Abstract
Neuregulin-1 (NRG1) and ErbB receptors have been associated with psychopathology, and NRG1-ErbB3 signaling has been shown to increase hippocampal neurogenesis and induce antidepressant-like effects. In this study, we aimed to determine whether deficits in NRG1 or ErbBs might be present in the hippocampus of suicide completers. In well-characterized postmortem hippocampal samples from suicides and matched sudden-death controls, we assessed gene expression and methylation using qRT-PCR and EpiTYPER, respectively. Moreover, in hippocampal tissues stained with cresyl violet, stereology was used to quantify numbers of granule cells and of glia. Granule cell body size was examined with a nucleator probe, and granule cell layer volume with a Cavalieri probe. Unmedicated suicides showed sharply decreased hippocampal ErbB3 expression and decreased numbers of ErbB3-expressing granule cell neurons in the anterior dentate gyrus; a phenomenon seemingly reversed by antidepressant treatment. Furthermore, we found ErbB3 expression to be significantly decreased in the dentate gyrus of adult mice exposed to chronic social defeat stress. Taken together, these results reveal novel suicidal endophenotypes in the hippocampus, as well as a putative etiological mechanism underlying suicidality, and suggest that antidepressant or NRG1 treatment may reverse a potential deficit in anterior dentate gyrus granule cell neurons in individuals at risk of dying by suicide.
Similar content being viewed by others
Introduction
Suicide is the leading cause of injury mortality in the United States.1 However, our understanding of suicidal etiology remains limited. Although studies have reported genetic and epigenetic associations with suicidality,2 a clear etiological mechanism has yet to emerge.
Neuregulin-1 (NRG1) has been associated with hippocampal plasticity, affective behavior and psychopathology in multiple human populations and animal models. NRG1 administration in adult mice rapidly induces cell proliferation in the ventral dentate gyrus (DG), leading to increased ventral DG neurogenesis,3 adding new granule cell (GC) neurons to the DG. This is accompanied by antidepressant-like behavior when these cells are functional hyperplastic neurons.3, 4 Neurogenic and affective effects of NRG1 may be mediated through activation of the NRG1 receptor ErbB3, which is expressed by neurogenic cells from division to functional maturity. Intriguingly, we have recently found that NRG1 administration selectively increases ErbB3 phosphorylation in the ventral DG, supporting the role of this subregion and signaling pathway in affective regulation.5 Conversely, ErbB4, the other primary NRG1 receptor to which the ligand binds directly, is not detectable in early neurogenic cells.3 Altering NRG1 activity also changes behavior in tasks modeling schizophrenia, anxiety and antidepressant response, and disrupts behavioral and hypothalamic-pituitary-adrenal (HPA) axis response to stress.3, 6, 7, 8 Notably, NRG1 readily crosses the blood-brain barrier and affects brain activity.3, 9
In humans, genetic association studies have linked NRG1 with schizophrenia and bipolar disorder.10 Postmortem investigations showed altered NRG1 cleavage in the prefrontal cortex in schizophrenia, decreased density of NRG1-expressing neurons in schizophrenia and major depressive disorder (MDD), and reduced hippocampal NRG1 expression in schizophrenia and bipolar disorder.11, 12 ErbB receptors have also been associated with psychiatric illness, particularly in the prefrontal and temporal cortices.13, 14 However, to date the potential role of NRG1/ErbB signaling in the hippocampus in suicide completers is unknown. Hippocampal neurogenesis has been strongly implicated in antidepressant response, stress response, and affective behavior.15 In addition, deficits in hippocampal neurogenesis may precipitate vulnerability to stress and psychopathology, and chronic stress reduces neurogenesis and induces depressive-like behavioral phenotypes.15 As a result, the investigation of the human DG and GCs has become of great interest for the field, and the few studies published so far on samples from psychiatric populations have provided fundamental psychopathological insight. In particular, recent postmortem studies from Boldrini and colleagues have suggested that antidepressants increase numbers of neural progenitor cells in depressed patients, and indicated that GCs are decreased in the anterior and mid DG of untreated MDD patients.16, 17, 18 However, GC numbers in suicide completers compared to controls have yet to be thoroughly assessed.
