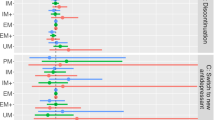
Abstract
Numerous studies have reported on pharmacogenetics of antidepressant response in depression. In contrast, little is known of response predictors in obsessive-compulsive disorder (OCD), a disorder with among the lowest proportion of responders to medication (40–60%). Our study is the largest investigation to date (N=184) of treatment response and side effects to antidepressants in OCD based on metabolizer status for CYP2D6 and CYP2C19. We observed significantly more failed medication trials in CYP2D6 non-extensive compared with extensive metabolizers (P=0.007). CYP2D6 metabolizer status was associated with side effects to venlafaxine (P=0.022). There were nonsignificant trends for association of CYP2D6 metabolizer status with response to fluoxetine (P=0.056) and of CYP2C19 metabolizer status with response to sertraline (P=0.064). Our study is the first to indicate that CYP genes may have a role in antidepressant response in OCD. More research is required for a future clinical application of genetic testing, which could lead to improved treatment outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ruscio AM, Stein DJ, Chiu WT, Kessler RC . The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry 2010; 15: 53–63.
APA. Practice Guideline for the Treatment of Patients with Obsessive-Compulsive Disorder. American Psychiatric Press, Inc.: Arlington, VA, USA, 2007.
Fineberg NA, Gale TM . Evidence-based pharmacotherapy of obsessive-compulsive disorder. Int J Neuropsychopharmacol 2005; 8: 107–129.
Jenike MA . Clinical practice. Obsessive-compulsive disorder. N Engl J Med 2004; 350: 259–265.
Pallanti S, Hollander E, Bienstock C, Koran L, Leckman J, Marazziti D et al. Treatment non-response in OCD: methodological issues and operational definitions. Int J Neuropsychopharmacol 2002; 5: 181–191.
Bloch MH, McGuire J, Landeros-Weisenberger A, Leckman JF, Pittenger C . Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry 2010; 15: 850–855.
Fineberg NA, Brown A, Reghunandanan S, Pampaloni I . Evidence-based pharmacotherapy of obsessive-compulsive disorder. Int J Neuropsychopharmacol 2012; 15: 1173–1191.
Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C et al. Brain activation of patients with obsessive-compulsive disorder during neuropsychological and symptom provocation tasks before and after symptom improvement: a functional magnetic resonance imaging study. Biol Psychiatry 2005; 57: 901–910.
Lazaro L, Caldu X, Junque C, Bargallo N, Andres S, Morer A et al. Cerebral activation in children and adolescents with obsessive-compulsive disorder before and after treatment: a functional MRI study. J Psychiatr Res 2008; 42: 1051–1059.
Saxena S, Rauch SL . Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatr Clin North Am 2000; 23: 563–586.
Pauls DL . The genetics of obsessive-compulsive disorder: a review. Dialogues Clin Neurosci 2010; 12: 149–163.
Porcelli S, Drago A, Fabbri C, Gibiino S, Calati R, Serretti A . Pharmacogenetics of antidepressant response. J Psychiatry Neurosci 2011; 36: 87–113.
Kato M, Serretti A . Review and meta-analysis of antidepressant pharmacogenetic findings in major depressive disorder. Mol Psychiatry 2010; 15: 473–500.
Horstmann S, Binder EB . Pharmacogenomics of antidepressant drugs. Pharmacol Ther 2009; 124: 57–73.
Gvozdic K, Brandl EJ, Taylor DL, Muller DJ . Genetics and personalized medicine in antidepressant treatment. Curr Pharm Des 2012; 18: 5853–5878.
Brandl EJ, Muller DJ, Richter MA . Pharmacogenetics of obsessive-compulsive disorders. Pharmacogenomics 2012; 13: 71–81.
Bertilsson L . Metabolism of antidepressant and neuroleptic drugs by cytochrome p450s: clinical and interethnic aspects. Clin Pharmacol Ther 2007; 82: 606–609.
