Abstract
Several studies have reported that adults with major depressive disorder have shorter telomere length and reduced hippocampal volumes. Moreover, studies of adult populations without major depressive disorder suggest a relationship between peripheral telomere length and hippocampal volume. However, the relationship of these findings in adolescents with major depressive disorder has yet to be explored. We examined whether adolescent major depressive disorder is associated with altered peripheral telomere length and hippocampal volume, and whether these measures relate to one another. In 54 unmedicated adolescents (13–18 years) with major depressive disorder and 63 well-matched healthy controls, telomere length was assessed from saliva using quantitative polymerase chain reaction methods, and bilateral hippocampal volumes were measured with magnetic resonance imaging. After adjusting for age and sex (and total brain volume in the hippocampal analysis), adolescents with major depressive disorder exhibited significantly shorter telomere length and significantly smaller right, but not left hippocampal volume. When corrected for age, sex, diagnostic group and total brain volume, telomere length was not significantly associated with left or right hippocampal volume, suggesting that these cellular and neural processes may be mechanistically distinct during adolescence. Our findings suggest that shortening of telomere length and reduction of hippocampal volume are already present in early-onset major depressive disorder and thus unlikely to be only a result of accumulated years of exposure to major depressive disorder.
Similar content being viewed by others
Introduction
Major depressive disorder (MDD) and its associated peripheral and central effects is relatively understudied in adolescents, compared with adults, despite the fact that adolescence is a vulnerable period for depression onset.1 The prevalence of MDD increases dramatically around puberty,2 and the lifetime prevalence of depression in the United States among 13–18-year olds was recently estimated to be 14.3%.3 MDD is now considered one of the largest contributors to the United States disease burden in terms of quantified mortality and disability,4 and in 2010, depression symptoms were ranked as the second largest contributor worldwide to ‘years lived with disabilities’.5 So far, prevention and treatment strategies have not been successful in decreasing the prevalence of adolescent MDD. Potential biomarkers may elucidate risk factors and pathophysiological pathways and aid the development of more targeted and effective preventions and treatments, ideally before the recurrent course of depression is established and associated systemic effects have manifested.
Recently, telomere length (TL), which is considered to be a measure of human cellular aging,6, 7 has received considerable attention as a possible biomarker in psychiatric illnesses, offering an explanation for why patients with MDD exhibit an increased risk of developing comorbid and aging-related diseases,8 including diabetes,9 dementia,10 certain types of cancer11 and cardiovascular diseases.12 Telomeric DNA is comprised of tandem repeat DNA sequences that, together with associated proteins, forms the telomere that caps the chromosome end, providing protection from genome-destabilizing DNA damage responses.13 Critical shortening of TL may result in cellular senescence or cell death, and mutations causing insufficient telomere maintenance result in a spectrum of diseases showing overlaps with diseases occurring with population aging.14 TL is regarded as a measure of cellular aging in humans as it (a) progressively shortens with every cell division, unless acted upon by the telomere repair enzyme, telomerase;15 (b) on average, decreases with advancing age in humans;6 and (c) is correlated with current and future physical diseases associated with aging.7
Several studies have examined whether accelerated cellular aging is present in depressed adults, yet findings remain inconsistent. Some studies find shorter TL of white blood cells of the peripheral circulation, such as leukocytes or peripheral blood mononuclear cells in MDD,8, 16, 17 whereas other studies have not replicated these findings.18, 19, 20 Shortening of TL has been reported to be proportional to the total lifetime exposure and duration of MDD, suggesting that accelerated telomere attrition reflects cumulative systemic effects of MDD.17, 21, 22, 23 However, another study did not find such a ‘dose-response’ relationship24 and it was also absent in a late-life cohort study.25 Furthermore, TL has been associated with lifestyle factors, for example, poor diet, smoking and decreased physical activity.26 To date, it is uncertain whether telomere shortening is the result of chronic depressive illness, lifestyle factors, chromosomal risk factors for developing MDD or a combination of these factors.
In addition to TL shortening, a large body of literature suggests volumetric hippocampal reductions in adult MDD,27, 28, 29, 30 but mixed results are also reported.31, 32 The hippocampal volume (HV) reduction is especially evident in elderly or chronically-ill samples,33 and smaller HV generally seem more apparent in patients suffering from prolonged and/or recurrent episodes of depression.29, 34 To date, studies on HV in pediatric MDD show inconsistent findings (for a review see ref. 35), reporting both HV reduction in the MDD samples36, 37, 38, 39 and no HV differences between depressed youth and healthy controls (HCs).40, 41, 42 However, non-depressed adolescents at high risk for depression also showed smaller HV compared with low-risk participants.43 Thus, inconsistencies exist among and between studies in adults and adolescents, and discrepancies of HV differences associated with MDD, remain unresolved.
