Abstract
The recent use of estrogen-based therapies as adjunctive treatments for the cognitive impairments of schizophrenia has produced promising results; however the mechanism behind estrogen-based cognitive enhancement is relatively unknown. Brain-derived neurotrophic factor (BDNF) regulates learning and memory and its expression is highly responsive to estradiol. We recently found that estradiol modulates the expression of hippocampal parvalbumin-positive GABAergic interneurons, known to regulate neuronal synchrony and cognitive function. What is unknown is whether disruptions to the aforementioned estradiol–parvalbumin pathway alter learning and memory, and whether BDNF may mediate these events. Wild-type (WT) and BDNF heterozygous (+/−) mice were ovariectomized (OVX) at 5 weeks of age and simultaneously received empty, estradiol- or progesterone-filled implants for 7 weeks. At young adulthood, mice were tested for spatial and recognition memory in the Y-maze and novel-object recognition test, respectively. Hippocampal protein expression of BDNF and GABAergic interneuron markers, including parvalbumin, were assessed. WT OVX mice show impaired performance on Y-maze and novel-object recognition test. Estradiol replacement in OVX mice prevented the Y-maze impairment, a Behavioral abnormality of dorsal hippocampal origin. BDNF and parvalbumin protein expression in the dorsal hippocampus and parvalbumin-positive cell number in the dorsal CA1 were significantly reduced by OVX in WT mice, while E2 replacement prevented these deficits. In contrast, BDNF+/− mice showed either no response or an opposite response to hormone manipulation in both behavioral and molecular indices. Our data suggest that BDNF status is an important biomarker for predicting responsiveness to estrogenic compounds which have emerged as promising adjunctive therapeutics for schizophrenia patients.
Similar content being viewed by others
Introduction
Adolescence is a time of increasing incidence for many psychiatric disorders including schizophrenia. Although childhood-onset (before age 13) schizophrenia is rare,1 the incidence of schizophrenia rises sharply after puberty which is marked by a significant surge in sex steroid hormone release.2 A robust difference in age of onset between the sexes is well established, with a peak onset age of 15–24 in men and 20–29 in women.3, 4 Moreover, women have been found to have a second peak of onset after age 45, which may correspond with the emergence of menopause and the associated rapid decline of ovarian hormone levels.5 Data from a number of groups suggest lower cognitive function in men with schizophrenia relative to women.6 Cohen et al.7 found that an earlier age of menarche was associated with a later age of onset of psychotic symptoms and first hospitalization, whereas no significant correlation was found between puberty and disease onset in men. Psychopathology scores in premenopausal women with schizophrenia fluctuate across the menstrual cycle, with symptom deterioration during the low-estrogen phases of the cycle.8 Reports also indicate that chronic psychoses improve during pregnancy, when E2 levels are 200-fold higher than normal.9 Conversely, women become more vulnerable to psychosis at the perimenstrual phase of the menstrual cycle, post menopause10 and following abortion,11 all representing periods of estrogen withdrawal. Premenopausal women have been found to show a less severe course of illness, displaying less psychopathology and disability, and a better response to antipsychotics compared with men.12, 13 However, it is unclear whether these findings are due to the later age of onset or simply the better compliance to medication in women. Alternatively, their premenopausal sex hormones may serve as an endogenous antipsychotic agent. Indeed, Seeman14 found that women with schizophrenia at 20–40 years were protected from relapse and achieved maximal functioning at lower doses of antipsychotics than older women or men of comparable age.14 It is therefore not surprising that their rapid decline may result in detrimental changes in central neurotransmission and thus psychotic, negative and cognitive symptoms. Female sex hormones thus appear to have a significant role in buffering disease onset and severity.