Rudberg I, Hermann M, Refsum H, Molden E . Serum concentrations of sertraline and N-desmethyl sertraline in relation to CYP2C19 genotype in psychiatric patients. Eur J Clin Pharmacol 2008; 64: 1181–1188.
Sawamura K, Suzuki Y, Someya T . Effects of dosage and CYP2D6-mutated allele on plasma concentration of paroxetine. Eur J Clin Pharmacol 2004; 60: 553–557.
McAlpine DE, Biernacka JM, Mrazek DA, O’Kane DJ, Stevens SR, Langman LJ et al. Effect of cytochrome P450 enzyme polymorphisms on pharmacokinetics of venlafaxine. Ther Drug Monit 2011; 33: 14–20.
Suzuki Y, Sugai T, Fukui N, Watanabe J, Ono S, Inoue Y et al. CYP2D6 genotype and smoking influence fluvoxamine steady-state concentration in Japanese psychiatric patients: lessons for genotype-phenotype association study design in translational pharmacogenetics. J Psychopharmacol 2011; 25: 908–914.
Ueda M, Hirokane G, Morita S, Okawa M, Watanabe T, Akiyama K et al. The impact of CYP2D6 genotypes on the plasma concentration of paroxetine in Japanese psychiatric patients. Prog Neuropsychopharmacol Biol Psychiatry 2006; 30: 486–491.
Grasmader K, Verwohlt PL, Rietschel M, Dragicevic A, Muller M, Hiemke C et al. Impact of polymorphisms of cytochrome-P450 isoenzymes 2C9, 2C19 and 2D6 on plasma concentrations and clinical effects of antidepressants in a naturalistic clinical setting. Eur J Clin Pharmacol 2004; 60: 329–336.
Mihara K, Otani K, Tybring G, Dahl ML, Bertilsson L, Kaneko S . The CYP2D6 genotype and plasma concentrations of mianserin enantiomers in relation to therapeutic response to mianserin in depressed Japanese patients. J Clin Psychopharmacol 1997; 17: 467–471.
Schenk PW, van Fessem MA, Verploegh-Van Rij S, Mathot RA, van Gelder T, Vulto AG et al. Association of graded allele-specific changes in CYP2D6 function with imipramine dose requirement in a large group of depressed patients. Mol Psychiatry 2008; 13: 597–605.
Huezo-Diaz P, Perroud N, Spencer EP, Smith R, Sim S, Virding S et al. CYP2C19 genotype predicts steady state escitalopram concentration in GENDEP. J Psychopharmacol 2012; 26: 398–407.
Schenk PW, van Vliet M, Mathot RA, van Gelder T, Vulto AG, van Fessem MA et al. The CYP2C19*17 genotype is associated with lower imipramine plasma concentrations in a large group of depressed patients. Pharmacogenomics J 2010; 10: 219–225.
Van Nieuwerburgh FC, Denys DA, Westenberg HG, Deforce DL . Response to serotonin reuptake inhibitors in OCD is not influenced by common CYP2D6 polymorphisms. Int J Psychiatry Clin Pract 2009; 13: 345–348.
Müller DJ, Brandl EJ, Hwang R, Tiwari AK, Sturgess JE, Zai CC et al. The AmpliChip((R)) CYP450 Test and response to treatment in schizophrenia and obsessive compulsive disorder: A Pilot Study and focus on cases with abnormal CYP2D6 drug metabolism. Genet Test Mol Biomarkers 2012; 16: 897–903.
First MB, Spitzer RL, Gibbon M, Williams JBW . Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P). Biometrics Research, New York State Psychiatric Institute: New York, 2002.
Guy W . ECDEU Assessment Manual for Psychopharmacology-Revised (DHEW Publ No ADM 76-338). Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research Programs: Rockville, MD, USA, pp 218–222 1976.
Lahiri DK, Nurnberger JI . A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res 1991; 19: 5444.
Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS . The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther 2008; 83: 234–242.
Borges S, Desta Z, Jin Y, Faouzi A, Robarge JD, Philips S et al. Composite functional genetic and comedication CYP2D6 activity score in predicting tamoxifen drug exposure among breast cancer patients. J Clin Pharmacol 2010; 50: 450–458.
Tsai MH, Lin KM, Hsiao MC, Shen WW, Lu ML, Tang HS et al. Genetic polymorphisms of cytochrome P450 enzymes influence metabolism of the antidepressant escitalopram and treatment response. Pharmacogenomics 2010; 11: 537–546.
Mrazek DA, Biernacka JM, O’Kane DJ, Black JL, Cunningham JM, Drews MS et al. CYP2C19 variation and citalopram response. Pharmacogenet Genomics 2011; 21: 1–9.
Gex-Fabry M, Eap CB, Oneda B, Gervasoni N, Aubry JM, Bondolfi G et al. CYP2D6 and ABCB1 genetic variability: influence on paroxetine plasma level and therapeutic response. Ther Drug Monit 2008; 30: 474–482.
Rau T, Wohlleben G, Wuttke H, Thuerauf N, Lunkenheimer J, Lanczik M et al. CYP2D6 genotype: impact on adverse effects and nonresponse during treatment with antidepressants-a pilot study. Clin Pharmacol Ther 2004; 75: 386–393.
Kawanishi C, Lundgren S, Agren H, Bertilsson L . Increased incidence of CYP2D6 gene duplication in patients with persistent mood disorders: ultrarapid metabolism of antidepressants as a cause of nonresponse. A pilot study. Eur J Clin Pharmacol 2004; 59: 803–807.
Lobello KW, Preskorn SH, Guico-Pabia CJ, Jiang Q, Paul J, Nichols AI et al. Cytochrome P450 2D6 phenotype predicts antidepressant efficacy of venlafaxine: a secondary analysis of 4 studies in major depressive disorder. J Clin Psychiatry 2010; 71: 1482–1487.
Chou WH, Yan FX, de Leon J, Barnhill J, Rogers T, Cronin M et al. Extension of a pilot study: impact from the cytochrome P450 2D6 polymorphism on outcome and costs associated with severe mental illness. J Clin Psychopharmacol 2000; 20: 246–251.
Peters EJ, Slager SL, Kraft JB, Jenkins GD, Reinalda MS, McGrath PJ et al. Pharmacokinetic genes do not influence response or tolerance to citalopram in the STAR*D sample. PLoS One 2008; 3: e1872.
Steimer W, Zopf K, von Amelunxen S, Pfeiffer H, Bachofer J, Popp J et al. Amitriptyline or not, that is the question: pharmacogenetic testing of CYP2D6 and CYP2C19 identifies patients with low or high risk for side effects in amitriptyline therapy. Clin Chem 2005; 51: 376–385.
Serretti A, Calati R, Massat I, Linotte S, Kasper S, Lecrubier Y et al. Cytochrome P450 CYP1A2, CYP2C9, CYP2C19 and CYP2D6 genes are not associated with response and remission in a sample of depressive patients. Int Clin Psychopharmacol 2009; 24: 250–256.
Murphy GM, Kremer C, Rodrigues HE, Schatzberg AF . Pharmacogenetics of antidepressant medication intolerance. Am J Psychiatry 2003; 160: 1830–1835.
Shams ME, Arneth B, Hiemke C, Dragicevic A, Muller MJ, Kaiser R et al. CYP2D6 polymorphism and clinical effect of the antidepressant venlafaxine. J Clin Pharm Ther 2006; 31: 493–502.
Parker G, Rowe M, Mehta F, Kumar S . Will a new genotyping test help the clinician predict response to antidepressant drugs? Australas Psychiat 2010; 18: 413–416.