A series of recent studies has indicated an association between telomere length in both peripheral leukocytes and peripheral blood mononuclear cells and structural brain changes in the hippocampus in populations of adults without MDD10, 44, 45 and the only study investigating the relationship between peripheral blood mononuclear cell TL and HV in depressed adults showed no correlation between them.46 Whether the telomere shortening and the HV reduction seen in MDD are involved in driving pathophysiological processes of MDD is unclear, nor is it clear whether and how these processes are related. For example, peripheral TL may serve as a proxy for hippocampal TL and hippocampal TL shortening may be the underlying mechanism by which HV is reduced in MDD. As telomere shortening (in mice) may retard neuritogenesis even in adult mouse brain tissue,47 and as newly generated neurons are hypersensitive to telomere damage,48 we hypothesize that a potential shortening of TL in hippocampal neurons may disrupt or dampen plasticity and, over time, cause a reduction of HV. Alternatively, both shortened peripheral TL and reduced HV may be the result of a common mediating factor, such as oxidative stress.49, 50 Many factors may thus potentially influence both the TL and HV in depressed populations, such as duration of illness, age of onset, number of recurrent episodes, ongoing or previous medication, psychiatric comorbidity and lifestyle factors.51 Studying early onset of MDD allows most of these potential confounders to be minimized. Investigations of the association between peripheral TL and changes of HV, especially early in the disease progression, may help further elucidate the pathophysiological pathways of early MDD and could potentially identify new treatment targets.
In this study, TL of saliva sample cells and HV are compared between adolescent MDD and HC with the following aims: (a) to investigate whether depressed unmedicated adolescents show shorter salivary TL as compared with well-matched HC, (b) to determine whether adolescent MDD is associated with reduced HV relative to HC and (c) to examine the relationship between peripheral TL and HV in adolescent MDD. The question of whether there is an association between TL and HV in MDD has never been examined in adolescents, and based on studies of adults there is rationale for expecting both a positive association between TL and HV as well as no such association. One previous study on adult MDD46 showed that shorter TL was not related to reduced HV, but such a relationship has been shown in other types of samples.10, 44, 45
Materials and methods
Participants
A total sample of 117 participants (age range 13–18 years) was selected from a larger ongoing study at the University of California, San Diego.52, 53 HC adolescents were recruited from the San Diego area through posted flyers, email and the Internet. MDD adolescents were recruited from 35 adolescent psychiatric clinics dispersed through the San Diego County area. After application of the exclusion criteria (see Supplementary Information), the data from 54 adolescents with a Diagnostic and Statistical Manual of Mental Disorders, fourth edition diagnosis of MDD established by the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version54 and 63 well-matched HCs were used in the statistical analyses. In all the participants, depression severity was clinically assessed with the Children’s Depression Rating Scale-Revised (CDRS-R)55 and self-assessment scales (see Supplementary Information) at the time of magnetic resonance imaging (MRI) scanning. Saliva samples were also collected at the time of MRI scanning. Early adversity was assessed by retroactive self-report using the Childhood Trauma Questionnaire,56 and collected later in the study, to test the hypothesis that childhood trauma may be related to TL shortening, HV reduction and adolescent depression.57, 58 For a complete description of the assessments, see Supplementary Information. The institutional review boards of the University of California, San Diego and University of California, San Francisco, Rady Children’s Hospital and the County of San Diego approved this study. Participants and their parents/legal guardians provided written informed assent and consent, respectively. Participants received monetary compensation for their time.
Telomere length measurement
Genomic DNA was purified from 500 μl of saliva collected in an Oragene DNA kit (DNA Genotek, Kanata, Ontario, Canada) with the DNA Agencourt DNAdvance kit (cat #A48705, Beckman Coulter Genomics, Brea, CA, USA) according to the instruction of the manufacturer. DNA was quantified by Quant-iT PicoGreen dsDNA Assay Kit (cat #P7589, Life Technologies, Grand Island, NY, USA) and run on 0.8% agarose gels for integrity check. Degraded DNA samples were excluded from TL analysis. The quantitative PCR TL measurement assay was adapted from the original method,59 and was performed by the Blackburn Lab at the Department of Biochemistry and Biophysics at University of California, San Francisco, and is described in detail elsewhere.60 The personnel who performed the assay received de-identified samples and were blind to all other measurements. For a more detailed description of the procedure, see Supplementary Information.
Image data acquisition and preprocessing
All the MRI data were acquired on a 3 T MR750 GE scanner (GE Healthcare, Milwaukee, WI, USA) at the University of California, San Diego Center for functional MRI. A fast-spoiled gradient-recalled echo sequence was used to collect T1-weighted images: TR=8.1 ms, TE=3.17ms, TI=450ms, flip angle=12°, 256 × 256 matrix, FOV=250 × 250 mm, 168 sagittal slices with a voxel size of 0.98 × 0.98 × 1 mm. Data were preprocessed and analyzed with AFNI61 and FSL.62 AFNI61 was only used to remove non-brain tissue. The resultant images were segmented into gray and white matter, and cerebrospinal fluid using FAST.63 Total brain volume (TBV) was calculated from the gray and white matter segments. Bilateral HVs were estimated with FSL-FIRST.64 FSL version 5.0.8 was used to analyze all the participants, and all the segmentations were visually inspected.