In the present study, we examined NRG1/ErbB expression in the hippocampus of suicide completers and controls, expecting particularly that ErbB3 would be decreased in these psychiatric cases given the role of ErbB3 in modulating hippocampal neuroplasticity and affective behavior,3, 5 and found a major downregulation of ErbB3 in suicides. As ErbB3 is ubiquitously expressed by GCs in the DG, we quantified these cells and found a strong reduction in their number in the anterior DG for unmedicated (but not medicated) suicides. Finally, we showed that chronic stress, which reduces production of GCs and induces depressive phenotypes, is sufficient to reduce DG ErbB3 expression. Together, these results identify a novel suicidal endophenotype in the reduction of hippocampal ErbB3, as well as a putative mechanism for this effect, in that fewer ErbB3-expressing GCs are present in the anterior DG, potentially as a consequence of chronic stress.
Materials and methods
Postmortem hippocampal samples
This study was approved by the Douglas Mental Health University Institute’s Institutional Review Board, and postmortem hippocampal samples from suicides and matched sudden-death controls (who had not been diagnosed primarily with a psychiatric disorder apart from substance use issues, nor had died by suicide), having undergone psychological autopsy assessments,19 were obtained from the Douglas-Bell Canada Brain Bank (Verdun, QC, Canada). Medication status was obtained from toxicological assay and medication history. For all quantitative human postmortem experiments, potentially confounding covariables were controlled by matching subjects by age, postmortem interval (PMI), and brain pH, such that overall groups did not differ statistically for these parameters (Supplementary Table S1). Detailed subject information is presented in Supplementary Table S1.
Gene expression and methylation
RNA was extracted from frozen hippocampal samples (49 suicides, 14 controls) using RNeasy kits (Qiagen, Toronto, ON, Canada), and cDNA produced using oligo dT primers. Amplification and quantification used quantitative reverse transcription PCR (qRT-PCR; Life Technologies, Carlsbad, CA, USA; 7900HD) and TaqMan probes (Hs00247620_m1, Hs01076078_m1, Hs01001580_m1, Hs00176538_m1, Hs00955525_m1, cat. #4310884E). Expression values are mean quantities of sample replicates normalized to GAPDH.
For epigenetic analyses, DNA was extracted (31 suicides, 9 controls), then bisulfite-converted using an EpiTect Bisulfite kit (Qiagen), and samples were processed for EpiTYPER (Sequenom, San Diego, CA, USA) quantification at Genome Quebec (Montreal, QC, Canada). Measuring ErbB3 methylation, CpGs were analyzed in 3 500 bp regions (spanning from positions 56472794-56474049 on chromosome 12) within the promoter of the ErbB3 gene. Data are represented as methylation percentage, with missing values calculated to allow mixed-model analysis.
ErbB3 immunohistochemistry
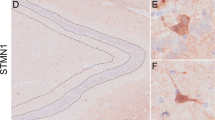
Wash buffer (Dako, Agilent, Mississauga, ON, Canada) washes were performed between all steps except between blocking and primary antibody addition. On the basis of a previously established ErbB3 IHC protocol,3 paraffin-embedded tissue from two control subjects (a 60-year-old male with a postmortem interval of 7.25 h, and a 74-year-old female with a postmortem interval of 11 h) was dewaxed and rehydrated. Tissue was incubated in a proteinase-K solution (1:1000; Qiagen) for 10 min, phosphate-buffered saline (PBS)-containing 0.2% Triton X-100 (Fisher Scientific, Ottawa, ON, Canada; PBS-T) for 1 h, 3% H2O2 for 10 min, blocking solution (2% normal goat serum (Vector Labs, Burlingame, CA, USA) in wash buffer) for 1 h, then overnight at 4 °C in blocking solution with rabbit anti-ErbB3 antibody (1:25-100, Santa Cruz Biotechnology, Dallas TX, USA; SC-285), an antibody that has been validated previously, and whose DG GC staining matches separately-produced antibodies.3, 20, 21 Tissue was then incubated in biotinylated goat anti-rabbit secondary antibody (1:500; Vector) in blocking solution for 90 min, and labeling was revealed using the Vectastain Elite and DAB kits (Vector), dehydrated, then coverslipped using Permount (Fisher). No-primary control did not produce staining. Sections were visualized on an Olympus BX51 microscope. To confirm our results, human ErbB3 immunohistochemistry data were also obtained from the Human Protein Atlas for a rabbit anti-ErbB3 antibody (HPA045396) with a demonstrated high degree of specificity.22, 23 In addition, ErbB3 in situ hybridization data (which had been verified with positive and negative controls) were obtained from the Allen Human Brain Atlas (http://human.brain-map.org/ish/search).24, 25
Stereological quantifications of hippocampal granule cells
Fixed hippocampal samples from 14 suicides and 9 controls were immersed in 30% sucrose until sinking, flash-frozen in isopentane (~−45 °C), sliced coronally at 50 μm on a freezing sliding microtome in PBS, placed on a shaker for 1 h, then transferred to a cryopreservative solution (glycerol : ethylene glycol : PBS, 3 : 3 : 4) for storage at −20 °C. Slices were mounted in PBS-T, and after drying were rehydrated in dH2O, stained with a heated cresyl violet solution for three minutes, rinsed in dH2O for 5 min, then dehydrated and coverslipped. DGs were carefully traced tightly around the GC layer, to assess population and volume as accurately as possible. The hippocampal head was considered as the anterior hippocampus.26 GCs and glia were distinguished as described previously.27, 28 Stereology, cell body measurement, and volume assessment were performed using StereoInvestigator software (MBF Bioscience, Williston, VT, USA). Stereology parameters were: counting frame: 30 μm × 30 μm; grid size: 350 μm × 350 μm; dissector height: 10 μm; guard zone height: 3 μm; measuring thickness at each site. GC bodies were assessed with the nucleator probe (eight-ray isotropic uniform random sections). Volume was assessed using the Cavalieri estimator (grid spacing: 20 μm) corrected for overprojection. Population and volumetric data were averaged across sections to account for variation in number of sections. The microscope was parcentric/parfocal and lens grid tune calibrated.
