Abstract
Pregnenolone sulfate, an endogenous neurosteroid in the central nervous system, is a positive allosteric modulator of the NMDA receptor, and plays a role in the modulation of learning and memory. Here, we study the actions of pregnenolone sulfate using the dopamine transporter knockout (DAT-KO) mice, which exhibit endophenotypes that recapitulate certain symptoms of schizophrenia, including the psychomotor agitation, stereotypy, prepulse inhibition (PPI) deficits and cognitive impairments. We found that acute treatment with pregnenolone sulfate normalized the hyperlocomotion and stereotypic bouts, and rescued the PPI deficits of DAT-KO mice. In addition, long-term treatment with pregnenolone sulfate rescued the cognitive deficits of DAT-KO mice in the novel object recognition and social transmission of food preference tests. We also showed that pregnenolone sulfate normalized behavioral abnormalities in MK801-treated wild-type mice, whereas pregnenolone, its precursor, only partially rescued MK801-induced behavioral abnormalities. This indicates that there are distinct mechanisms of action between pregnenolone sulfate and pregnenolone, and the involvement of NMDA receptor signaling in the action of pregnenolone sulfate. Moreover, we found that acute treatment with pregnenolone sulfate increased the phosphorylation levels of striatal AKT and GSK3β in DAT-KO mice, and that long-term treatment with pregnenolone sulfate increased expression levels of NR1 subunit of the NMDA receptor in hippocampus. Thus, pregnenolone sulfate was able to rescue the behavioral anomalies of DAT-KO mice through the NMDA receptor-mediated, AKT/GSK3β signaling pathway.
Similar content being viewed by others
Introduction
Schizophrenia is a debilitating mental disorder, which affects ~1% of the world population.1 Understanding the pathophysiology of schizophrenia is the key for its effective diagnosis and treatment. The dopamine hypothesis is one of the more established theories of schizophrenia to date, and is supported by clinical observations of amphetamine-induced psychosis and dopamine D2 receptor-mediated antipsychotic treatment.2, 3 Although antipsychotics are commonly used to treat positive symptoms of schizophrenia, there are few effective tools that can be used to combat the cognitive deficits and negative symptoms of schizophrenia.4, 5
Another prominent hypothesis suggests that the hypofunction of glutamatergic NMDA receptors is an underlying cause of schizophrenia.6, 7, 8, 9 This hypothesis arose from clinical observations that NMDA receptor antagonists, such as ketamine and phenylcyclidine, can induce schizophrenia-like symptoms,10, 11 and that schizophrenia patients exhibit decreased levels of NMDA receptors.12 Given that NMDA receptors regulate dopaminergic neurotransmission, the dopaminergic and glutamatergic models are not mutually exclusive, and hypofunction of NMDA receptors may in fact be responsible for the abnormal dopamine activity observed in schizophrenia.8 These two neurotransmitter systems also converge on many levels, one of which is the AKT/GSK3β pathway through dopamine D2 receptor signaling13 or NMDA/PI3K signaling.14 AKT phosphorylation regulates various downstream molecules, including GSK3β.15 A constitutively active kinase that has many downstream targets that modulate synaptic plasticity, GSK3β plays an important role in learning and memory.16 Dysfunction of the AKT/GSK3β signaling pathway has been implicated in schizophrenia.17, 18
In addition to antipsychotics, there have been recent advances in the treatment of schizophrenia using alternative pharmacological agents,19 such as neurosteroids.20, 21 Neurosteroids are synthesized in the central nervous system, and have effects on anxiety, cognition and memory.22, 23, 24, 25, 26 Altered levels of neurosteroids such as pregnenolone (Preg) and dehydroepiandrosterone have been observed in patients with schizophrenia,27 and administration of Preg has been shown to rescue certain schizophrenia symptoms in proof-of-concept, randomized controlled clinical trials.20 Recently, it has been shown that Preg can rescue schizophrenia-like behavior in dopamine transporter knockout (DAT-KO) mice.28 DAT-KO mice have behavioral manifestations that mirror the positive and negative symptoms associated with schizophrenia,29, 30, 31 suggesting that the DAT-KO mouse is an ideal mouse model for use to study schizophrenia. In addition, elevated GSK3β activity through dopamine D2 receptor has also been observed in DAT-KO mice,13 thus recapitulating the impaired AKT/GSK3β signaling pathway in schizophrenia patients.17, 18
As Preg can be converted to its soluble form of pregnenolone sulfate (PregS), PregS may also have the potential to alleviate symptoms of schizophrenia. Moreover, PregS is a known positive allosteric NMDA receptor modulator,32, 33, 34, 35, 36 and may exert its effects by ameliorating NMDA receptor hypofunction. In this study, we propose that PregS possesses a distinct mechanism of action from its precursor, Preg, and is able to rescue schizophrenia-like behavior in DAT-KO mice by modulating AKT/GSK3β signaling via the NMDA receptor. Therefore, PregS may be a potential alternative therapeutic agent in the treatment of schizophrenia.
Materials and methods
Animals
Male and female, wild-type (WT) and DAT-KO mice (8–10 weeks) were bred from C57BL/6J DAT heterozygous mice. Genotyping was carried out as previously described.28 Mice were housed in a specific pathogen-free environment, with ad libitum access to food and water, on a 12-h light/dark cycle (lights on at 0700 h). Animal procedures were approved by the Institutional Animal Care and Use Committees of NUS and Duke-NUS Graduate Medical School Singapore, in accordance with national guidelines for the care and use of laboratory animals for scientific purposes.
Drug preparation and adminstration
PregS (Steraloids, Newport, RI, USA) was first dissolved in ethanol and diluted in autoclaved water to a final ethanol concentration of 0.6%. PregS or 0.6% ethanol vehicle was injected intraperitoneally with a 5 ml kg−1 injection volume. Preg (60 mg kg−1, Sigma-Aldrich, St. Louis, MO, USA) was administered as a suspension in peanut oil either intraperitoneally (for acute injections) or subcutaneously (for long-term injections) with a 5 ml kg−1 volume. Controls were injected with peanut oil vehicle. For long-term injections, PregS was administered for 10 consecutive days, while Preg was administered for 14 consecutive days.