Statistical analysis
Statistical analyses were performed using R.65 Between-group comparisons of the demographic variables were conducted by Welch t-tests, χ2 tests and Wilcoxon rank-sum tests. To investigate between-group differences in TL, we conducted an analysis of covariance with diagnostic group (MDD, HC) as a between-subjects factor and sex and age (in years) as within-subjects factors. Analysis of HV (left and right separately) was conducted similarly, insofar as we used analyses of covariance with group as between-subjects and age and sex as within-subject factors. TBV was also included in these models to account for inter-individual differences in brain volume.66 Examination of TBV between groups was performed as for the TL analysis with an additional model investigating the interaction of group and age on TBV. To investigate the association of HV and peripheral TL, a linear regression model was computed with HV (left, right separately) as the dependent variable and TL, age, sex, diagnostic group (for across group analysis), and TBV as predictors. Finally, linear regressions were conducted to examine associations between demographic (age) and clinical characteristics (CDRS-R, CTQ) on both TL and HV. This was performed separately within MDDs and HCs and HV regressions were controlled for TBV. Analyses of covariance and linear regressions for TL and TBV were considered significant at P<0.05. The analyses of HVs were Bonferroni corrected for two hemispheres and were consequently considered significant at P<0.025. Personnel performing these analyses were not blind to participant grouping.
Results
Sample characteristics
The mean age of our total sample was 15.8 years (s.d.=1.32 years, range 13.1–18.1 years), consisting of 54.7% of females. The groups did not differ significantly in age, sex, pubertal status and socioeconomic status (all P>0.05), and no left-handed persons were included in the sample. For a detailed description of these assessments, see Supplementary Information Comorbid psychiatric disorders were assessed during the KSAD-PL interview, a diagnostic interview the MDD participants had to undergo to confirm a primary diagnosis of MDD, and, therefore, eligibility for the current study. For a detailed description of current comorbidities in our MDD sample, see Table 1. To investigate the effect of the number of comorbidities on TL and HV, we conducted analyses of variance with the number of comorbidities (0, 1, 2+) as a between-subjects factor (and total brain volume as a within-subject factor in case of the hippocampal volume analyses), but found no effect of the number of comorbidities on either TL or HV in the MDDs (all P>0.4). See Supplementary Information for more details. As anticipated, MDD subjects reported higher levels of depression (CDRS-R) and childhood trauma (CTQ) than the HCs (both P<0.001; see Table 1).
Telomere length
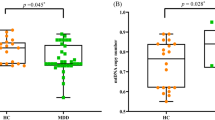
Significantly shorter salivary TL was observed in the depressed subjects compared with HCs (mean T/S ratio±s.d. MDD: 1.39±0.24, HC: 1.51±0.25; F(1,113)= 6.59, P<0.05). There was also a main effect of age (F(1,113)=4.85, P<0.05), with older subjects showing shorter TL. Mean TL of both groups is presented in Figure 1. TL was not significantly associated with CDRS-R scores or CTQ total score in either group (all P>0.19).
Hippocampal volume
MDD subjects had significantly smaller right HVs than HCs after adjusting for age, sex and TBV (mean HV±s.d. MDD: 3890 μl±426, HC: 4040 μl±336; F(1,112)= 6.91, P<0.01), but no difference in the left HV was observed (F(1,112)= 0.23, P=0.63); see Figure 2. A main effect of sex was seen for both left and right HV (both P<0.001), with males showing larger right and left hippocampi than females. TBV was lower in adolescents with MDD compared with HCs (mean TBV±SD MDD: 1 074 655 μl±111 958, HC: 1 142 252 μl±96 964; F(1,113)=19.7, P<0.001), after controlling for age and sex. However, an additional model showed a significant interaction between group and age on TBV (F(1,112)=4.22, P<0.05), after controlling for sex. HV (left, right) was not significantly associated with CDRS-R (both P>0.033) or CTQ total score (both P>0.36) in either group.
Left and right hippocampal volumes (mean±s.e.m.) adjusted for age, sex and total brain volume in adolescents with major depressive disorder (MDD) and healthy controls (HCs). Although there was no significant group difference observed for the left hippocampus (a), the depressed group exhibited significantly smaller right hippocampal volumes, F1,112=6.91, **P=0.009 (b).
Relationship between HV and TL
We examined the association between HV (left and right separately) and TL across groups and found that TL did not significantly predict the left (β=−24.4, t(111)=−0.18, P>0.86) or right HV (β=53, t(111)=−0.44, P>0.66), when correcting for age, sex, diagnostic group and TBV. Separate within-group analyses did not detect any significant relationship between HV and TL (all P>0.08). Additional models not adjusting for covariates also did not reveal any significant associations between TL and HV (see Supplementary Information).