Hippocampal ErbB3 expression in mice exposed to chronic social defeat stress
Adult (~P60) male C57Bl/6 mice (Charles River, QC, Canada) were subjected to social defeat stress following an established protocol.29 Animal experiments were in accordance with the guidelines and policies of the Canadian Council on Animal Care, and approved by McGill University's Animal Care Committee. Briefly, defeated mice (n=12) were exposed to daily 5 min physical interactions with an aggressive male CD1 mouse (~P60; Charles River) during 10 consecutive days. CD1 mice were otherwise housed individually and had been previously screened for 3d to verify aggressive behavior. Defeated mice remained in the home cage of the aggressor mouse for 24 h post-defeat, separated physically by a perforated plexiglass divider, and were rotated between CD1 aggressors for each session. Control undefeated mice (n=6) were pair-housed, separated by a plexiglass divider. Animals were decapitated, and brains were frozen in −40 °C isopentane and stored at −80 °C. In brief, brains were placed in a cryostat at −20 °C and separated into serial 1 mm-thick sections using a mouse brain matrix and razor blades. 0.5 mm diameter micropunches were obtained from the dorsal and ventral DG bilaterally, and stored at −80 °C until use. To measure ErbB3 expression in the DG, RNA was extracted from micropunches using RNeasy Micro (Qiagen) kits with elution in RNAse-free water. RNA was assessed using a NanoDrop 1000 system (Thermo Scientific, Waltham, MA, USA). cDNA was synthesized with oligo dT primers. Messenger RNA (mRNA) was measured by qRT-PCR using TaqMan probes (Life Technologies; Mm01159999_m1, Mm99999915_g1) for ErbB3 and GAPDH, including water and RNA-free control wells that did not give a positive signal. Expression values as shown are mean relative fold change quantities of sample replicates normalized to GAPDH.
Statistical analyses
Statistical tests were two-tailed. Pairwise comparisons were made by Mann–Whitney or t-tests with Welch’s correction when required. Social defeat expression data were analyzed by two-way mixed-model ANOVA. Three-group analyses were performed by Kruskal–Wallis or ANOVA with Fisher’s post hoc tests. Timepoint/CpG × group analyses were performed with two-way mixed-model ANOVAs and Sidak post hoc tests. Experimental group identity was blinded when applicable. Statistical outliers were identified by Grubbs’ tests and removed. P-values ⩽0.05 were considered significant. Data are presented as mean±s.e.m., and numbers in bars represent sample sizes for those subjects included in analyses.
Results
Hippocampal ErbB3 expression is strongly decreased in suicide completers
Hippocampal NRG1 expression (assessed by qRT-PCR) was unchanged in suicide completers (Figure 1a). However, ErbB3 expression was markedly decreased in suicide completers compared to controls (Figure 1d; F(2,53)=4.39, P=0.017; unmedicated by antidepressants t(53)=2.80, P=0.0071; medicated by antidepressants t(53)=2.37, P=0.021; pooled suicides vs controlst=1.78, P<0.05), whereas ErbB1 (EGFR; Figure 1b), ErbB2 (Figure 1c), and ErbB4 (Figure 1e) expression were unchanged between groups. Suicide completers with a diagnosis of MDD tended to have higher ErbB3 expression than non-MDD suicide completers, but this did not reach statistical significance (U=145; P=0.060). As five of the suicide completers had taken antipsychotic medication, we compared ErbB3 expression in these subjects to suicide completers who had not taken antidepressants or antipsychotics, and found that antipsychotics did not induce significantly altered ErbB3 expression between these groups (t(34)=0.37, P=0.72).