Open field activity
Locomotor activity was recorded using an automated Omnitech Digiscan apparatus (AccuScan Instruments, Columbus, OH, USA) under ~180 lux illumination. The mice were placed into the chamber for 30 min to obtain baseline activity, followed by an injection (intraperitoneally) with vehicle or PregS at 40 mg kg−1 or 80 mg kg−1, then returned to the chamber for 120 min. Activity was measured in terms of total distance traveled, rearing as vertical activity and stereotypy as the numbers of repeated beambreaks with intervals of <1 s.
PPI of acoustic startle
The mice were injected (intraperitoneally) with vehicle, or PregS at 20 mg kg−1 or 40 mg kg−1 and were placed into startle chambers (SR-LAB, San Diego Instruments, San Diego, CA, USA) for 10 min of acclimatization. The mice were then exposed to a series of trials as previously described,28 with three different prepulse stimuli, which were 4, 8 or 12 dB above the white-noise background. Prepulse inhibition (PPI) responses were calculated as percentage scores for each prepulse intensity, where %PPI=[1−(prepulse trials/startle-only trials)] × 100.
Novel object recognition test
The mice were injected (intraperitoneally) with vehicle or PregS at 40 mg kg−1 daily for 10 days. On the next day, the novel object recognition test was carried out as previously described.28 In brief, they were trained with identical ‘familiar’ objects for 5 min and then assessed for short-term, long-term and remote memory, which were conducted 20 min, 24 h and 14 days after training, respectively. All the tests were video recorded and scored using the TopScan Behaviour Analyzing system (CleverSys, Reston, VA, USA). Preference score was calculated as (Time spent with novel object−Time spent with familiar object)/(Total time spent with both objects).
Social transmission of food preference test
Mice were injected (intraperitoneally), with vehicle or 40 mg kg−1 PregS, daily for 10 days. After the last injection, the animals were food deprived for 16–18 hours before testing.28 The familiar diet was the standard mouse diet (5LJ5, LabDiet, St. Louis, MO, USA) mixed with 1% of ground oregano (McCormick, Hunt Valley, MD, USA). Novel diets for short-term, long-term and remote memory tests were flavored with 1% ground thyme, marjoram or cumin, respectively. The mice were then tested at 20 min, 24 h and 14 days after exposure to assess for short-term, long-term and remote memory, respectively. Preference scores were calculated as (Amount of familiar diet consumed−Amount of novel diet consumed)/(Total amount of both diets consumed).
Western blotting
Tissues of striatum and hippocampus were rapidly dissected and homogenized at 4 °C in lysis buffer (20 mM Tris; pH 8.0, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100). Thirty micrograms of protein extracts were resolved on 10% SDS-polyacrylamide gels and transferred onto nitrocellulose membranes (Bio-Rad, Richmond, CA, USA). Immunoblots of striatal samples were probed with the following primary antibodies: AKT (1:10 000); phospho-AKT (Thr308) (1:2000); GSK3β (1:30 000); phospho-GSK3β (1:5000); GAPDH (1:30 000; Cell Signaling Technology, Beverly, MA, USA). Immunoblots of hippocampal samples were probed with antibodies against NR1 (1:4000; Synaptic Systems, Gottingen, Germany) and Actin (1:20 000; Merck Millipore, Billerica, MA, USA). Image J software (National Institutes of Health, USA) was used for the densitometric analyses.
Statistical analysis
R statistical program (R Foundation for Statistical Computing, Vienna, Austria) was used for data analyses. Data were expressed as means±s.e.m. and P<0.05 was considered statistically significant. A two-way analysis of variance was used for open field cumulative activities, and null and startle activity in PPI. Mixed factorial design analysis of variance was used to analyze PPI, novel object recognition and social transmission of food preference tests. For all tests, genotype and treatment were between-subject factors; while inhibition across prepulse intensities for PPI, and test sessions for both cognitive tests were within-subject factors. A t-test was used to analyze the preference scores in the novel object recognition and social transmission of food preference tests versus ‘0’, and for densitometric analyses. Bonferroni-corrected pair-wise comparisons were used as post hoc tests.
Results
PregS normalizes hyperlocomotor activities in DAT-KO mice
WT and DAT-KO mice were treated with vehicle or PregS (40 and 80 mg kg−1) and tested for locomotor activities. The locomotor, rearing and stereotypic activities were aggregated over baseline (0–30 min) and the post-injection (31–150 min) periods. The cumulative baseline activity showed a significant main effect of genotype, but no significant main effect of treatment nor genotype by treatment interaction, for locomotion, rearing and stereotypical activity (F1,59=234; 129; and 541, respectively, Ps<0.05). As has been previously shown,30 DAT-KO mice had significantly higher cumulative baseline activities than WT mice (Figures 1a to c). For the cumulative post-injection period, there were significant main effects of genotype (locomotion: F1,59=117; rearing: F1,59=35.8; stereotypy: F1,59=234, Ps<0.001) and treatment (locomotion: F2,59=41.9; rearing: F2,59=14.4; stereotypy: F2,59=31.9, Ps<0.001), with a significant genotype by treatment interaction (locomotion: F2,59=35.3; rearing: F2,59=11.1; stereotypy: F2,59=10.4, Ps<0.001). Pair-wise comparisons showed that the activities of WT mice were unaffected by 40 or 80 mg kg−1 of PregS (Figures 1d to f). However, relative to vehicle controls, the activities of DAT-KO mice were partially suppressed with 40 mg kg−1 PregS (Ps<0.005) and further suppressed with 80 mg kg−1 PregS (Ps<0.001). Activities of DAT-KO mice treated with 80 mg kg−1 PregS were suppressed to levels that were comparable to WT vehicle control.
Pregnenolone sulfate (PregS) suppresses hyperlocomotor activities in DAT-KO mice. Baseline activities (pre-injection baseline) were measured for 30 min before mice were injected (intraperitoneally) with vehicle (Veh), or 40 or 60 mg kg−1 PregS, and locomotor activities were monitored for a further 2 h (post-injection). Cumulative distance traveled (a), vertical activity (b) and stereotypical activity (c) at baseline are shown. Cumulative post-injection activities are shown for horizontal activity (d), rearing (e) and stereotypy (f). N=10–16 mice per genotype per treatment condition; aP<0.05, WT-Veh versus DAT-KO-Veh; bP<0.05, within treatment groups WT versus DAT-KO; cP<0.05, within groups versus Veh; dP<0.05, within groups PregS40 versus PregS80. DAT-KO, dopamine transporter knockout; WT, wild type.