Estradiol (E2) and progesterone (P4) are major ovarian hormones predominantly found in females, but also present in lower concentrations in males. E2 and P4 have been shown to act via genomic and non-genomic signaling mechanisms to regulate several key functions including mammalian brain development, cognition, memory and mood.15, 16 Further, serum levels of E2 are associated with heightened well-being and enhanced performance on certain cognitive tasks in healthy individuals17 as well as those with schizophrenia.18, 19, 20 Both E2 and P4 serum levels have been reported to be low in schizophrenia patients on admission.20, 21, 22, 23 Data from a number of clinical trials conducted by Kulkarni and others suggest that adjunctive E2 results in an improvement of symptomatology in women of child-bearing age with schizophrenia.22, 24, 25, 26 Recent reports on selective-estrogen receptor modulators as a safer option compared with E2 due to lower risk of peripheral side-effects, have yielded encouraging results in both pre- and postmenopausal women, particularly with regard to cognitive function.27, 28, 29 However, the mechanisms governing estrogen-based cognitive enhancement in schizophrenia remain unclear, and a better understanding of what mediates optimal treatment response is needed.
The molecular targets of E2 and P4 are diverse but accumulating animal and human data suggest that brain-derived neurotrophic factor (BDNF) is a key mediator in regulating the cognitive-enhancing effects of estradiol.30, 31, 32, 33 Early hypotheses of an interaction between estrogen and BDNF were made on the basis of the colocalized expression of estrogen receptors with BDNF and its affinity receptor, tropomyosin-related kinase B, in the hippocampus of rats and mice.34 Later, the rat BDNF gene was found to contain a sequence with close homology to the estrogen response element indicating that estrogen may directly modulate BDNF expression via activating this putative estrogen response element.35 Further, estrogen signaling has common downstream pathways as tropomyosin-related kinase B signaling, such as the MAPK cascade, which can activate CREB to drive BDNF transcription.36 Alternatively, E2 may interact with GABAergic interneurons to increase BDNF synthesis by an activity-dependent mechanism.37 Studies in adult female rats have shown that ovariectomy (OVX) decreases hippocampal BDNF mRNA33, 38 and protein39 expression while E2 replacement restored this deficit. Studies in humans and rodents also point to a critical role of BDNF in the development and function of organs in the endocrine and reproductive systems.40 Reduced BDNF level has been reported in the hippocampus of schizophrenia patients.41, 42 In addition, the Val66Met BDNF polymorphism, which results in disrupted intracellular trafficking and release of BDNF43 has been associated with aspects of schizophrenia including antipsychotic treatment response44, 45 and cognitive functioning.46 Furthermore, serum BDNF levels of schizophrenia patients show positive correlations with verbal working memory scores.47
A loss of BDNF (BDNF KO) is accompanied by reduced expression of a number of GABA neuron markers.48, 49 Dysfunction of inhibitory GABAergic circuits has emerged as a key factor contributing to cognitive impairments in schizophrenia, now regarded as the core clinical feature of the illness.50 Specifically, parvalbumin (PV)-containing interneurons are involved in mediating inhibitory-based synchronized gamma rhythms, which are associated with cognition and sensory processing.51 Reduced number of hippocampal PV-expressing interneurons as well as lower mRNA and protein expression of PV and glutamate decarboxylase 67 (GAD67) have been documented in postmortem studies of schizophrenia.52, 53, 54 Markers for other GABA neuron subtypes including somatostatin (SST) have also been implicated in schizophrenia55 whereas calretinin (CR) expression, which is independent of BDNF signaling, appears to be spared.54, 55
Recent data from our group suggest that circulating levels of E2 may regulate the protein expression of hippocampal PV during adolescent development in female but not male mice through ER-α signaling.56 Moreover, PV expression in the dorsal hippocampus (DHP) of female mice was more responsive to changes in serum E2 levels compared with the ventral hippocampus (VHP). This may be behaviorally relevant given the known function of the dorsal segment in mediating affect-neutral spatial memory and the role of the ventral segment in modulating anxiety-related behaviors.57 It should be noted, however, that circulating E2 levels may not reflect CNS levels. Indeed, E2 levels in the plasma (<0.02 pg μl−1) of adult male rats are much lower compared with E2 levels in the hippocampus (~0.05 pg μl−1), whereas in adult female rats, E2 levels in plasma (~0.37 pg μl−1) reflect E2 levels in the hippocampus (~0.4 pg μl−1).58 Nevertheless, E2 levels in the hippocampus of adult female rats are considerably higher than that of males.58 To dissect and analyze the mechanisms of female sex steroids in bolstering cognition, we performed OVX and hormone replacement with either estradiol or progesterone at pre-pubescence in health and in a state of BDNF deficiency, using wild-type (WT) and BDNF heterozygous (+/−) mutant mice, respectively. In adulthood, we then assessed spatial and recognition memory followed by protein analysis of hippocampal PV, SST, CR and GAD67.