Hippocampal and peripheral NRG1/ErbB gene expression in cases and controls. Hippocampal expression of NRG1 (a), ErbB1 (EGFR; b), ErbB2 (c), and ErbB4 (e) does not differ between controls and suicides regardless of antidepressant treatment; however, ErbB3 expression (d) is reduced. Reduced hippocampal expression of ErbB3 in suicides is not due to changes in ErbB3 methylation (f), as unmedicated suicides did not differ from controls in terms of CpG methylation. However, antidepressant usage was associated with increased methylation in a cluster of CpGs located around the ErbB3 promoter region near the transcription start site, in an area enriched in transcription factor binding sites. Numbers in bars denote numbers of subjects included in analysis. *P<0.05; **P<0.01; ****P<0.0001. AD, antidepressant; CTRL, control.
Decreased ErbB3 expression is not due to changes in gene methylation
To explore the mechanism underlying the decreased hippocampal ErbB3 expression observed in suicide completers, we next sought to determine whether epigenetic modifications to ErbB3, in particular differential methylation, could underlie this deficit. We found no overall group differences in hippocampal ErbB3 methylation, as assessed by EpiTYPER. However, there was a group × CpG interaction, in that a cluster of CpGs in the third fragment, surrounding the transcription start site, was hypermethylated in suicides who had taken antidepressants (F(34,626)=2.04, P=0.0006; CpG 22–24 ps<0.0001; CpG 25-28 t(663)=3.41, P=0.0021 vs unmedicated suicides, t(663)=2.59, P=0.029 vs controls; Figure 1f).
ErbB3 is ubiquitously expressed in human DG granule cells
We recently reported that ErbB3 is ubiquitously expressed in murine DG GCs, and sought to confirm in the human DG this expression pattern, which has been previously mentioned in a study focused on Alzheimer's disease.30 We indeed found a similar distribution in humans, with strong and ubiquitous ErbB3 expression associated with GCs throughout the GC layer. This was evident in our immunohistochemical experiments (Supplementary Figure S1A) as well as in open-access in situ hybridization data (Supplementary Figure S1C and D). These observations were further supported by data from the Human Protein Atlas22, 23 (Supplementary Figure S1B).
Quantification of DG granule cells in suicides
As decreased hippocampal ErbB3 in suicides was not attributable to changes in gene methylation, and given the expression pattern of ErbB3 in the human DG, we hypothesized that a loss of GCs may account for this phenomenon, and tested this hypothesis through detailed tracing and stereology in human DG samples (Supplementary Figure S2A and B). Stereology, cell body nucleator, and Cavalieri coefficients of error (CEs) were low (mean optical fractionator CE=0.045; mean nucleator CE=0.0066; mean Cavalieri CE=0.028).
Number of GCs was sharply and selectively decreased in the anterior DG for unmedicated suicides compared to controls (F(2,10)=5.18, P=0.029; t(10)=2.78, P=0.020; Figure 2a, Supplementary Figure S3A and B) and medicated suicides (t(10)=2.85, P=0.017), with the latter not differing from controls (t(10)=0.23, P=0.83). Similarly, GC layer volume was selectively reduced in the anterior DG for unmedicated suicides relative to controls (F(2,10)=4.50, P=0.04; t(10)=2.70, P=0.022; Figure 2b, Supplementary Figure S3C and D) and medicated suicides (t(10)=2.53, P=0.030; controls vs medicated suicides t(10)=0.036, P=0.97). Neuronal density was unchanged between groups (Figure 2c, Supplementary Figure S3E and F). Neuronal cell body size was increased in the posterior DG of suicides (t(9)=2.54; P=0.032; Figure 2d), but not in the anterior or overall (Supplementary Figure S3G and H) DG. Number of glial cells did not differ between groups overall (Supplementary Figure S3I) or in the posterior DG (Supplementary Figure S3J), but were altered in the anterior DG (F(2,10)=4.32; P=0.045; Figure 2e), with decreased glial cells in unmedicated suicides vs controls (t(10)=2.91, P=0.016). Glial density did not differ between groups, either overall or in the anterior or posterior DG (Supplementary Figure S3K–M). Neuron-glial ratio did not differ, either overall (Figure 2f) or in the anterior (Supplementary Figure S3N) or posterior (Supplementary Figure S3O) DG.