PregS rescues PPI deficits in DAT-KO mice
The mice received treatments of either 20 or 40 mg kg−1 PregS to investigate the effects of PregS on PPI. There were no genotype differences for the percentage of null activity (WT: 1.32–10.31%; DAT-KO: 2.23–10.76%) and for the startle response. In addition, the treatment did not affect startle responses (Figure 2b). There were significant main effects of genotype, treatment and prepulse (F1,204=82.2, F2,204=22.9 and F2,204=161, respectively, Ps<0.001), with significant treatment by genotype interaction (F2,204=21.8, P<0.001). DAT-KO mice showed distinct PPI deficits compared with WT controls at all prepulse intensities (Ps<0.005). WT mice were not affected by 20 or 40 mg kg−1 PregS treatment. Relative to WT controls, PPI deficits of DAT-KO mice were partially rescued with 20 mg kg−1 PregS and fully rescued with 40 mg kg−1 PregS (Ps<0.05, Figure 2a).
Pregnenolone sulfate (PregS) rescues PPI deficits in DAT-KO mice. WT and DAT-KO mice were injected (intraperitoneally) with vehicle (Veh), or 20 or 40 mg kg−1 PregS before being tested. (a) PPI levels of WT (left) and DAT-KO (right) mice. (b) Amplitude of startle responses of WT and DAT-KO mice. N=9–17 mice per genotype per treatment condition; aP<0.05, WT-Veh versus DAT-KO-Veh; bP<0.05, within treatment groups WT versus DAT-KO; cP<0.05, within groups versus Veh; dP<0.05, within groups PregS40 versus PregS80. DAT-KO, dopamine transporter knockout; PPI, prepulse inhibition; WT, wild type.
PregS alleviates the cognitive deficits in DAT-KO mice
To evaluate the effect of PregS on cognition, WT and DAT-KO mice were administered 40 mg kg−1 PregS for 10 days, and then examined in the novel object recognition and social transmission of food preference tests. For the novel object recognition test, there were significant main effects of genotype, treatment and test session (F1,176=21.5, F1,176=21.5 and F3,176=13.4, respectively, Ps<0.001), with significant genotype by treatment interaction (F1,176=14.0, Ps<0.001). During training, no preference was shown for either identical object used by genotype or treatment group (Figure 3a). During the testing, vehicle-treated WT mice showed preference for the novel object for all the three test sessions (t(>0.318)<4.46, Ps<0.01). PregS treatment did not affect the preference scores of WT mice, with them showing preference for the novel object (t(>0.170)<7.15, Ps<0.001). In contrast, vehicle-treated DAT-KO mice showed no preference for either the novel or familiar object during each of the three test sessions. For all the three test sessions, PregS-treated DAT-KO mice show increased preference for the novel object relative to vehicle-treated DAT-KO mice (Ps<0.001), to levels similar to vehicle- or PregS-treated WT mice (Figure 3a). For short-term, long-term and remote memory, a one-sample t-test showed that the preference scores of PregS-treated DAT-KO mice were significantly higher than 0 (t(>4.14)<6.17, Ps<0.001). For the total duration of object exploration, the only significant main effect was that of test session (F3,96=5.72, P<0.01), with no other significant main effects or interactions (Figure 3b). Thus, the differences in preference scores were not due to genotypic differences in the total duration of object exploration. As such, the reduced preference for the novel object by the DAT-KO mice is not due to genotype differences in durations of object explorations.
Pregnenolone sulfate (PregS) alleviates cognitive deficits in DAT-KO mice. WT and DAT-KO mice were treated with vehicle (Veh) or 40 mg kg−1 PregS for 10 consecutive days before being tested in the paradigms of novel object recognition (a) and social transmission of food preference (c) for short-term (STM), long-term (LTM) and remote memory. Total duration of contacts with both objects (b) in the novel object recognition test and the total amount of food consumed (d) in the social transmission of food preference test were also calculated. N=9–12 mice per genotype per treatment condition for each test. aP<0.05, WT-Veh versus DAT-KO-Veh; cP<0.05, within groups versus Veh. DAT-KO, dopamine transporter knockout; WT, wild type.
For the social transmission of food preference test, there were significant main effects of genotype, treatment and test session (F1,105=16.9 and 17.7, respectively, Ps<0.001), and significant genotype by treatment interaction (F1,105=20.4, Ps<0.001). Vehicle-treated WT mice showed positive preference, indicating that they preferred the familiar diet over the novel diet across all the three test sessions (t(>6.23)<6.56, Ps<0.001). Preference scores of WT mice were not affected by PregS treatment (t(>4.53)<7.45, Ps<0.001, Figure 3c). Vehicle-treated DAT-KO mice showed no preference for either diet across the three sessions, whereas those treated with 40 mg kg−1 PregS preferred the familiar diet for all the three test sessions (t(>5.6)<9.01, Ps<0.001), with preference scores that were significantly higher than that of their DAT-KO controls (Ps<0.05) and that were similar to vehicle- or PregS-treated WT mice (Figure 3c). To ensure that the preference scores were not biased by genotype differences in motivation to consume food, the total amount of food consumed by each group of mice for each test session were calculated. The only significant main effect detected was that of test session (F2,105=9.37, P<0.01; Figure 3d). Therefore, the differences in preference scores between the test groups are not due to differing motivations for consuming the different diets.
PregS antagonizes MK801-induced behavioral deficits in WT mice
As PregS is a known positive allosteric modulator of NMDA receptors, WT mice were administered MK801 to induce behavioral deficits, and then treated with PregS or its precursor, Preg (Figure 4). Both locomotor activity and PPI paradigms were used to determine the efficacy of PregS versus Preg. Cumulative post-injection distance traveled showed a significant main effect of treatment for distance traveled in the open field test (F5,53=54.1, P<0.001; Figures 4a and b). WT mice treated with 0.1 mg kg−1 MK801 had significantly increased locomotion relative to vehicle-treated mice (P<0.001). MK801-induced hyperlocomotion was fully suppressed by 80 mg kg−1 PregS (P<0.001), but was partially suppressed by 60 mg kg−1 Preg (P<0.001) to levels that were still higher than that of vehicle-treated WT mice (Ps<0.05).