Gerstenberg G, Aoshima T, Fukasawa T, Yoshida K, Takahashi H, Higuchi H et al. Effects of the CYP 2D6 genotype and cigarette smoking on the steady-state plasma concentrations of fluvoxamine and its major metabolite fluvoxamino acid in Japanese depressed patients. Ther Drug Monit 2003; 25: 463–468.
Zourkova A, Ceskova E, Hadasova E, Ravcukova B . Links among paroxetine-induced sexual dysfunctions, gender, and CYP2D6 activity. J Sex Marital Ther 2007; 33: 343–355.
Bouchez J, Dumur V, Lhermitte M, Goudemand M . Genotypes of cytochrome P450 and clinical response to clomipramine in patients with major depression. Eur Psychiat 1995; 10: 410–412.
Spina E, Gitto C, Avenoso A, Campo GM, Caputi AP, Perucca E . Relationship between plasma desipramine levels, CYP2D6 phenotype and clinical response to desipramine: a prospective study. Eur J Clin Pharmacol 1997; 51: 395–398.
McAlpine DE, O’Kane DJ, Black JL, Mrazek DA . Cytochrome P450 2D6 genotype variation and venlafaxine dosage. Mayo Clin Proc 2007; 82: 1065–1068.
Bijl MJ, Visser LE, Hofman A, Vulto AG, van Gelder T, Stricker BH et al. Influence of the CYP2D6*4 polymorphism on dose, switching and discontinuation of antidepressants. Br J Clin Pharmacol 2008; 65: 558–564.
Kwadijk-de Gijsel S, Bijl MJ, Visser LE, van Schaik RH, Hofman A, Vulto AG et al. Variation in the CYP2D6 gene is associated with a lower serum sodium concentration in patients on antidepressants. Br J Clin Pharmacol 2009; 68: 221–225.
Vandel P, Haffen E, Vandel S, Bonin B, Nezelof S, Sechter D et al. Drug extrapyramidal side effects. CYP2D6 genotypes and phenotypes. Eur J Clin Pharmacol 1999; 55: 659–665.
Allgulander C, Nilsson B . A prospective study of 86 new patients with social anxiety disorder. Acta Psychiatr Scand 2001; 103: 447–452.
Chen S, Chou WH, Blouin RA, Mao Z, Humphries LL, Meek QC et al. The cytochrome P450 2D6 (CYP2D6) enzyme polymorphism: screening costs and influence on clinical outcomes in psychiatry. Clin Pharmacol Ther 1996; 60: 522–534.
Suzuki Y, Sawamura K, Someya T . Polymorphisms in the 5-hydroxytryptamine 2A receptor and CytochromeP4502D6 genes synergistically predict fluvoxamine-induced side effects in japanese depressed patients. Neuropsychopharmacology 2006; 31: 825–831.
Ramaekers JG, Conen S, de Kam PJ, Braat S, Peeters P, Theunissen EL et al. Residual effects of esmirtazapine on actual driving performance: overall findings and an exploratory analysis into the role of CYP2D6 phenotype. Psychopharmacology (Berl) 2011; 215: 321–332.
Roberts RL, Luty SE, Mulder RT, Joyce PR, Kennedy MA . Association between cytochrome P450 2D6 genotype and harm avoidance. Am J Med Genet B Neuropsychiatr Genet 2004; 127B: 90–93.
Hedenmalm K, Guzey C, Dahl ML, Yue QY, Spigset O . Risk factors for extrapyramidal symptoms during treatment with selective serotonin reuptake inhibitors, including cytochrome P-450 enzyme, and serotonin and dopamine transporter and receptor polymorphisms. J Clin Psychopharmacol 2006; 26: 192–197.
Nichols AI, Lobello K, Guico-Pabia CJ, Paul J, Preskorn SH . Venlafaxine metabolism as a marker of cytochrome P450 enzyme 2D6 metabolizer status. J Clin Psychopharmacol 2009; 29: 383–386.