Discussion
To our knowledge, this is the first study to examine peripheral TL and HV in adolescent depression. The study yielded three main findings: first, peripheral TL was significantly shorter in adolescents with MDD compared with HCs. Second, MDD subjects had reduced right, but not left, HV compared with HC. Finally, no significant associations between TL and HV, within or across groups, were found.
The first finding provides evidence that shorter salivary TL is present in a sample of adolescents diagnosed with MDD as compared with HC. As the depressed adolescents were not receiving any psychotropic medication, our observation of TL shortening, in the MDD group, suggests that shortened telomeres may be a marker that is associated with adolescent onset MDD and not solely result from extended MDD exposure or the cumulative effect of MDD, as previously suggested in adult samples.22, 23 Importantly, even though the adolescents may have had limited exposure to lifestyle factors associated with cellular aging as compared with older samples, the shorter TL in our depressed sample may still be related to lifestyle or other life factors. The lack of an association between depression severity scores and TL in our sample also supports the notion that TL shortening may either be a part of depression etiology, or else reflective of other damaging factors that are part of its etiology or both. Interestingly, non-depressed girls with depressed mothers, that is, girls at high risk for developing MDD also show shorter salivary TL compared with age-matched low-risk girls.60 These findings could be explained by a possible genetic effect on TL and/or increased exposure to factors that could influence TL even before the onset of MDD, such as maternal stress during pregnancy67 and early-life stress in the MDD group.60 The present study, however, found no significant correlations between either TL or HV and childhood trauma.
The second finding of decreased right, although not left, HV in adolescent MDD is consistent with the consensus of reduced HV in adult MDD27, 28, 29 and what is generally reported in adolescent MDD, (for a review, see ref. 35). Analogous to our TL results, this suggests that the hippocampus may be negatively affected early in the course of MDD. Furthermore, HV volume changes may also be involved in the etiology of the disorder and not only after extended duration and/or recurrent episodes of depression as has previously27, 29, 68, 69 but not consistently31, 70 been reported. Consistent with our results, some studies show HV reductions in adolescents at risk of developing MDD even before clinical manifestation of the disease.37, 38 Recent findings show associations between hippocampal volume abnormalities and several mechanisms involved in stress-related disorders and MDD, such as the messenger RNA expression of glucocorticoid inducible genes,71 glucocorticoid receptor methylation,72 genetic polymorphisms associated with variation in pro-inflammatory cytokine levels73 and increased oxidative stress.49
The asymmetrical right-sided HV reduction found in our MDD sample was present independent of age and sex. Previous literature on adolescents at risk for MDD and adults with MDD report inconsistent findings with regard to the lateralization of hippocampal volume reductions with both left-sided,36 right-sided36 and bilateral volume reductions.37, 38 Furthermore, hippocampus asymmetries are also prevalent in the normal population independent of MDD and the neurodevelopmental processes; other possible mechanisms and clinical implications of these asymmetries are unknown.74 More research into the causes and implications of lateralization of structural changes in the hippocampus is warranted in general and in MDD in particular.
The third finding showed a lack of an association between TL and HV, replicating the one similar study performed in adults. This may suggest that these cellular and neural features may be mechanistically distinct, a least in this age group. The relationship between peripheral TL and HV has only been previously investigated in one small study with an MDD population wherein leukocyte TL was found to be unrelated to HV in both MDD and HC.46 Interestingly, TL has been linked to variance in HV in the general population,75 in early-stage dementia10 and in adolescents with early-childhood stress.76 In addition, experimental studies have shown that oxidative stress is associated with telomere shortness in the periphery, and associated with reduced neurogenesis and increased neuronal death in the hippocampus.77 Several, but not all, studies of MDD report high levels of peripheral markers of oxidative stress, (for a review, see ref. 78). Peripheral blood circulation cell telomerase activity, rather than TL, was recently found to be positively correlated with HV in adult MDD79 but not in HC, suggesting that decreased telomerase activity, which is a cellular enzyme compensating for the loss of telomeric repeats and preserving TL13, 20 may thus be more directly related to HV reduction than TL itself, at least regarding peripheral TL and telomerase measures.