Pregnenolone sulfate (PregS) rescues MK801-induced hyperlocomotion and PPI deficit. (a) WT mice were treated with vehicle or MK801, followed by an injection of vehicle (Veh) or PregS 80 mg kg−1 before being monitored for horizontal activity for 90 min. (b) PregS 80 mg kg−1 fully suppressed MK801-induced hyperactivity, whereas 60 mg kg−1 Preg showed partial suppression. (c) WT mice were treated with vehicle or MK801, followed by vehicle, PregS 40 mg kg−1 or Preg 60 mg kg−1, and subsequently tested in PPI. PregS 40 mg kg−1 fully rescued MK801-induced PPI deficits, whereas 60 mg kg−1 Preg showed partial effects (c). N=9–14 mice per treatment condition for each test. aP<0.05, versus WT Veh-Veh; bP<0.05, versus WT MK801-Veh; cP<0.05, MK801-PregS-treated groups versus MK801-Preg treated groups. PPI, prepulse inhibition; WT, wild type.
In PPI, the main effects of treatment and prepulse intensity were significant (F5,222=36.0 and F2,222=214, respectively, Ps<0.001), but the treatment and prepulse intensity interaction were not. MK801 treatment suppressed PPI relative to vehicle-treated WT for all the three prepulse intensities (Ps<0.001; Figure 4c). At 40 mg kg−1, PregS restored PPI of MK801-treated WT mice (Ps<0.001) to levels of the vehicle-treated WT mice at all the three prepulse intensities, whereas 60 mg kg−1 Preg only partially rescued the MK801-induced PPI deficits at the 4 and 8 db prepulse intensities (Ps<0.01). At the 12 db prepulse intensity, 60 mg kg−1 Preg could not fully rescue PPI deficits of MK801-treated WT mice (Ps<0.05).
Acute treatment of PregS, but not Preg, activates AKT/GSK signaling pathway
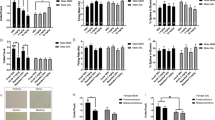
As PregS could antagonize MK801-induced hyperlocomotion, we tested whether acute treatment with PregS affected NMDA receptor-related downstream signaling pathways, such as the AKT/GSK3β pathway, in a time-dependent manner. We found that there was significant main effect of time for phosphorylation levels of AKT and GSK3β (F3,13=10.7; 12.4, respectively, Ps<0.001; Figures 5a to c) in striatal tissues of PregS-treated WT mice. Maximal phosphorylation levels of AKT and GSK3β occurred at 15 min after 80 mg kg−1 PregS treatment. At 15 min, pAKT and pGSK3β showed a 2.08 (±0.185) and 1.87 (±0.212) fold increase relative to vehicle (n=4; Ps<0.01).
Modulatory effects of PregS on NMDA signaling. Effects of acute treatment of 80 mg kg−1 PregS on AKT/GSK3β signaling in striatum were analyzed in WT (a–c) and DAT-KO (d–f) mice by antibodies against phospho-AKT (Thr308), phospho-GSK3β (Ser9) and their respective non-phosphorylated forms. (g–i) Long-term effects of chronic treatment of 40 mg kg−1 PregS on NR1 subunit of NMDA receptor in hippocampus were characterized in WT and DAT-KO (g–i) mice. GAPDH (a,d) and actin (g) were used as loading controls for densitometry measures. Quantified ratios of phosphorylated/non-phosphorylated forms were normalized to 0 min. aP<0.05, versus 0 min or vehicle (Veh) group. DAT-KO, dopamine transporter knockout; PregS, pregnenolone sulfate; WT, wild type.
For the DAT-KO treated groups, the phosphorylation levels of AKT and GSK3β showed a significant main effect of time (F1,21=77.2; 29.2, respectively, Ps<0.001, Figures 5d to f). At 15 and 30 min, pAKT and pGSK3β levels were higher than that of baseline (P s<0.005). Maximal levels of pAKT and pGSK3β occurred at 30 min after 80 mg kg−1 PregS treatment, which were significantly higher than at 15 and 60 min (P s<0.001). Phosphorylated GSK3β levels returned to baseline levels 60 min after PregS treatment, whereas pAKT levels still remained elevated (P s<0.001).
In contrast, Preg, the precursor of PregS, showed no significant effect on pAKT in DAT-KO mice (Supplementary Figures S1A and B). There was a significant main effect of time on the phosphorylation levels of GSK3β (F1,11=4.00, Ps=0.0375, Supplementary Figures S1A and C), with maximal levels of pGSK3β occurring at 30 min after 60 mg kg−1 Preg treatment (Ps=0.038, relative to baseline). Phosphorylation levels of pGSK3β at 15 min and 60 min were not significantly different from 0 min.
Long-term treatment of PregS, but not Preg, increases NMDA receptor NR1 subunit levels
As the 10-day treatment with PregS improved the performance of DAT-KO mice in learning and memory tests (Figure 3), we tested whether long-term PregS treatment changes NMDA receptor levels in the hippocampus of WT and DAT-KO mice (Figure 5g). In WT mice, there was no significant main effect of treatment on the expression of the NR1 subunit of the NMDA receptor (Figure 5h), whereas in DAT-KO mice, long-term PregS treatment significantly increased NR1 levels in the hippocampus relative to vehicle treatment (F1,7=37.62, P<0.001; Figure 5i). In contrast, although long-term Preg treatment has also been shown to improve cognitive performance in DAT-KO mice,28 hippocampal NR1 expression levels did not increase with treatment in both WT and DAT-KO mice (Supplementary Figure S1D to F).