Assessment of granule cells, glia, and granule cell layer in dentate gyrus (DG) of suicides and controls. Unmedicated suicides have decreased numbers of granule cells in the anterior DG (a), as well as decreased volume in the anterior DG (b). Granule cell neuronal density was unchanged in the anterior DG (c). Granule cell body size was increased in suicides in the posterior DG (d). Number of glial cells was decreased for unmedicated suicides compared to controls in the anterior DG (e). Ratio of granule cell neurons to glia in the overall DG did not differ between groups (f). *P<0.05. AD, antidepressant.
DG ErbB3 expression is significantly decreased following chronic social defeat stress
Given that chronic stress has been suggested to reduce the production of GCs and induce depressive phenotypes,15 we examined whether hippocampal ErbB3 expression (assessed by qRT-PCR) is reduced in an animal chronic stress model. Mice subjected to chronic social defeat stress displayed a significant reduction in the expression of DG ErbB3 compared to unstressed mice (main effect of experimental condition F(1,16)=6.16; P=0.025; Figure 3).
Discussion
To our knowledge, our results are the first to associate NRG1-ErbB3 with suicidality, revealing disrupted hippocampal ErbB3 in suicide completers, likely owing to deficits in DG GCs in unmedicated suicides. ErbB3 has previously been associated with multiple psychiatric conditions.13, 31, 32, 33, 34 Although previously considered to be kinase-insufficient (c.f. Cao et al.35) based largely on a report of reduced autophosphorylation compared to EGFR in insect cells with induced ErbB3 expression,36 recent studies have suggested that ErbB3 has sufficient kinase activity for signaling,37, 38, 39 and its downstream signaling primarily involves PI3K/AKT.40 ErbB3 has also recently been found to be required for certain types of cell proliferation in the brain, likely due to downstream PI3K signaling.40 Peripheral NRG1 administration (shown to be safe in humans41, 42) increases neurogenesis, producing additional GCs expressing ErbB3, with concomitant antidepressant-like effects.3 As such, this represents a promising putative psychiatric therapeutic target, particularly for patients with a suicidal phenotype.
That DG ErbB3 is decreased by chronic stress inversely mirrors the antidepressant-like effects of augmented NRG1-ErbB3 signaling,3 suggesting that affective regulation by hippocampal NRG1-ErbB3 may be bidirectional. Given the expression of ErbB3 in neurogenic and mature GCs, this finding also aligns with previous studies showing that a rodent chronic stress model of depression reduces DG neurogenesis across the septotemporal axis.43
Although our epigenetic analysis reveals that decreased hippocampal ErbB3 expression in suicides is likely not related to differential methylation, we found in antidepressant-medicated individuals a cluster of differentially methylated CpGs located around the transcription start site of the ErbB3 gene, in a region enriched in transcription factor binding sites. Though preliminary, this potentially highlights a mechanism for the positive effects of antidepressants on neurogenesis, especially as increased antidepressant-mediated gene methylation has been shown in previous studies to mediate antidepressant effects.44 Future investigation could determine if methylation at these sites affects the response of neural progenitor cells to antidepressant medication.
The decrease in hippocampal ErbB3 can plausibly be attributed to a loss of GCs in the anterior DG, particularly in unmedicated suicides, as we (and others3, 23, 24, 30) show that these cells ubiquitously express ErbB3. These results are also in line with previous studies showing decreased numbers of GCs and neural progenitors in the anterior hippocampus in postmortem samples from individuals with MDD, as well as increased numbers of mitotic cells in this subregion with antidepressant treatment.16, 17, 18 The anatomical specificity of the effects is noteworthy, as the anterior hippocampus is more associated with emotional modulation45 and, in non-human primates, depression-like behaviors are inversely correlated with anterior DG neurogenesis.46 This subregion is analogous to the ventral hippocampus in rodents, which has been associated with affective regulation.47 More specifically, the ventral DG and DG neurogenesis in particular have been associated with response to stress and antidepressants, as well as affective behavior.15 It is also the selective subregion in which NRG1 disruption leads to decreased communication efficacy with the nucleus accumbens,48 and in which increased ErbB3 phosphorylation and neurogenesis occurs following NRG1 administration.3, 5 Thus it seems that chronic