Our relatively large sample size lends confidence to our findings and a major strength of this study is the reduction of confounding factors on TL and HV, such as psychotropic medications. Nevertheless, our study is not without limitations. First, the relatively young age range of our MDD sample limited the possible duration of MDD as compared with adult samples, but unfortunately, we lack more precise data on the age of onset or the duration of depression in our samples. Second, the design was cross-sectional in nature, yet the cellular and neural outcomes associated with MDD are likely to be influenced by neurodevelopment. Third, telomerase cannot be analyzed from salivary samples; therefore, the previously described relationship between telomerase activity in peripheral blood mononuclear cells such as leukocytes and HV could not be validated. Saliva contains both epithelial cells and leukocytes, which are not possible to separate in our analysis but known to be highly correlated, suggesting systematic effects of TL regulation.80 Therefore, the use of saliva as the source of DNA for TL measurement is justified. TL in human tissues generally shows significant correlations within individuals,81 even though human postmortem investigations shows no strong correlations between TL in peripheral leukocytes and other tissues including brain tissue.82 It is not fully known how the TL in peripheral cells relates to TL in the hippocampal neurons. However, shorter hippocampal telomere length has been found in well-defined depression models of rats.83
Certain limitations arise from our choice of FSL-FIRST to perform subcortical segmentation. Although Freesurfer84 estimates of HV have been shown to have higher correlation with the volumes derived from manual segmentations,85 Freesurfer tends to overestimate HV in younger age groups compared with manual segmentations.86 In addition, although the data set used to train FSL-FIRST to perform automated segmentation consisted of a large sample (n=336) with a wide age range (4.2–87 years) of controls and participants with different pathophysiologies,64 we cannot rule out the presence of a systematic bias attributable to the mismatch between the sample used in the present study and that used to train FSL-FIRST.
Finally, the original study was not designed to assess early adversity and the CTQ was therefore added later in the study. Consequently, CTQ data were not available from the full samples (see Table 1), which reduced our power to detect associations. Although significantly higher CTQ scores were found in the MDD sample compared with the HC, no associations were found between CTQ-score and TL or HV within the MDD sample. Our finding does not support the hypothesis that TL and HV alterations observed in adolescent MDD are explained by childhood trauma alone, but rather suggests that MDD may influence these outcomes.
To fully capture how TL and HV changes relate to MDD pathophysiology and disease progression, future studies with prospective longitudinal designs are needed, ideally combining MRI with assessments of clinical data including adverse childhood experiences, putative biochemical mediators of TL shortening and/or HV diminution, for example, oxidative stress, systemic inflammation and telomerase activity.
In conclusion, this is the first study to reveal shorter salivary TL in adolescent MDD, despite their relatively short illness history and limited exposure to lifestyle factors associated with cellular aging. This novel finding supports the notion that telomere shortening is not only associated with chronic depressive illness, but present early in the course of MDD, possibly representing a genetic and/or acquired risk factor for MDD development. We also show that the right HV is reduced in early-onset MDD. This suggests that HV reduction is present before MDD or that the hippocampus may be negatively affected early in disease progression and not only after recurrent and/or elongated periods of depression. The absence of a significant relationship between peripheral TL and HV in our sample suggests that these cellular and neural outcomes associated with MDD may be mechanistically distinct, at least during adolescence. Given that this association may be different in other age groups, further exploration is warranted.
References
Angold A, Costello EJ, Worthman CM . Puberty and depression: the roles of age, pubertal status and pubertal timing. Psychol Med 1998; 28: 51–61.
Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE . Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. J Abnorm Psychol 1998; 107: 128–140.
Avenevoli S, Swendsen J, He JP, Burstein M, Merikangas KR . Major depression in the national comorbidity survey-adolescent supplement: prevalence, correlates, and treatment. J Am Acad Child Adolesc Psychiatry 2015; 54: 37–44.
Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ et al. Burden of depressive disorders by country, sex, age, and year: findings from the Global Burden of Disease Study 2010. PLoS Med 2013; 10: e1001547.
Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2163–2196.
Blackburn EH . Telomere states and cell fates. Nature 2000; 408: 53–56.
Wolkowitz OM, Reus VI, Mellon SH . Of sound mind and body: depression, disease, and accelerated aging. Dialogues Clin Neurosci 2011; 13: 25–39.
Wolkowitz OM, Epel ES, Reus VI, Mellon SH . Depression gets old fast: do stress and depression accelerate cell aging? Depress Anxiety 2010; 27: 327–338.
McIntyre RS, Soczynska JK, Konarski JZ, Woldeyohannes HO, Law CW, Miranda A et al. Should depressive syndromes be reclassified as "Metabolic Syndrome Type II"? Ann Clin Psychiatry 2007; 19: 257–264.
Grodstein F, van Oijen M, Irizarry MC, Rosas HD, Hyman BT, Growdon JH et al. Shorter telomeres may mark early risk of dementia: preliminary analysis of 62 participants from the nurses' health study. PLoS One 2008; 3: e1590.
Willeit P, Willeit J, Mayr A, Weger S, Oberhollenzer F, Brandstatter A et al. Telomere length and risk of incident cancer and cancer mortality. JAMA 2010; 304: 69–75.
Epel ES, Lin J, Wilhelm FH, Wolkowitz OM, Cawthon R, Adler NE et al. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology 2006; 31: 277–287.
Blackburn EH . Switching and signaling at the telomere. Cell 2001; 106: 661–673.
Armanios M, Blackburn EH . The telomere syndromes. Nat Rev Genet 2012; 13: 693–704.
Blackburn EH . Structure and function of telomeres. Nature 1991; 350: 569–573.
