, Van TVL . Gene expression profiling may improve diagnosis in patients with carcinoma of unknown primary. Br J Cancer 2008; 98: 1425–1430.
Buckhaults P, Zhang Z, Chen YC, Wang TL St, Croix B, Saha S et al. Identifying tumor origin using a gene expression-based classification map. Cancer Res 2003; 63: 4144–4149.
Horlings HM, van Laar RK, Kerst JM, Helgason HH, Wesseling J, van der Hoeven JJ et al. Gene expression profiling to identify the histogenetic origin of metastatic adenocarcinomas of unknown primary. J Clin Oncol 2008; 26: 4435–4441.
Ma XJ, Patel R, Wang X, Salunga R, Murage J, Desai R et al. Molecular classification of human cancers using a 92-gene real-time quantitative polymerase chain reaction assay. Arch Pathol Lab Med 2006; 130: 465–473.
Monzon FA, Lyons-Weiler M, Buturovic LJ, Rigl CT, Henner WD, Sciulli C et al. Multicenter validation of a 1,550-gene expression profile for identification of tumor tissue of origin. J Clin Oncol 2009; 27: 2503–2508.
Monzon FA, Koen TJ . Diagnosis of metastatic neoplasms: molecular approaches for identification of tissue of origin. Arch Pathol Lab Med 2010; 134: 216–224.
Rosenfeld N, Aharonov R, Meiri E, Rosenwald S, Spector Y, Zepeniuk M et al. MicroRNAs accurately identify cancer tissue origin. Nat Biotechnol 2008; 26: 462–469.
Su AI, Welsh JB, Sapinoso LM, Kern SG, Dimitrov P, Lapp H et al. Molecular classification of human carcinomas by use of gene expression signatures. Cancer Res 2001; 61: 7388–7393.
Talantov D, Baden J, Jatkoe T, Hahn K, Yu J, Rajpurohit Y et al. A quantitative reverse transcriptase-polymerase chain reaction assay to identify metastatic carcinoma tissue of origin. J Mol Diagn 2006; 8: 320–329.
van Laar RK, Ma XJ, de Jong D, Wehkamp D, Floore AN, Warmoes MO et al. Implementation of a novel microarray-based diagnostic test for cancer of unknown primary. Int J Cancer 2009; 125: 1390–1397.
Rosenwald S, Gilad S, Benjamin S, Lebanony D, Dromi N, Faerman A et al. Validation of a microRNA-based qRT-PCR test for accurate identification of tumor tissue origin. Mod Pathol 2010; 23: 814–823.
Pillai R, Deeter R, Rigl CT, Nystrom JS, Miller MH, Buturovic L et al. Validation and reproducibility of a microarray-based gene expression test for tumor identification in formalin-fixed, paraffin-embedded specimens. J Mol Diagn 2011; 13: 48–56.
U.S. FDA 510(k) Decision Summary for the Pathwork Tissue of Origin Test Kit – FFPE, May 17, 2012. http://www.accessdata.fda.gov/cdrh_docs/pdf12/K120489pdf. Accessed May 10, 2014.
Dumur CI, Fuller CE, Blevins TL, Schaum JC, Wilkinson DS, Garrett CT et al. Clinical verification of the performance of the pathwork tissue of origin test: utility and limitations. Am J Clin Pathol 2011; 136: 924–933.
Wu AH, Drees JC, Wang H, VandenBerg SR, Lal A, Henner WD et al. Gene expression profiles help identify the tissue of origin for metastatic brain cancers. Diagn Pathol 2010; 5: 26.
Monzon FA, Medeiros F, Lyons-Weiler M, Henner WD . Identification of tissue of origin in carcinoma of unknown primary with a microarray-based gene expression test. Diagn Pathol 2010; 5: 3.
The ResponseDX: Tissue of Origin Test. Response Genetics, Inc http://www.responsegenetics.com/products-services/tissue-of-origin-testing/. Accessed May 28, 2014.
Laupacis A, Feeny D, Detsky AS, Tugwell PX . How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ 1992; 146: 473–481.
Inadomi JM . Decision analysis and economic modelling: a primer. Eur J Gastroenterol Hepatol 2004; 16: 535–542.
Hainsworth JD, RP, Henner WD, Halks-Miller M, Lane C, Greco FA . Molecular Tumor Profiling in the Diagnosis of Patients with Carcinoma of Unknown Primary Site: Retrospective Evaluation of Gene Microarray Assay. J Mol Biomark Diagn 2011; 2: 2.
Greco FA, Spigel DR, Yardley DA, Erlander MG, Ma XJ, Hainsworth JD . Molecular profiling in unknown primary cancer: accuracy of tissue of origin prediction. Oncologist 2010; 15: 500–506.