stress or other deleterious factors may contribute to suicidality by reducing the number of ErbB3-expressing GCs in this emotion-related subregion of the hippocampus, whereas this endophenotype is reversed by administration of antidepressants (and potentially NRG1). This could be supported by subsequent validation including psychiatric non-suicides, to verify whether the association between hippocampal ErbB3 and psychopathology is specifically relevant to suicidality. The mechanism by which reduced numbers of GCs could contribute etiologically to suicidality may also be stress-related, as DG GCs inhibit HPA axis activity, and in their absence response to stress is augmented, further reducing numbers of GCs and exacerbating other effects of stress,15 although notably recovery of numbers of anterior GCs by antidepressant usage is not sufficient to completely prevent suicide, as demonstrated by medicated individuals that had normal numbers of GCs yet died by suicide. The fact that medicated suicides had significantly more GCs than unmedicated suicides, but not higher hippocampal ErbB3 expression, was surprising; however, there are at least three potential explanations. First, although adultborn rodent GCs express ErbB3 soon after division,3 it is possible that adultborn human GCs express ErbB3 later in maturation, in accordance to their potentially lengthened maturation profile in primates,46, 49 with a larger proportion of GCs being born in response to antidepressants. In fact, this has been shown previously, with increased ErbB3 expression in DCX+ immature neurons in comparison to SOX2+ early neurogenic cells.50 Alternatively, we show that antidepressants hypermethylate a region of the ErbB3 gene, which could subsequently suppress ErbB3 expression per cell. Finally, the mRNA expression data were from the hippocampus as a whole, whereas the stereological assessment was specific to the GC layer, which may account for the discrepancy, and may further indicate that the DG-specific restoration of ErbB3-expressing GC numbers may be a more antidepressant-relevant response than a restoration of overall hippocampal ErbB3 expression. Given the potential relevance of ErbB3 to neurogenesis, affective behavior, and psychopathology, and its ubiquitous expression profile in the GC layer, ErbB3 may represent a promising target of future investigation in the etiology and treatment of psychiatrically relevant phenotypes.
References
Rockett IR, Regier MD, Kapusta ND, Coben JH, Miller TR, Hanzlick RL et al. Leading causes of unintentional and intentional injury mortality: United States, 2000-2009. Am J Public Health 2012; 102: e84–e92.
Turecki G . The molecular bases of the suicidal brain. Nat Rev Neurosci 2014; 15: 802–816.
Mahar I, Tan S, Davoli MA, Dominguez-Lopez S, Qiang C, Rachalski A et al. Subchronic peripheral neuregulin-1 increases ventral hippocampal neurogenesis and induces antidepressant-like effects. PLoS ONE 2011; 6: e26610.
Ge S, Yang CH, Hsu KS, Ming GL, Song H . A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron 2007; 54: 559–566.
Mahar I, MacIsaac A, Kim JJ, Qiang C, Davoli MA, Turecki G et al. Effects of neuregulin-1 administration on neurogenesis in the adult mouse hippocampus, and characterization of immature neurons along the septotemporal axis. Sci Rep 2016; 6: 30467.
O'Tuathaigh CM, Harte M, O'Leary C, O'Sullivan GJ, Blau C, Lai D et al. Schizophrenia-related endophenotypes in heterozygous neuregulin-1 'knockout' mice. Eur J Neurosci 2010; 31: 349–358.
Chohan TW, Boucher AA, Spencer JR, Kassem MS, Hamdi AA, Karl T et al. Partial genetic deletion of neuregulin 1 modulates the effects of stress on sensorimotor gating, dendritic morphology, and HPA axis activity in adolescent mice. Schizophr Bull 2014; 40: 1272–1284.
Bi LL, Sun XD, Zhang J, Lu YS, Chen YH, Wang J et al. Amygdala NRG1-ErbB4 is critical for the modulation of anxiety-like behaviors. Neuropsychopharmacology 2015; 40: 974–986.
Kastin AJ, Akerstrom V, Pan W . Neuregulin-1-beta1 enters brain and spinal cord by receptor-mediated transport. J Neurochem 2004; 88: 965–970.
Georgieva L, Dimitrova A, Ivanov D, Nikolov I, Williams NM, Grozeva D et al. Support for neuregulin 1 as a susceptibility gene for bipolar disorder and schizophrenia. Biol Psychiatry 2008; 64: 419–427.
Marballi K, Cruz D, Thompson P, Walss-Bass C . Differential neuregulin 1 cleavage in the prefrontal cortex and hippocampus in schizophrenia and bipolar disorder: preliminary findings. PLoS ONE 2012; 7: e36431.
Bertram I, Bernstein HG, Lendeckel U, Bukowska A, Dobrowolny H, Keilhoff G et al. Immunohistochemical evidence for impaired neuregulin-1 signaling in the prefrontal cortex in schizophrenia and in unipolar depression. Ann N Y Acad Sci 2007; 1096: 147–156.