Garcia-Rizo C, Fernandez-Egea E, Miller BJ, Oliveira C, Justicia A, Griffith JK et al. Abnormal glucose tolerance, white blood cell count, and telomere length in newly diagnosed, antidepressant-naive patients with depression. Brain Behav Immun 2013; 28: 49–53.
Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH et al. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry 2006; 60: 432–435.
Shaffer JA, Epel E, Kang MS, Ye S, Schwartz JE, Davidson KW et al. Depressive symptoms are not associated with leukocyte telomere length: findings from the Nova Scotia Health Survey (NSHS95), a population-based study. PLoS One 2012; 7: e48318.
Hoen PW, Rosmalen JG, Schoevers RA, Huzen J, van der Harst P, de Jonge P . Association between anxiety but not depressive disorders and leukocyte telomere length after 2 years of follow-up in a population-based sample. Psychol Med 2013; 43: 689–697.
Needham BL, Mezuk B, Bareis N, Lin J, Blackburn EH, Epel ES . Depression, anxiety and telomere length in young adults: evidence from the National Health and Nutrition Examination Survey. Mol Psychiatry 2014; 20: 520–528.
Wikgren M, Maripuu M, Karlsson T, Nordfjall K, Bergdahl J, Hultdin J et al. Short telomeres in depression and the general population are associated with a hypocortisolemic state. Biol Psychiatry 2012; 71: 294–300.
Wolkowitz OM, Mellon SH, Epel ES, Lin J, Dhabhar FS, Su Y et al. Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress—preliminary findings. PLoS One 2011; 6: e17837.
Verhoeven JE, Revesz D, Epel ES, Lin J, Wolkowitz OM, Penninx BW . Major depressive disorder and accelerated cellular aging: results from a large psychiatric cohort study. Mol Psychiatry 2014; 19: 895–901.
Hartmann N, Boehner M, Groenen F, Kalb R . Telomere length of patients with major depression is shortened but independent from therapy and severity of the disease. Depress Anxiety 2010; 27: 1111–1116.
Schaakxs R, Verhoeven JE, Oude Voshaar RC, Comijs HC, Penninx BW . Leukocyte telomere length and late-life depression. Am J Geriatr Psychiatry 2015; 23: 423–432.
Lin J, Epel E, Blackburn E . Telomeres and lifestyle factors: roles in cellular aging. Mutat Res 2012; 730: 85–89.
Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS . Hippocampal volume reduction in major depression. Am J Psychiatry 2000; 157: 115–118.
Frodl T, Meisenzahl EM, Zetzsche T, Born C, Groll C, Jager M et al. Hippocampal changes in patients with a first episode of major depression. Am J Psychiatry 2002; 159: 1112–1118.
MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci USA 2003; 100: 1387–1392.
Cole J, Costafreda SG, McGuffin P, Fu CH . Hippocampal atrophy in first episode depression: a meta-analysis of magnetic resonance imaging studies. J Affect Disord 2011; 134: 483–487.
McKinnon MC, Yucel K, Nazarov A, MacQueen GM . A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci 2009; 34: 41–54.
Treadway MT, Waskom ML, Dillon DG, Holmes AJ, Park MT, Chakravarty MM et al. Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biol Psychiatry 2015; 77: 285–294.
Savitz JB, Drevets WC . Imaging phenotypes of major depressive disorder: genetic correlates. Neuroscience 2009; 164: 300–330.
Lorenzetti V, Allen NB, Fornito A, Yucel M . Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J Affect Disord 2009; 117: 1–17.
Hulvershorn LA, Cullen K, Anand A . Toward dysfunctional connectivity: a review of neuroimaging findings in pediatric major depressive disorder. Brain Imaging Behav 2011; 5: 307–328.
MacMaster FP, Kusumakar V . Hippocampal volume in early onset depression. BMC Med 2004; 2: 2.
MacMaster FP, Mirza Y, Szeszko PR, Kmiecik LE, Easter PC, Taormina SP et al. Amygdala and hippocampal volumes in familial early onset major depressive disorder. Biol Psychiatry 2008; 63: 385–390.
Rao U, Chen LA, Bidesi AS, Shad MU, Thomas MA, Hammen CL . Hippocampal changes associated with early-life adversity and vulnerability to depression. Biol Psychiatry 2010; 67: 357–364.
Caetano SC, Fonseca M, Hatch JP, Olvera RL, Nicoletti M, Hunter K et al. Medial temporal lobe abnormalities in pediatric unipolar depression. Neurosci Lett 2007; 427: 142–147.
MacMillan S, Szeszko PR, Moore GJ, Madden R, Lorch E, Ivey J et al. Increased amygdala: hippocampal volume ratios associated with severity of anxiety in pediatric major depression. J Child Adolesc Psychopharmacol 2003; 13: 65–73.