Materials and methods
Animals
Female WT and BDNF+/− mice on a C57Bl/6 background were derived from a breeding colony at the Florey Institute of Neuroscience and Mental Health. Mice were housed in groups of 3–5 in individually ventilated cages with a 12/12 h light/dark cycle and ad libitum access to water and standard mouse chow (Specialty Feeds, Glen Forrest, WA, Australia). Behavioral testing for all animals was conducted at the same time of the year (within 3 months apart). All experimental procedures were approved by the Animal Experimentation Ethics Committee of the Florey Institute of Neuroscience and Mental Health, University of Melbourne at Parkville, Victoria, Australia.
Surgical techniques
Mice were either sham-operated or ovariectomized (OVX) at pre-pubescence (5 weeks) and were simultaneously implanted with 0.5 cm of silastic tubing filled with E2, P4 or received placebo treatment (empty implant). See Supplementary 1 for surgical technique details.
Behavioral studies
At 10 weeks of age, short-term spatial memory was assessed using the Y-maze. At 11 weeks of age, recognition memory was assessed using the novel-object recognition test and this was followed by the open-field test to examine spontaneous locomotor activity and the general level of anxiety. Behavioral testing procedures and analysis were performed as previously described59, 60 and are detailed in Supplementary 1.
Western blot analysis
Following Behavioral testing, seven mice per group were killed at 12 weeks of age by cervical dislocation between 1200 and 1700 h and their brains were harvested. The whole hippocampus was dissected from the brain, divided into the dorsal and ventral section with weight ratio of 50/50, then snap-frozen for protein extraction followed by western blot analysis as described previously.56 See Supplementary 1 for antibody details.
Immunofluorescence labeling of PV+ cells
Following Behavioral testing at 12 weeks of age, four to five mice per group were anaesthetized and transcardially perfused with 4% paraformaldehyde solution. Brains were removed, postfixed, cryoprotected and snap-frozen. Serial (1:6) 20 μm-thick coronal sections of the hippocampus were cut on a cryostat between −1.46 and −3.40 mm relative to bregma and mounted on gelatine-coated slides. The immunofluorescence labeling was processed on the same day by the same individual. Coronal sections from WT animals including intact, OVX, OVX+E2 and OVX+P4 treatment groups were stained for PV+ cells and counted by an individual blind to the experimental groups. The representative sections were imaged using a Leica DFC310 FX camera. See Supplementary 1 for antibody details.
Statistical analysis
Two-way analysis of variance (ANOVA) was performed with genotype (two levels: WT and BDNF+/−) and treatment (four levels: intact, OVX, OVX+E2 and OVX+P4) as the main factors. If significant effects of genotype or treatment, or an interaction were found, separate two-way ANOVAs for genotype × OVX (OVX vs intact), genotype × E2 (E2 vs OVX) and genotype × P4 (P4 vs OVX) effects were performed. Correlation analysis was conducted on individual western blot protein expression and Y-maze ratio data from each animal (N =7 per group). PV cell counts were analyzed by one-way ANOVA followed by Bonferroni-corrected post hoc comparisons. If P<0.05, differences were considered statistically significant and if 0.05<P<0.1, this was considered to represent a trend.
Results
Y-maze
Two-way ANOVA revealed a significant interaction between genotype and treatment (F(3,83)=4.22, P=0.008). We therefore proceeded to assess genotype × OVX, genotype × estradiol and genotype × progesterone effects.