Aston C, Jiang L, Sokolov BP . Transcriptional profiling reveals evidence for signaling and oligodendroglial abnormalities in the temporal cortex from patients with major depressive disorder. Mol Psychiatry 2005; 10: 309–322.
Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH et al. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med 2006; 12: 824–828.
Mahar I, Bambico FR, Mechawar N, Nobrega JN . Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci Biobehav Rev 2014; 38: 173–192.
Boldrini M, Butt TH, Santiago AN, Tamir H, Dwork AJ, Rosoklija GB et al. Benzodiazepines and the potential trophic effect of antidepressants on dentate gyrus cells in mood disorders. Int J Neuropsychopharmacol 2014; 17: 1923–1933.
Boldrini M, Santiago AN, Hen R, Dwork AJ, Rosoklija GB, Tamir H et al. Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacology 2013; 38: 1068–1077.
Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John Mann J et al. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology 2009; 34: 2376–2389.
Dumais A, Lesage AD, Lalovic A, Seguin M, Tousignant M, Chawky N et al. Is violent method of suicide a behavioral marker of lifetime aggression? Am J Psychiatry 2005; 162: 1375–1378.
Jackson-Fisher AJ, Bellinger G, Breindel JL, Tavassoli FA, Booth CJ, Duong JK et al. ErbB3 is required for ductal morphogenesis in the mouse mammary gland. Breast Cancer Res 2008; 10: R96.
Lee D, Yu M, Lee E, Kim H, Yang Y, Kim K et al. Tumor-specific apoptosis caused by deletion of the ERBB3 pseudo-kinase in mouse intestinal epithelium. J Clin Invest 2009; 119: 2702–2713.
Human Protein Atlas (2015). Human Protein Atlas. www.proteinatlas.org.
Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A et al. Proteomics. Tissue-based map of the human proteome. Science 2015; 347: 1260419.
Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature 2012; 489: 391–399.
Allen Institute for Brain Science (2014). Allen Human Brain Atlas. http://www.brain-map.org.
Duvernoy HM . The Human Hippocampus: An Atlas of Applied Anatomy. Springer-Verlag: Munich, 1988.
Ramos RL, Smith PT, Croll SD, Brumberg JC . Demonstrating cerebral vascular networks: a comparison of methods for the teaching laboratory. J Undergrad Neurosci Educ 2008; 6: A53–A59.
Seress L . Pyramid-like basket cells in the granular layer of the dentate gyrus in the rat. J Anat 1978; 127 (Pt 1): 163–168.
Golden SA, Covington HE 3rd, Berton O, Russo SJ . A standardized protocol for repeated social defeat stress in mice. Nat Protoc 2011; 6: 1183–1191.
Chaudhury AR, Gerecke KM, Wyss JM, Morgan DG, Gordon MN, Carroll SL . Neuregulin-1 and erbB4 immunoreactivity is associated with neuritic plaques in Alzheimer disease brain and in a transgenic model of Alzheimer disease. J Neuropathol Exp Neurol 2003; 62: 42–54.
Aston C, Jiang L, Sokolov BP . Microarray analysis of postmortem temporal cortex from patients with schizophrenia. J Neurosci Res 2004; 77: 858–866.
Le-Niculescu H, Kurian SM, Yehyawi N, Dike C, Patel SD, Edenberg HJ et al. Identifying blood biomarkers for mood disorders using convergent functional genomics. Mol Psychiatry 2009; 14: 156–174.
Li D, Feng G, He L . Case-control study of association between the functional candidate gene ERBB3 and schizophrenia in Caucasian population. World J Biol Psychiatry 2009; 10 (4 Pt 2): 595–598.
Milanesi E, Minelli A, Cattane N, Cattaneo A, Mora C, Barbon A et al. ErbB3 mRNA leukocyte levels as a biomarker for major depressive disorder. BMC Psychiatry 2012; 12: 145.
Cao Z, Wu X, Yen L, Sweeney C, Carraway KL 3rd . Neuregulin-induced ErbB3 downregulation is mediated by a protein stability cascade involving the E3 ubiquitin ligase Nrdp1. Mol Cell Biol 2007; 27: 2180–2188.
Guy PM, Platko JV, Cantley LC, Cerione RA, Carraway KL 3rd . Insect cell-expressed p180erbB3 possesses an impaired tyrosine kinase activity. Proc Natl Acad Sci USA 1994; 91: 8132–8136.
Deng C, Pan B, Engel M, Huang XF . Neuregulin-1 signalling and antipsychotic treatment: potential therapeutic targets in a schizophrenia candidate signalling pathway. Psychopharmacology (Berl) 2013; 226: 201–215.