Veefkind AH, Haffmans PM, Hoencamp E . Venlafaxine serum levels and CYP2D6 genotype. Ther Drug Monit 2000; 22: 202–208.
Whyte EM, Romkes M, Mulsant BH, Kirshne MA, Begley AE, Reynolds CF et al. CYP2D6 genotype and venlafaxine-XR concentrations in depressed elderly. Int J Geriatr Psychiat 2006; 21: 542–549.
Morinobu S, Tanaka T, Kawakatsu S, Totsuka S, Koyama E, Chiba K et al. Effects of genetic defects in the CYP2C19 gene on the N-demethylation of imipramine, and clinical outcome of imipramine therapy. Psychiat Clin Neurosci 1997; 51: 253–257.
D’Empaire I, Guico-Pabia CJ, Preskorn SH . Antidepressant treatment and altered CYP2D6 activity: are pharmacokinetic variations clinically relevant? J Psychiatr Pract 2011; 17: 330–339.
Perlis RH, Patrick A, Smoller JW, Wang PS . When is pharmacogenetic testing for antidepressant response ready for the clinic? A cost-effectiveness analysis based on data from the STAR*D study. Neuropsychopharmacology 2009; 34: 2227–2236.
Vetti HH, Molven A, Eliassen AK, Steen VM . Is pharmacogenetic CYP2D6 testing useful? Tidsskr Nor Laegeforen 2010; 130: 2224–2228.
Penas-Lledo EM, Dorado P, Aguera Z, Gratacos M, Estivill X, Fernandez-Aranda F et al. CYP2D6 polymorphism in patients with eating disorders. Pharmacogenomics J 2012; 12: 173–175.
Zackrisson AL, Lindblom B, Ahlner J . High frequency of occurrence of CYP2D6 gene duplication/multiduplication indicating ultrarapid metabolism among suicide cases. Clin Pharmacol Ther 2010; 88: 354–359.
Kirchheiner J, Seeringer A, Godoy AL, Ohmle B, Maier C, Beschoner P et al. CYP2D6 in the brain: genotype effects on resting brain perfusion. Mol Psychiatry 2011; 16: 333–341.
Stingl JC, Brockmoller J, Viviani R . Genetic variability of drug-metabolizing enzymes: the dual impact on psychiatric therapy and regulation of brain function. Mol Psychiatry 2013; 18: 273–287.
Acknowledgements
We thank Dr Andrea Gaedigk for her advice regarding the use of the activity score. AKT: NARSAD Young Investigator Award. DJM: Ministry of Research and Innovation of Ontario Early Researcher Award; CIHR operating grant, NARSAD Young Investigator Award, CIHR Michael Smith New Investigator Salary Prize for Research in Schizophrenia, OMHF New Investigator Fellowship. JLK: CIHR operating grant. MAR: Ontario Mental Health Foundation grant, and International OCD Foundation grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
JLK has been a consultant to GSK, Sanofi-Aventis and Dainippon-Sumitomo. All other authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the The Pharmacogenomics Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Brandl, E., Tiwari, A., Zhou, X. et al. Influence of CYP2D6 and CYP2C19 gene variants on antidepressant response in obsessive-compulsive disorder. Pharmacogenomics J 14, 176–181 (2014). https://doi.org/10.1038/tpj.2013.12
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tpj.2013.12
Keywords
This article is cited by
-
A cross-sectional study of the relationship between CYP2D6 and CYP2C19 variations and depression symptoms, for women taking SSRIs during pregnancy
Archives of Women's Mental Health (2022)
-
Global genetic variation of select opiate metabolism genes in self-reported healthy individuals
The Pharmacogenomics Journal (2018)
-
Pharmacogenetics and Imaging–Pharmacogenetics of Antidepressant Response: Towards Translational Strategies
CNS Drugs (2016)
-
Whole-genome association analysis of treatment response in obsessive-compulsive disorder
Molecular Psychiatry (2016)