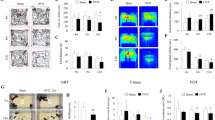
A significant genotype × OVX interaction was found (Figure 1a), F(1,41)=10.5, P=0.002, whereby OVX reduced Y-maze discrimination ratio (preference for novel arm over the other arms) in WT mice, but increased this ratio in BDNF+/− mice. A significant genotype × E2 interaction was found (Figure 1a), F(1,41)=9.4, P=0.004, with E2 treatment increasing the discrimination ratio in WT OVX mice, but reducing it in BDNF+/− OVX mice. No significant effects of P4 and no significant interactions between P4 and genotype were found.
Behavior of WT and BDNF heterozygous (+/−) mice at young adulthood following pre-pubescent ovariectomy (OVX), OVX and estradiol (E2) implant (OVX+E2) or OVX and progesterone (P4) implant (OVX+P4). Intact controls (sham) received sham surgery and an empty implant. (a) Y-maze performance is expressed as a discrimination ratio (preference for novel arm over the start arm and other arm). (b) Novel-object recognition test (NORT) score is expressed as percentage of time spent with the novel object. (c) Open field behavior is expressed as percentage of time spent in the center of the square arena. (d) Spontaneous locomotor activity is expressed as distance traveled in centimeters during the open-field test. N =10–12 per treatment group per genotype, short horizontal lines without end brackets =genotype × OVX effect, short horizontal lines with end brackets=genotype × estradiol effect, **P<0.01, #P<0.1. BDNF, brain-derived neurotrophic factor; WT, wild type.
Novel-object recognition test
Two-way ANOVA revealed a trend for a genotype × treatment interaction (F(3,73)=2.3, P=0.07). Further analysis revealed a significant genotype × OVX interaction (Figure 1b), F(1,38)=8.7, P=0.005, whereby OVX reduced the time spent with the novel object in WT mice but had no effect in BDNF+/− mice. E2 treatment appeared to partially prevent the effect of OVX in WT mice (Figure 1b), however, this did not reach statistical significance and no interaction between genotype and E2 was found. Similarly, there was no significant effect of genotype on P4 treatment.
Open field
There were no significant effects of either genotype or treatment in the percentage of time spent in the center of the open-field arena (Figure 1c).
Spontaneous locomotor activity
A significant treatment effect on spontaneous locomotor activity was detected (Figure 1d), F(3,85)=3.9, P=0.01. Further analysis revealed no significant effect of OVX or genotype, but a trend for an effect of E2 treatment (F(1,42)=3.3, P=0.07), whereby E2-treated WT and BDNF+/− OVX mice appear to show less spontaneous locomotor activity.
BDNF expression in the hippocampus
In the DHP (Figure 2a), an expected genotype effect (F(1,45)=7.10, P=0.01) as well as a treatment effect (F(3,45)=3.26, P=0.03) and a treatment × genotype interaction (F(3,45)=4.72, P=0.006) were found.
Hippocampal BDNF and PV protein expression in WT and BDNF heterozygous (het) (+/−) mice at young adulthood following pre-pubescent ovariectomy (OVX), OVX and estradiol (E2) implant (OVX+E2) or OVX and progesterone (P4) implant (OVX+P4). Intact controls (sham) received sham surgery and an empty implant. (a) BDNF expression in the dorsal (DHP) and (b) ventral (VHP) hippocampus. (c) PV expression in the dorsal (DHP) and (d) ventral (VHP) hippocampus. N=5–7 per treatment group per genotype, short horizontal lines without end brackets=genotype × OVX effect, short horizontal lines with end brackets=genotype × estradiol effect, gray horizontal lines=genotype × progesterone effects, *P<0.05 and **P<0.01. BDNF, brain-derived neurotrophic factor; WT, wild type.