Shi F, Telesco SE, Liu Y, Radhakrishnan R, Lemmon MA . ErbB3/HER3 intracellular domain is competent to bind ATP and catalyze autophosphorylation. Proc Natl Acad Sci USA 2010; 107: 7692–7697.
Steinkamp MP, Low-Nam ST, Yang S, Lidke KA, Lidke DS, Wilson BS . erbB3 is an active tyrosine kinase capable of homo- and heterointeractions. Mol Cell Biol 2014; 34: 965–977.
Sathyamurthy A, Yin DM, Barik A, Shen C, Bean JC, Figueiredo D et al. ERBB3-mediated regulation of Bergmann glia proliferation in cerebellar lamination. Development 2015; 142: 522–532.
Gao R, Zhang J, Cheng L, Wu X, Dong W, Yang X et al. A Phase II, randomized, double-blind, multicenter, based on standard therapy, placebo-controlled study of the efficacy and safety of recombinant human neuregulin-1 in patients with chronic heart failure. J Am Coll Cardiol 2010; 55: 1907–1914.
Jabbour A, Hayward CS, Keogh AM, Kotlyar E, McCrohon JA, England JF et al. Parenteral administration of recombinant human neuregulin-1 to patients with stable chronic heart failure produces favourable acute and chronic haemodynamic responses. Eur J Heart Fail 2011; 13: 83–92.
Tanti A, Westphal WP, Girault V, Brizard B, Devers S, Leguisquet AM et al. Region-dependent and stage-specific effects of stress, environmental enrichment, and antidepressant treatment on hippocampal neurogenesis. Hippocampus 2013; 23: 797–811.
Robison AJ, Vialou V, Sun HS, Labonte B, Golden SA, Dias C et al. Fluoxetine epigenetically alters the CaMKIIalpha promoter in nucleus accumbens to regulate DeltaFosB binding and antidepressant effects. Neuropsychopharmacology 2014; 39: 1178–1186.
Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T et al. Regional dissociations within the hippocampus—memory and anxiety. Neurosci Biobehav Rev 2004; 28: 273–283.
Perera TD, Dwork AJ, Keegan KA, Thirumangalakudi L, Lipira CM, Joyce N et al. Necessity of hippocampal neurogenesis for the therapeutic action of antidepressants in adult nonhuman primates. PLoS One 2011; 6: e17600.
Fanselow MS, Dong HW . Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 2010; 65: 7–19.
Nason MW Jr., Adhikari A, Bozinoski M, Gordon JA, Role LW . Disrupted activity in the hippocampal-accumbens circuit of type III neuregulin 1 mutant mice. Neuropsychopharmacology 2011; 36: 488–496.
Kohler SJ, Williams NI, Stanton GB, Cameron JL, Greenough WT . Maturation time of new granule cells in the dentate gyrus of adult macaque monkeys exceeds six months. Proc Natl Acad Sci USA 2011; 108: 10326–10331.
Bracko O, Singer T, Aigner S, Knobloch M, Winner B, Ray J et al. Gene expression profiling of neural stem cells and their neuronal progeny reveals IGF2 as a regulator of adult hippocampal neurogenesis. J Neurosci 2012; 32: 3376–3387.
Acknowledgements
This research was supported by CIHR (MOP-111022) and NSERC RTI (345952-07) grants to NM. IM and EI were supported by FRQS. NM was a CIHR New Investigator and FRQ-S scholar during the course of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Translational Psychiatry website
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Mahar, I., Labonte, B., Yogendran, S. et al. Disrupted hippocampal neuregulin-1/ErbB3 signaling and dentate gyrus granule cell alterations in suicide. Transl Psychiatry 7, e1161 (2017). https://doi.org/10.1038/tp.2017.132
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2017.132
This article is cited by
-
Short-term exposure to an obesogenic diet during adolescence elicits anxiety-related behavior and neuroinflammation: modulatory effects of exogenous neuregulin-1
Translational Psychiatry (2022)
-
Genetic analyses identify pleiotropy and causality for blood proteins and highlight Wnt/β-catenin signalling in migraine
Nature Communications (2022)
-
Spine impairment in mice high-expressing neuregulin 1 due to LIMK1 activation
Cell Death & Disease (2021)
-
Extreme Glycemic Fluctuations Debilitate NRG1, ErbB Receptors and Olig1 Function: Association with Regeneration, Cognition and Mood Alterations During Diabetes
Molecular Neurobiology (2021)