Rosso IM, Cintron CM, Steingard RJ, Renshaw PF, Young AD, Yurgelun-Todd DA . Amygdala and hippocampus volumes in pediatric major depression. Biol Psychiatry 2005; 57: 21–26.
Lupien SJ, Parent S, Evans AC, Tremblay RE, Zelazo PD, Corbo V et al. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc Natl Acad Sci USA 2011; 108: 14324–14329.
Chen MC, Hamilton JP, Gotlib IH . Decreased hippocampal volume in healthy girls at risk of depression. Arch Gen Psychiatry 2010; 67: 270–276.
Jacobs EG, Epel ES, Lin J, Blackburn EH, Rasgon NL . Relationship between leukocyte telomere length, telomerase activity, and hippocampal volume in early aging. JAMA Neurol 2014; 71: 921–923.
Wikgren M, Karlsson T, Lind J, Nilbrink T, Hultdin J, Sleegers K et al. Longer leukocyte telomere length is associated with smaller hippocampal volume among non-demented APOE epsilon3/epsilon3 subjects. PLoS One 2012; 7: e34292.
Wolkowitz OM, Mellon SH, Lindqvist D, Epel ES, Blackburn EH, Lin J et al. PBMC telomerase activity, but not leukocyte telomere length, correlates with hippocampal volume in major depression. Psychiatry Res 2015; 232: 58–64.
Ferron SR, Marques-Torrejon MA, Mira H, Flores I, Taylor K, Blasco MA et al. Telomere shortening in neural stem cells disrupts neuronal differentiation and neuritogenesis. J Neurosci 2009; 29: 14394–14407.
Cheng A, Shin-ya K, Wan R, Tang SC, Miura T, Tang H et al. Telomere protection mechanisms change during neurogenesis and neuronal maturation: newly generated neurons are hypersensitive to telomere and DNA damage. J Neurosci 2007; 27: 3722–3733.
Lindqvist D, Mueller S, Mellon SH, Su Y, Epel ES, Reus VI et al. Peripheral antioxidant markers are associated with total hippocampal and CA3/dentate gyrus volume in MDD and healthy controls-preliminary findings. Psychiatry Res 2014; 224: 168–174.
von Zglinicki T . Oxidative stress shortens telomeres. Trends Biochem Sci 2002; 27: 339–344.
Malykhin NV, Carter R, Seres P, Coupland NJ . Structural changes in the hippocampus in major depressive disorder: contributions of disease and treatment. J Psychiatry Neurosci 2010; 35: 337–343.
Connolly CG, Wu J, Ho TC, Hoeft F, Wolkowitz O, Eisendrath S et al. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol Psychiatry 2013; 74: 898–907.
Ho TC, Connolly CG, Henje Blom E, LeWinn KZ, Strigo IA, Paulus MP et al. Emotion-dependent functional connectivity of the default mode network in adolescent depression. Biol Psychiatry 2014; 78: 635–646.
Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36: 980–988.
Poznanski EO . Children's Depression Rating Scale-Revised (CDRS-R) Manual. Western Psychological Services: Los Angeles, 1996, pp 76.
Bernstein DP, Ahluvalia T, Pogge D, Handelsman L . Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. J Am Acad Child Adolesc Psychiatry 1997; 36: 340–348.
Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry 2012; 71: 286–293.
Shalev I . Early life stress and telomere length: Investigating the connection and possible mechanisms: a critical survey of the evidence base, research methodology and basic biology. BioEssays 2012; 34: 943–952.
Cawthon RM . Telomere measurement by quantitative PCR. Nucleic Acids Res 2002; 30: e47.
Gotlib IH, LeMoult J, Colich NL, Foland-Ross LC, Hallmayer J, Joormann J et al. Telomere length and cortisol reactivity in children of depressed mothers. Mol Psychiatry 2014; 20: 615–620.
Cox RW . AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29: 162–173.
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23: S208–S219.
Zhang Y, Brady M, Smith S . Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging 2001; 20: 45–57.
Patenaude B, Smith SM, Kennedy DN, Jenkinson M . A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 2011; 56: 907–922.
R Development Core Team R: A Language and Environment for Statistical Computing Vol.1). R Foundation for Statistical Computing: Vienna, Austria, 2014.
O'Brien LM, Ziegler DA, Deutsch CK, Frazier JA, Herbert MR, Locascio JJ . Statistical adjustments for brain size in volumetric neuroimaging studies: some practical implications in methods. Psychiatry Res 2011; 193: 113–122.
Entringer S, Epel ES, Lin J, Buss C, Shahbaba B, Blackburn EH et al. Maternal psychosocial stress during pregnancy is associated with newborn leukocyte telomere length. Am J Obstet Gynecol 2013; 208: 134.e1–134.e7.
Stratmann M, Konrad C, Kugel H, Krug A, Schöning S, Ohrmann P et al. Insular and hippocampal gray matter volume reductions in patients with major depressive disorder. PLoS One 2014; 9: e102692.