A significant effect of OVX (F(1,23)=5.3, P=0.03) and a significant genotype × OVX interaction (F(1,23)=14.3, P=0.001) were found, whereby OVX significantly reduced BDNF expression in the DHP of WT but not BDNF+/− mice (Figure 2a). In addition, a significant effect of E2 (F(1,22)=12.4, P=0.002) and a significant genotype × E2 interaction (F(1,22)=11.4, P=0.003) were detected. E2 treatment in WT OVX mice significantly increased BDNF expression compared with WT OVX, but had no effect on BDNF expression in BDNF+/− OVX mice (Figure 2a). In marked contrast to the DHP, BDNF protein levels remained unchanged in the VHP following OVX and hormone replacement in WT mice (Figure 2b). Similarly, BDNF expression in OVX and hormone-replaced BDNF+/- mice did not significantly differ from intact BDNF+/− controls (Figure 2b).
PV expression in the hippocampus
Two-way ANOVA of PV expression in the DHP revealed main effects of genotype (Figure 2c); F(1,45)=5.6, P=0.022 and treatment (F(3,45)=6.2, P=0.001) and a trend for a treatment × genotype interaction (F(3,45)=2.4, P=0.07). Separate two-way ANOVA revealed a significant genotype × OVX interaction (F(1,24)=11.5, P=0.002) with OVX causing a significant reduction in PV expression in WT but not BDNF+/− mice. A significant genotype × E2 effect (F(1,24)=4.8, P=0.03) was also found, whereby E2 treatment in WT OVX mice increased PV expression to that of intact controls, but had no effect on PV expression in BDNF+/− mice. Further, a significant genotype × P4 effect on PV expression in the DHP (F(1,24)=8.0, P=0.009) was detected. P4 treatment increased PV expression in WT OVX mice, but had no effect in BDNF+/− OVX mice relative to their untreated OVX counterparts (Figure 2c). In contrast to the DHP, no significant effect of genotype or treatment was found for PV expression in the VHP (Figure 2d).
Association of Y-maze performance and BDNF/PV expression in the DHP
There was a significant correlation between BDNF expression in the DHP and performance on the Y-maze in WT mice (Figure 3a), r2=0.17, P=0.03, whereas no such correlation was found in BDNF+/− mice (Figure 3b). Similarly, we found a significant correlation between Y-maze performance and PV expression in the DHP in WT mice (Figure 3c), r2=0.28, P=0.0048, but no such correlation in BDNF+/− mice (Figure 3d).
Correlation analysis of BDNF and PV protein expression with Y-maze performance expressed as a discrimination ratio (novel:other arms) in the dorsal hippocampus (DHP) of WT and BDNF heterozygous (+/−) mice. (a) WT mice display a significant positive correlation between BDNF expression and Y-maze performance (r2=0.17, P=0.03) and this is not found in BDNF+/− mice (b). (c) WT mice display a significant positive correlation between PV expression and Y-maze performance (r2=0.28, P=0.0048) and this is not found in BDNF+/− mice (d). BDNF, brain-derived neurotrophic factor; PV, parvalbumin; WT, wild type.
PV cell number in the hippocampus
One-way ANOVA showed a strong trend for a significant difference in total PV cell count across treatment groups in the DHP (P=0.055). In the dorsal CA1, one-way ANOVA revealed significant differences among treatment groups (P=0.0096). Bonferroni-corrected post hoc analysis showed a significant difference between intact and OVX mice (P=0.024), with OVX mice showing a reduction in PV cell number (Figure 4a–c). In addition, E2 treatment significantly prevented this deficit where a significant difference in PV cell number between OVX and OVX+E2 was detected (P=0.048, Figure 4a, c and d). No significant differences in PV cell number were found in CA2, CA3 or dentate gyrus (Figure 4a). In addition, no significant differences in PV cell number were detected throughout the VHP (data not shown).
(a) Total PV+ cell count and across each subfield of the dorsal hippocampus including cornus ammonis (CA) 1, CA2, CA3 and dentate gyrus (DG) in WT mice at young adulthood following pre-pubescent ovariectomy (OVX), OVX and estradiol (E2) implant (OVX+E2) or OVX and progesterone (P4) implant (OVX+P4). Intact controls (sham) received sham surgery and an empty implant. (b) Representative image of the dorsal CA1 of an intact WT control. (c) Representative image of the dorsal CA1 of a WT OVX mouse. (d) Representative image of the dorsal CA1 of a WT OVX+E2-treated mouse. (e) Representative image of the dorsal CA1 of a WT OVX+P4-treated mouse. Negative control in the right hand corner of e. Scale bar, 100 μm, × 20 magnification. N=4–5 per treatment group, *P<0.05. PV, parvalbumin; WT, wild type.