Discussion
In this study, we characterized the efficacy of PregS on alleviating the schizophrenia-like behaviors in DAT-KO mice. DAT-KO mice show an elevated dopaminergic tone and as a result, exhibit certain schizophrenia-like endophenotypes, including hyperlocomotion, stereotypy,29 deficits in prepulse inhibition28, 31 and impaired spatial memory.28 Previous studies have also shown that dysregulation in the AKT/GSK3β pathway is the underlying cause of hyperlocomotion in DAT-KO mice.13
Here, we showed that acute administration of PregS in DAT-KO mice dose-dependently suppressed their hyperlocomotion and stereotypic activity (Figure 1), and rescued prepulse inhibition deficits (Figure 2). Long-term administration of 40 mg kg−1 PregS alleviated the cognitive deficits of DAT-KO mice in the novel object recognition and social transmission of food preference paradigms (Figure 3). Therefore, increasing the circulating levels of PregS alleviates the positive and negative schizophrenia-like endophenotypes in DAT-KO mice without adversely affecting WT mice.
PregS rescues the aberrant behaviors of DAT-KO mice by acting on the central nervous system
PregS is a neurosteroid that is converted from Preg by a sulfotransferase.37, 38, 39, 40, 41 However, the level of PregS in human brain is controversial due to technical limitations, as the level of PregS is below the detection limit of 0.3 ng per gram tissue.34 Even then, it is likely that the brain tissue levels of PregS are likely to be lower than its precursor, Preg.37
When systemically administered, PregS was able to cross the blood–brain barrier to increase brain levels of PregS.42 Here, we show that systemic administration of PregS changes phosphorylation levels of signaling molecules in brain tissues (Figure 5). This confirms that the observed behavioral effects following acute PregS administration are due to the actions of PregS on the central nervous system.
PregS modulates NMDA signaling to rescue cognitive deficits
PregS has important regulatory effects on several neurotransmission signaling systems, such as those regulated by sigma-1 (ref. 43) and GABAA44 receptors. More importantly, PregS is a positive allosteric modulator of NMDA neurotransmission, possibly through its interaction with NR1/NR2B subunits.35 Here, we showed that acute PregS treatment had antagonistic effects to a NMDA receptor antagonist, MK801, on locomotor activity and acoustic startle reflex behavior (Figure 4).
In addition, the concentration of PregS is known to be higher in the hippocampus, an area primarily associated with memory, than in the cortex,45 which may give rise to the role of PregS in learning and memory. Previous studies have shown that PregS is an effective memory enhancer for rodent models.22, 46, 47 For example, the infusion of PregS into the hippocampus restored the memory deficits of aged rats in the Y maze.48 PregS has also been shown to antagonize the memory deficits induced by NMDA receptor antagonists.49, 50 Here, we showed that long-term treatment of DAT-KO mice with PregS rescued their impaired episodic memory and poor discriminative abilities (Figure 3), and increased the expression of the NR1 subunit of the NMDA receptor in the hippocampus (Figure 5i). As NMDA receptor signaling is important for long-term potentiation,51, 52 we postulate that the increased NR1 expression may be the underlying mechanism of the rescued cognitive deficits of DAT-KO mice. These data are also in agreement with recent findings that PregS can stimulate NMDA receptor surface expression.53
Acute PregS treatment has modulatory effects on the AKT/GSK3β signaling pathway
It has been proposed that DAT-KO mice have dysregulated AKT/GSK3β signaling due to overstimulation of dopamine D2 receptor, which results in increased striatal GSK3β activation, that is, decreased GSK3β phosphorylation.13 Therefore, inhibition of GSK3β activity, (i.e. increased GSK3β phosphorylation), by a GSK3β inhibitor, such as 4-benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione, lithium or dopamine D2 receptor antagonists, reduces hyperlocomotion in DAT-KO mice.13 Interestingly, hyperdopamine-induced behavioral abnormalities of DAT-KO mice were further enhanced by MK801, but were attenuated by positive modulators of AMPA receptors, suggesting dysregulation of glutamatergic function in DAT-KO mice.54 As NMDA receptor antagonists also affect AKT/GSK3β signaling,55, 56, 57 the antagonistic action of PregS on MK801 effects has led us to hypothesize that PregS may modulate the NMDA receptor signaling through the phosphatidylinositol 3-kinase/AKT signaling.58 To investigate this possibility, we treated WT and DAT-KO mice with 80 mg kg−1 PregS for 15, 30 and 60 min, and measured the non-phosphorylated and phosphorylated forms of AKT and GSK3β in the striatum (Figures 5a to f). In WT mice, we found that PregS treatment caused an increase in the phosphorylation levels of AKT and GSK3β 15 min after treatment, and the phosphorylation levels returned to baseline after 30 min. In PregS-treated DAT-KO mice, there was a delayed activation of the AKT/GSK3β phosphorylation levels, and AKT phosphorylation was persistent even at 60 min, concomitant with the attenuation of hyperlocomotion and stereotypic activity. Although PregS treatment increases phosphorylation levels of AKT and GSK3β in both WT and DAT-KO mice, it is observed that there was a temporal difference in their responses. We propose that this temporal difference may be due to the dysregulated AKT/GSK3β signaling in DAT-KO mice, evident from the decreased levels of AKT and GSK3β phosphorylation at baseline.13
It is also worthwhile to note that in comparison to vehicle-treated controls, DAT-KO mice treated long term with PregS still exhibited hyperlocomotion, and showed no changes in pAKT/AKT and pGSK3β/GSK3β levels when assayed the day after the last PregS injection (data not shown). These results suggest that the different treatment regimens of PregS, acute versus long term, bring about different biological responses. The effect of PregS on striatal-driven behaviors, such as locomotion, is fast-acting and short-lasting; whereas the effect of PregS on hippocampal-driven behaviors, such as memory, requires prolonged treatment and is more long-lasting.
PregS and Preg have differing mechanisms of action
PregS and Preg are inter-convertible.38, 39, 40, 41, 59 Therefore, given our results presented here and that of Wong et al.,28 where Preg and PregS treatment rescued the abberrant behaviors of DAT-KO mice, we carried out further experiments to determine whether the observed behavioral rescue was due to inter-conversion between PregS and Preg, or distinct PregS and Preg mechanisms of actions that converged on similar behavioral results.