Laupacis A . Economic evaluations in the canadian common drug review. Pharmacoeconomics 2006; 24: 1157–1162.
Sonnenberg FA, Beck JR . Markov models in medical decision making: a practical guide. Med Decis Making 1993; 13: 322–338.
Briggs AH, Ades AE, Price MJ . Probabilistic sensitivity analysis for decision trees with multiple branches: use of the Dirichlet distribution in a Bayesian framework. Med Decis Making 2003; 23: 341–350.
Hyphantis T, Papadimitriou I, Petrakis D, Fountzilas G, Repana D, Assimakopoulos K et al. Psychiatric manifestations, personality traits and health-related quality of life in cancer of unknown primary site. Psychooncology 2013; 22: 2009–2015.
Lloyd A, Nafees B, Narewska J, Dewilde S, Watkins J . Health state utilities for metastatic breast cancer. Br J Cancer 2006; 95: 683–690.
Tappenden P, Jones R, Paisley S, Carroll C . Systematic review and economic evaluation of bevacizumab and cetuximab for the treatment of metastatic colorectal cancer. Health Technol Assess 2007; 11: 1–128.
Spackman E, Rice S, Norman G, Suh DC, Eastwood A, Palmer S . Trastuzumab for the treatment of HER2-positive metastatic gastric cancer: a NICE single technology appraisal. Pharmacoeconomics 2013; 31: 185–194.
Chong CA, Gulamhussein A, Heathcote EJ, Lilly L, Sherman M, Naglie G et al. Health-state utilities and quality of life in hepatitis C patients. Am J Gastroenterol 2003; 98: 630–638.
Cella D, Li JZ, Cappelleri JC, Bushmakin A, Charbonneau C, Kim ST et al. Quality of life in patients with metastatic renal cell carcinoma treated with sunitinib or interferon alfa: results from a phase III randomized trial. J Clin Oncol 2008; 26: 3763–3769.
King SM, Bonaccorsi P, Bendeck S, Hadley J, Puttgen K, Kolm PG et al. Melanoma quality of life: pilot study using utility measurements. Arch Dermatol 2011; 147: 353–354.
Soini EJ, Martikainen JA, Nousiainen T . Treatment of follicular non-Hodgkin's lymphoma with or without rituximab: cost-effectiveness and value of information based on a 5-year follow-up. Ann Oncol 2011; 22: 1189–1197.
Trippoli S, Vaiani M, Lucioni C, Messori A . Quality of life and utility in patients with non-small cell lung cancer. Quality-of-life Study Group of the Master 2 Project in Pharmacoeconomics. Pharmacoeconomics 2001; 19: 855–863.
Dyer M, Richardson J, Robertson J, Adam J . NICE guidance on bevacizumab in combination with paclitaxel and carboplatin for the first-line treatment of advanced ovarian cancer. Lancet Oncol 2013; 14: 689–690.
Tam VC, Ko YJ, Mittmann N, Cheung MC, Kumar K, Hassan S et al. Cost-effectiveness of systemic therapies for metastatic pancreatic cancer. Curr Oncol 2013; 20: e90–e106.
Torvinen S, Farkkila N, Sintonen H, Saarto T, Roine RP, Taari K . Health-related quality of life in prostate cancer. Acta Oncol 2013; 52: 1094–1101.
Reichardt P, Leahy M, Garcia Del Muro X, Ferrari S, Martin J, Gelderblom H et al. Quality of Life and Utility in Patients with Metastatic Soft Tissue and Bone Sarcoma: The Sarcoma Treatment and Burden of Illness in North America and Europe (SABINE) Study. Sarcoma 2012; 2012: 740279.
Fossa SD, de Wit R, Roberts JT, Wilkinson PM, de Mulder PH, Mead GM et al. Quality of life in good prognosis patients with metastatic germ cell cancer: a prospective study of the European Organization for Research and Treatment of Cancer Genitourinary Group/Medical Research Council Testicular Cancer Study Group (30941/TE20). J Clin Oncol 2003; 21: 1107–1118.
Kim SH, Jo MW, Kim HJ, Ahn JH . Mapping EORTC QLQ-C30 onto EQ-5D for the assessment of cancer patients. Health Qual Life Outcomes 2012; 10: 151.
Singer S, Lincke T, Gamper E, Bhaskaran K, Schreiber S, Hinz A et al. Quality of life in patients with thyroid cancer compared with the general population. Thyroid 2012; 22: 117–124.