Mervaala E, Fohr J, Kononen M, Valkonen-Korhonen M, Vainio P, Partanen K et al. Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol Med 2000; 30: 117–125.
Vakili K, Pillay SS, Lafer B, Fava M, Renshaw PF, Bonello-Cintron CM et al. Hippocampal volume in primary unipolar major depression: a magnetic resonance imaging study. Biol Psychiatry 2000; 47: 1087–1090.
Frodl T, Carballedo A, Frey EM, O'Keane V, Skokauskas N, Morris D et al. Expression of glucocorticoid inducible genes is associated with reductions in cornu ammonis and dentate gyrus volumes in patients with major depressive disorder. Dev Psychopathol 2014; 26: 1209–1217.
Na KS, Chang HS, Won E, Han KM, Choi S, Tae WS et al. Association between glucocorticoid receptor methylation and hippocampal subfields in major depressive disorder. PLoS One 2014; 9: e85425.
Raz N, Daugherty AM, Bender AR, Dahle CL, Land S . Volume of the hippocampal subfields in healthy adults: differential associations with age and a pro-inflammatory genetic variant. Brain Struct Funct 2014; 220: 2663–2674.
Guadalupe T, Zwiers MP, Teumer A, Wittfeld K, Vasquez AA, Hoogman M et al. Measurement and genetics of human subcortical and hippocampal asymmetries in large datasets. Hum Brain Mapp 2014; 35: 3277–3289.
King KS, Kozlitina J, Rosenberg RN, Peshock RM, McColl RW, Garcia CK . Effect of leukocyte telomere length on total and regional brain volumes in a large population-based cohort. JAMA Neurol 2014; 71: 1247–1254.
Teicher MH, Anderson CM, Polcari A . Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci USA 2012; 109: E563–E572.
Huang TT, Zou Y, Corniola R . Oxidative stress and adult neurogenesis—effects of radiation and superoxide dismutase deficiency. Semin Cell Dev Biol 2012; 23: 738–744.
Ng F, Berk M, Dean O, Bush AI . Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol 2008; 11: 851–876.
Wolkowitz OM, Mellon SH, Epel ES, Lin J, Reus VI, Rosser R et al. Resting leukocyte telomerase activity is elevated in major depression and predicts treatment response. Mol Psychiatry 2012; 17: 164–172.
Gadalla SM, Cawthon R, Giri N, Alter BP, Savage SA . Telomere length in blood, buccal cells, and fibroblasts from patients with inherited bone marrow failure syndromes. Aging 2010; 2: 867–874.
Takubo K, Aida J, Izumiyama-Shimomura N, Ishikawa N, Sawabe M, Kurabayashi R et al. Changes of telomere length with aging. Geriatr Gerontol Int 2010; 10 (Suppl 1): S197–S206.
Dlouha D, Maluskova J, Kralova Lesna I, Lanska V, Hubacek JA . Comparison of the relative telomere length measured in leukocytes and eleven different human tissues. Physiol Res 2014; 63 (Suppl 3): S343–S350.
Wei YB, Backlund L, Wegener G, Mathe AA, Lavebratt C . Telomerase dysregulation in the hippocampus of a rat model of depression: normalization by lithium. Int J Neuropsychopharmacol 2015; 18: pyv002.
Fischl B . FreeSurfer. Neuroimage 2012; 62: 774–781.
Pardoe HR, Pell GS, Abbott DF, Jackson GD . Hippocampal volume assessment in temporal lobe epilepsy: how good is automated segmentation? Epilepsia 2009; 50: 2586–2592.
Wenger E, Martensson J, Noack H, Bodammer NC, Kuhn S, Schaefer S et al. Comparing manual and automatic segmentation of hippocampal volumes: reliability and validity issues in younger and older brains. Hum Brain Mapp 2014; 35: 4236–4248.
Acknowledgements
This work was supported by the Swedish Research Council (EHB, grant number 350-2012-303); the NARSAD foundation and the National Institute of Mental Health (TTY, grant numbers 7R01MH085734, 3R01MH085734-02S1 and R01MH085734-05S1); (KZL, grant number K01MH097978); OMW and ESE, grant number R01 MH083784 and the Tinberg Family Foundation. The funding agencies had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript. We thank James/Jing Wu, Kevin Han and Audrey Fortezzo for their help in collecting and managing the data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
JL is a consultant to Telomere Diagnostics, formerly Telome Health, and owns stock in the company. The company had no role in this research or in writing this review. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Translational Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Henje Blom, E., Han, L., Connolly, C. et al. Peripheral telomere length and hippocampal volume in adolescents with major depressive disorder. Transl Psychiatry 5, e676 (2015). https://doi.org/10.1038/tp.2015.172
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2015.172
This article is cited by
-
The indirect effect of peer problems on adolescent depression through nucleus accumbens volume alteration
Scientific Reports (2020)
-
Telomere length and human hippocampal neurogenesis
Neuropsychopharmacology (2020)