Previous studies have shown that acute PregS treatment increases levels of Preg, allopregnanolone and 5α-dihydroprogesterone in the brain.42, 50 It has also been shown that in the brain, the conversion rate of PregS to Preg is lower than that of Preg to PregS.42, 60 However, most older studies obtained the levels of PregS through indirect measurements, which are rather inaccurate,34 and direct assays have shown that the level of PregS in the brain is actually very low.61 Yet, we would like to highlight that the rate of inhibition of locomotor responses of DAT-KO mice is similar between acute PregS and Preg treatments, which should not be the case if the conversion of PregS to Preg is at a different rate from the conversion of Preg to PregS.42, 60 As such, it is unlikely that the inhibition of locomotor activity following PregS treatment is due to the conversion to Preg.
A previous study has shown that unlike PregS, Preg does not directly affect NMDA receptor actions.62 In addition, PregS, but not Preg, could potentiate NMDA-induced toxicity in vivo.63 These evidences suggest that PregS directly, rather than through the conversion to Preg, affected NMDA receptor actions. Our results are congruent with previous findings, where PregS, but not Preg, fully rescued MK801-induced hyperactivity and prepulse inhibition deficits (Figure 4).
When we investigated the effects of Preg on downstream signaling molecules, we found that Preg treatment did not increase pAKT levels in DAT-KO mice and the relative increase in pGSK levels is smaller than that of PregS-treated mice (Supplementary Figures S1A and C). These data suggest that even though PregS and Preg are inter-convertible, the observed effects on the AKT/GSK3β signaling pathway following the administration of PregS, is not due to the conversion of PregS to Preg. This also points to the presence of distinct mechanisms underlying the action of PregS and Preg in attenuating schizophrenia-like endophenotypes in DAT-KO mice. In addition, we showed that long-term treatment with PregS, but not Preg (Supplementary Figure S1) increased expression of hippocampal NR1 subunit of NMDA receptor in DAT-KO mice. Therefore, our biochemical and behavioral results suggest that PregS, but not Preg, acts through the NMDA receptor-modulated AKT/GSK3β signaling pathway to normalize the behaviors of DAT-KO mice.13
Concluding remarks
Dysregulation of dopaminergic and glutamatergic signaling are key players in the pathophysiology of schizophrenia.2, 3, 5, 6 Both of these signaling systems converge on the AKT/GSK3β pathway, where dysregulation in the AKT/GSK3β signaling pathway has been implicated in schizophrenia.18, 64 In mouse models, mice deficient in AKT1 (ref. 65) or overexpressing GSK3β66 show defects in working memory. The DAT-KO mice are known to have dysregulated AKT/GSK3β signaling,13 and recapitulate several schizophrenia-like endophenotypes. We have shown here that acute PregS treatment increases striatal GSK3β phosphorylation as a potential effect in normalizing the positive schizophrenia-like symptoms of DAT-KO mice. We have shown evidence that the actions of PregS is somewhat distinct from Preg, in terms of biochemical and temporal signaling patterns. However, the conversion of PregS to other neurosteroid metabolites, such as dehydroisoandrosterone sulfate,67 cannot be ruled out without further studies to determine the metabolism of systemically delivered PregS, which will involve direct assays to measure the levels of the downstream biosynthesized neurosteroids. We also showed that long-term administration of PregS ameliorates the cognitive deficits of DAT-KO mice, possibly through increasing hippocampal NR1 levels.
The results obtained with this study suggest that PregS may be able to directly address the NMDA receptor hypofunction in schizophrenic patients, which in turn leads to the amelioration of the associated negative symptoms and cognitive deficits.68, 69 This study also strengthens the potential use of neurosteroids as therapeutics in the treatment of neuropsychiatric disorders. However, more studies are required to understand the enzymatic kinetics that regulate the metabolism of Preg, PregS and the other downstream neurosteroids.
References
Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS et al. Grand challenges in global mental health. Nature 2011; 475: 27–30.
Snyder SH . Amphetamine psychosis: a ‘model’ schizophrenia mediated by catecholamines. Am J Psychiatry 1973; 130: 61–67.
Seeman P . Dopamine receptors and the dopamine hypothesis of schizophrenia. Synapse 1987; 1: 133–152.
Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT . Neurobiology of schizophrenia. Neuron 2006; 52: 139–153.
Lewis DA, Lieberman JA . Catching up on schizophrenia: natural history and neurobiology. Neuron 2000; 28: 325–334.
Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML . Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol 2001; 41: 237–260.
Coyle JT . Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol 2006; 26: 365–384.
Harrison PJ, Weinberger DR . Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry 2005; 10: 40–68.
Mohn AR, Gainetdinov RR, Caron MG, Koller BH . Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell 1999; 98: 427–436.
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 1994; 51: 199–214.
Newcomer JW, Farber NB, Jevtovic-Todorovic V, Selke G, Melson AK, Hershey T et al. Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology 1999; 20: 106–118.
Pilowsky LS, Bressan RA, Stone JM, Erlandsson K, Mulligan RS, Krystal JH et al. First in vivo evidence of an NMDA receptor deficit in medication-free schizophrenic patients. Mol Psychiatry 2006; 11: 118–119.
Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR et al. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci USA 2004; 101: 5099–5104.
Yoshii A, Constantine-Paton M . BDNF induces transport of PSD-95 to dendrites through PI3K-AKT signaling after NMDA receptor activation. Nat Neurosci 2007; 10: 702–711.
Scheid MP, Woodgett JR . PKB/AKT: functional insights from genetic models. Nat Rev Mol Cell Biol 2001; 2: 760–768.
Frame S, Cohen P . GSK3 takes centre stage more than 20 years after its discovery. Biochem J 2001; 359 (Pt 1): 1–16.
Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA . Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet 2004; 36: 131–137.
Freyberg Z, Ferrando SJ, Javitch JA . Roles of the Akt/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. Am J Psychiatry 2010; 167: 388–396.
Miyamoto S, Jarskog LF, Fleischhacker WW . Alternative pharmacologic targets for the treatment of schizophrenia: results from phase I and II trials. Curr Opin Psychiatry 2013; 26: 158–165.
Marx CE, Keefe RS, Buchanan RW, Hamer RM, Kilts JD, Bradford DW et al. Proof-of-concept trial with the neurosteroid pregnenolone targeting cognitive and negative symptoms in schizophrenia. Neuropsychopharmacology 2009; 34: 1885–1903.