Mesia R, Rivera F, Kawecki A, Rottey S, Hitt R, Kienzer H et al. Quality of life of patients receiving platinum-based chemotherapy plus cetuximab first line for recurrent and/or metastatic squamous cell carcinoma of the head and neck. Ann Oncol 2010; 21: 1967–1973.
Gotay CC, Ransom S, Pagano IS . Quality of life in survivors of multiple primary cancers compared with cancer survivor controls. Cancer 2007; 110: 2101–2109.
van den Hout WB, van der Linden YM, Steenland E, Wiggenraad RG, Kievit J, e Haes H et al. dSingle- versus multiple-fraction radiotherapy in patients with painful bone metastases: cost-utility analysis based on a randomized trial. J Natl Cancer Inst 2003; 95: 222–229.
Pentheroudakis G, Greco FA, Pavlidis N . Molecular assignment of tissue of origin in cancer of unknown primary may not predict response to therapy or outcome: a systematic literature review. Cancer Treat Rev 2009; 35: 221–227.
McKenna C, Claxton K . Addressing adoption and research design decisions simultaneously: the role of value of sample information analysis. Med Decis Making 2011; 31: 853–865.
Schmidt C . Researchers consider value-of-information theory for selecting trials. J Natl Cancer Inst 2010; 102: 144–146.
Hannouf MB, Xie B, Brackstone M, Zaric GS . Cost-effectiveness of a 21-gene recurrence score assay versus Canadian clinical practice in women with early-stage estrogen- or progesterone-receptor-positive, axillary lymph-node negative breast cancer. BMC Cancer 2012; 12: 447.
Tsoi DT, Inoue M, Kelly CM, Verma S, Pritchard KI . Cost-effectiveness analysis of recurrence score-guided treatment using a 21-gene assay in early breast cancer. Oncologist 2010; 15: 457–465.
Muszbek N, Shah S, Carroll S, McDonald H, Dale P, Maroun J et al. Economic evaluation of sorafenib in the treatment of hepatocellular carcinoma in Canada. Curr Med Res Opin 2008; 24: 3559–3569.
Chabot I, Rocchi A . How do cost-effectiveness analyses inform reimbursement decisions for oncology medicines in Canada? The example of sunitinib for first-line treatment of metastatic renal cell carcinoma. Value Health 2010; 13: 837–845.
Rawson NSB (2013b). Potential impact of delayed access to five oncology drugs in Canada. Vancouver: Fraser Institute, November 2013.
de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010; 376: 1147–1154.
Cortes J, O'Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K et al. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet 2011; 377: 914–923.
Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007; 356: 2271–2281.
Butts C, Kamel-Reid S, Batist G, Chia S, Blanke C, Moore M et al. Benefits, issues, and recommendations for personalized medicine in oncology in Canada. Curr Oncol 2013; 20: e475–e483.
Laupacis A, Feeny D, Detsky AS, Tugwell PX . Tentative guidelines for using clinical and economic evaluations revisited. CMAJ 1993; 148: 927–929.
Hornberger J, Degtiar I, Gutierrez H, Shewade A, Henner WD, Becker S et al. Cost-effectiveness of gene-expression profiling for tumor-site origin. Value Health 2013; 16: 46–56.
Greco FA . The impact of molecular testing on treatment of cancer of unknown primary origin. Oncology (Williston Park) 2013; 27: 815–817.
Chiang WM, Kapadia M, Laver NV, Nystrom JS . Cancer of unknown primary: from immunohistochemistry to gene expression profiling. J Clin Oncol 2012; 30: e300–e302.
Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012; 366: 883–892.
Acknowledgements
We thank the Department of Epidemiology and Cancer Registry of CancerCare Manitoba and Manitoba Health, Healthy Living and Seniors for its support throughout the study. The results and conclusions are those of the authors, and no official endorsement by Manitoba Health, Healthy Living and Seniors is intended or should be inferred. This work was supported by the Canadian Institutes of Health Research (CIHR) [Operating Grant #231890; GSZ (PI)]; the CIHR Strategic Training Program in Cancer Research and Technology Transfer (CaRTT) and Academic Development Grant from Western University to MBH; the Canada Research Chairs program to GSZ, PKR and SMM; and the Great-West Life, London Life and Canada Life Junior Investigator of the Canadian Cancer Society [Grant # 2011-700644] to SMM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the The Pharmacogenomics Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Hannouf, M., Winquist, E., Mahmud, S. et al. Cost-effectiveness of using a gene expression profiling test to aid in identifying the primary tumour in patients with cancer of unknown primary. Pharmacogenomics J 17, 286–300 (2017). https://doi.org/10.1038/tpj.2015.94
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tpj.2015.94