Zorumski CF, Mennerick S . Neurosteroids as therapeutic leads in psychiatry. JAMA Psychiatry 2013; 70: 659–660.
Flood JF, Morley JE, Roberts E . Memory-enhancing effects in male mice of pregnenolone and steroids metabolically derived from it. Proc Natl Acad Sci USA 1992; 89: 1567–1571.
Farr SA, Flood JF, Scherrer JF, Kaiser FE, Taylor GT, Morley JE . Effect of ovarian steroids on footshock avoidance learning and retention in female mice. Physiol Behav 1995; 58: 715–723.
Stein DG . Brain damage, sex hormones and recovery: a new role for progesterone and estrogen? Trends Neurosci 2001; 24: 386–391.
Eser D, Baghai TC, Schule C, Nothdurfter C, Rupprecht R . Neuroactive steroids as endogenous modulators of anxiety. Curr Pharm Des 2008; 14: 3525–3533.
Zheng P . Neuroactive steroid regulation of neurotransmitter release in the CNS: action, mechanism and possible significance. Prog Neurobiol 2009; 89: 134–152.
Ritsner M, Maayan R, Gibel A, Weizman A . Differences in blood pregnenolone and dehydroepiandrosterone levels between schizophrenia patients and healthy subjects. Eur Neuropsychopharmacol 2007; 17: 358–365.
Wong P, Chang CC, Marx CE, Caron MG, Wetsel WC, Zhang X . Pregnenolone rescues schizophrenia-like behavior in dopamine transporter knockout mice. PLoS One 2012; 7: e51455.
Giros B, Jaber M, Jones SR, Wightman RM, Caron MG . Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 1996; 379: 606–612.
Pogorelov VM, Rodriguiz RM, Insco ML, Caron MG, Wetsel WC . Novelty seeking and stereotypic activation of behavior in mice with disruption of the Dat1 gene. Neuropsychopharmacology 2005; 30: 1818–1831.
Ralph RJ, Paulus MP, Fumagalli F, Caron MG, Geyer MA . Prepulse inhibition deficits and perseverative motor patterns in dopamine transporter knock-out mice: differential effects of D1 and D2 receptor antagonists. J Neurosci 2001; 21: 305–313.
Wu FS, Gibbs TT, Farb DH . Pregnenolone sulfate: a positive allosteric modulator at the N-methyl-D-aspartate receptor. Mol Pharmacol 1991; 40: 333–336.
Fahey JM, Lindquist DG, Pritchard GA, Miller LG . Pregnenolone sulfate potentiation of NMDA-mediated increases in intracellular calcium in cultured chick cortical neurons. Brain Res 1995; 669: 183–188.
Gibbs TT, Russek SJ, Farb DH . Sulfated steroids as endogenous neuromodulators. Pharmacol Biochem Behav 2006; 84: 555–567.
Kostakis E, Jang MK, Russek SJ, Gibbs TT, Farb DH . A steroid modulatory domain in NR2A collaborates with NR1 exon-5 to control NMDAR modulation by pregnenolone sulfate and protons. J Neurochem 2011; 119: 486–496.
Irwin RP, Lin SZ, Rogawski MA, Purdy RH, Paul SM . Steroid potentiation and inhibition of N-methyl-D-aspartate receptor-mediated intracellular Ca++ responses: structure-activity studies. J Pharmacol Exp Ther 1994; 271: 677–682.
Robel P, Young J, Corpechot C, Mayo W, Perche F, Haug M et al. Biosynthesis and assay of neurosteroids in rats and mice: functional correlates. J Steroid Biochem Mol Biol 1995; 53: 355–360.
Baulieu E, Robel P, Vatier O, Haug M, Le Goascogne C, Bourreau E . Neurosteroids: pregnenolone and dehydrocpiandrosterone in the brain. In: Fuxe K, Agnati L (eds). Receptor-Receptor Interactions. MacMillan Press: Basingstoke, UK, 1987; 89–104.
Dufort I, Tremblay Y, Belanger A, Labrie F, Luu-The V . Isolation and characterization of a stereospecific 3beta-hydroxysteriod sulfotransferase (pregnenolone sulfotransferase) cDNA. DNA Cell Biol 1996; 15: 481–487.
Kohjitani A, Fuda H, Hanyu O, Strott CA . Regulation of SULT2B1a (pregnenolone sulfotransferase) expression in rat C6 glioma cells: relevance of AMPA receptor-mediated NO signaling. Neurosci Lett 2008; 430: 75–80.
Kriz L, Bicikova M, Hampl R . Roles of steroid sulfatase in brain and other tissues. Physiol Res 2008; 57: 657–668.
Wang MD, Wahlstrom G, Backstrom T . The regional brain distribution of the neurosteroids pregnenolone and pregnenolone sulfate following intravenous infusion. J Steroid Biochem Mol Biol 1997; 62: 299–306.
Hashimoto K, Fujita Y, Iyo M . Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of fluvoxamine: role of sigma-1 receptors. Neuropsychopharmacology 2007; 32: 514–521.
Akk G, Covey DF, Evers AS, Steinbach JH, Zorumski CF, Mennerick S . Mechanisms of neurosteroid interactions with GABA(A) receptors. Pharmacol Ther 2007; 116: 35–57.
Rustichelli C, Pinetti D, Lucchi C, Ravazzini F, Puia G . Simultaneous determination of pregnenolone sulphate, dehydroepiandrosterone and allopregnanolone in rat brain areas by liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2013; 930: 62–69.
Akwa Y, Ladurelle N, Covey DF, Baulieu EE . The synthetic enantiomer of pregnenolone sulfate is very active on memory in rats and mice, even more so than its physiological neurosteroid counterpart: distinct mechanisms? Proc Natl Acad Sci USA 2001; 98: 14033–14037.
Reddy DS, Kulkarni SK . The effects of neurosteroids on acquisition and retention of a modified passive-avoidance learning task in mice. Brain Res 1998; 791: 108–116.
Vallee M, Purdy RH, Mayo W, Koob GF, Le Moal M . Neuroactive steroids: new biomarkers of cognitive aging. J Steroid Biochem Mol Biol 2003; 85: 329–335.
Mathis C, Paul SM, Crawley JN . The neurosteroid pregnenolone sulfate blocks NMDA antagonist-induced deficits in a passive avoidance memory task. Psychopharmacology 1994; 116: 201–206.
Cheney DL, Uzunov D, Guidotti A . Pregnenolone sulfate antagonizes dizocilpine amnesia: role for allopregnanolone. Neuroreport 1995; 6: 1697–1700.
Malenka RC, Nicoll RA . Long-term potentiation—a decade of progress? Science 1999; 285: 1870–1874.
Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J et al. LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron 2007; 53: 703–717.
Kostakis E, Smith C, Jang MK, Martin SC, Richards KG, Russek SJ et al. The neuroactive steroid pregnenolone sulfate stimulates trafficking of functional N-methyl D-aspartate receptors to the cell surface via a noncanonical, G protein, and Ca2+-dependent mechanism. Mol Pharmacol 2013; 84: 261–274.
Gainetdinov RR, Mohn AR, Bohn LM, Caron MG . Glutamatergic modulation of hyperactivity in mice lacking the dopamine transporter. Proc Natl Acad Sci USA 2001; 98: 11047–11054.
Xi D, Li YC, Snyder MA, Gao RY, Adelman AE, Zhang W et al. Group II metabotropic glutamate receptor agonist ameliorates MK801-induced dysfunction of NMDA receptors via the Akt/GSK-3beta pathway in adult rat prefrontal cortex. Neuropsychopharmacology 2011; 36: 1260–1274.
De Sarno P, Bijur GN, Zmijewska AA, Li X, Jope RS . In vivo regulation of GSK3 phosphorylation by cholinergic and NMDA receptors. Neurobiol Aging 2006; 27: 413–422.
Lei G, Xia Y, Johnson KM . The role of Akt-GSK-3beta signaling and synaptic strength in phencyclidine-induced neurodegeneration. Neuropsychopharmacology 2008; 33: 1343–1353.
Wang YB, Wang JJ, Wang SH, Liu SS, Cao JY, Li XM et al. Adaptor protein APPL1 couples synaptic NMDA receptor with neuronal prosurvival phosphatidylinositol 3-kinase/Akt pathway. J Neurosci 2012; 32: 11919–11929.
Compagnone NA, Salido E, Shapiro LJ, Mellon SH . Expression of steroid sulfatase during embryogenesis. Endocrinology 1997; 138: 4768–4773.
Billiar RB, Jassani M, Saarikoski S, Little B . Pregnenolone and pregnenolone sulfate metabolism in vivo and uterine extraction at midgestation. J Clin Endocrinol Metab 1974; 39: 27–35.
Mitamura K, Yatera M, Shimada K . Quantitative determination of pregnenolone 3-sulfate in rat brains using liquid chromatography/electrospray ionization-mass spectrometry. Anal Sci 1999; 15: 951–956.
Weaver CE, Land MB, Purdy RH, Richards KG, Gibbs TT, Farb DH . Geometry and charge determine pharmacological effects of steroids on N-methyl-D-aspartate receptor-induced Ca(2+) accumulation and cell death. J Pharmacol Exp Ther 2000; 293: 747–754.
Guarneri P, Russo D, Cascio C, De Leo G, Piccoli T, Sciuto V et al. Pregnenolone sulfate modulates NMDA receptors, inducing and potentiating acute excitotoxicity in isolated retina. J Neurosci Res 1998; 54: 787–797.
Emamian ES . AKT/GSK3 signaling pathway and schizophrenia. Front Mol Neurosci 2012; 5: 33.
Balu DT, Carlson GC, Talbot K, Kazi H, Hill-Smith TE, Easton RM et al. Akt1 deficiency in schizophrenia and impairment of hippocampal plasticity and function. Hippocampus 2012; 22: 230–240.
Engel T, Hernandez F, Avila J, Lucas JJ . Full reversal of Alzheimer's disease-like phenotype in a mouse model with conditional overexpression of glycogen synthase kinase-3. J Neurosci 2006; 26: 5083–5090.
Calvin HI, Vandewiele RL, Lieberman S . Evidence that steroid sulfates serve as biosynthetic intermediates: in vivo conversion of pregnenolone-sulfate-S35 to dehydroisoandrosterone sulfate-S35. Biochemistry 1963; 2: 648–653.
Akbarian S, Sucher NJ, Bradley D, Tafazzoli A, Trinh D, Hetrick WP et al. Selective alterations in gene expression for NMDA receptor subunits in prefrontal cortex of schizophrenics. J Neurosci 1996; 16: 19–30.
Weinberger DR . Schizophrenia and the frontal lobe. Trends Neurosci 1988; 11: 367–370.
Acknowledgements
We thank the staffs at the Duke-NUS Behavioral Phenotyping Core Facility, Duke-NUS vivarium and SingHealth Experimental Medical Centre for their support. This research was supported in part by grants from Singapore National Medical Research Council (NMRC) Translational and Clinical Research Program (NMRC/TCR/003-GMS/2008) and Duke-NUS Block Funding to XZ, and NMRC Basic New Investigator Grant (BNIG13nov003) to PW. PW is also currently supported by the NMRC NUHS Centre Grant—Neuroscience Phenotyping Core (NMRC/CG/013/2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Translational Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Wong, P., Sze, Y., Chang, C. et al. Pregnenolone sulfate normalizes schizophrenia-like behaviors in dopamine transporter knockout mice through the AKT/GSK3β pathway. Transl Psychiatry 5, e528 (2015). https://doi.org/10.1038/tp.2015.21
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2015.21
This article is cited by
-
Temporal effects on death by suicide: empirical evidence and possible molecular correlates
Discover Mental Health (2023)
-
A synthetic pregnenolone analog promotes microtubule dynamics and neural development
Cell & Bioscience (2022)
-
Validation of impaired Transient Receptor Potential Melastatin 3 ion channel activity in natural killer cells from Chronic Fatigue Syndrome/ Myalgic Encephalomyelitis patients
Molecular Medicine (2019)
-
Synthesis and CYP17α hydroxylase inhibition activity of new 3α- and 3β-ester derivatives of pregnenolone and related ether analogues
Medicinal Chemistry Research (2016)