SST, CR and GAD67 expression in the hippocampus
No significant effects of genotype or treatment were found for SST, CR and GAD67 protein expression in either DHP or VHP of WT and BDNF+/− mice (Figure 5a and f).
Hippocampal SST, CR and GAD67 protein expression in WT and BDNF heterozygous (het) (+/−) mice at young adulthood following pre-pubescent ovariectomy (OVX), OVX and estradiol (E2) implant (OVX+E2) or OVX and progesterone (P4) implant (OVX+P4). Intact controls (sham) received sham surgery and an empty implant. (a) SST expression in the dorsal (DHP) and (b) ventral (VHP) hippocampus. (c) CR expression in the dorsal (DHP) and (d) ventral (VHP) hippocampus. (e) GAD67 expression in the dorsal (DHP) and (f) ventral (VHP) hippocampus. N=5–7 per treatment group per genotype. BDNF, brain-derived neurotrophic factor; CR, calretinin; SST, somatostatin; WT, wild type.
Discussion
We believe our data are the first to show that female BDNF+/− mice exhibit drastically altered responses to ovarian hormone depletion in both behavioral and molecular pathways involved in spatial memory. Genetic variation in BDNF is associated with antipsychotic treatment resistance in schizophrenia patients.44, 45 The present data suggest that BDNF may also have an important role in predicting response to estrogenic compounds, which have recently emerged as promising adjunctive therapeutics for schizophrenia patients.24, 26, 61 We find that hormone deprivation during adolescent development in WT mice disrupts spatial memory and recognition memory in young adulthood. Simultaneous E2 replacement prevented the disruption in spatial memory but not recognition memory in WT OVX mice, suggesting a critical role for E2 in modulating dorsal hippocampus-dependent tasks. We further found that circulating levels of E2 in WT mice regulate PV cell number and protein expression in a region-dependent manner. In contrast, protein expression of SST, CR and GAD67 appear to be insensitive to changes in serum levels of E2. Our correlative evidence suggests that spatial memory performance depends on BDNF and PV protein levels in the DHP of WT mice but not BDNF+/− mice. Together, these findings indicate an altered estrogenic response in BDNF+/− mice and this may be an important step toward understanding the effects of therapeutics which target estrogen signaling under impaired BDNF expression, as often observed in schizophrenia.
Our data demonstrate that higher circulating E2, during adolescence, is crucial for DHP-dependent spatial memory. This selective effect of E2 is reflected in our molecular analysis which showed E2 regulation of BDNF and PV protein levels in the DHP and not in the VHP of OVX mice. Further analysis of the hippocampal subregions revealed a significantly lower number of PV-expressing cells in the dorsal but not in the ventral CA1 of OVX mice compared with intact controls. Moreover, E2 treatment was able to prevent this deficit. Interestingly, male schizophrenia patients exhibit a greater PV cell reduction than females in each hippocampal subfield.54 The dorsal CA1 forms prominent projections to the splenial and anterior cingulated cortices that are primarily involved in cognitive processing of visuospatial information and memory as well as navigation and exploration.62, 63, 64, 65 Disruption of CA1 PV cells may result in unsynchronised gamma oscillations due to diminished inhibitory control of pyramidal cells51, 66 and thus contribute to spatial memory impairments, as observed in the current study. Indeed, a population of PV-expressing CA1 interneurons in the DHP of mice have been documented to selectively support the encoding of spatial working memory.67 Given that both verbal and spatial memories rely on the integrity of the hippocampus,68, 69, 70 testing spatial memory in animals may be a relevant model for evaluating the link between steroid hormones and more human-specific cognitive tasks like verbal memory. Recent work in humans suggests that the same place cells in the hippocampus that represent location during spatial navigation also code elements of verbal recall.71 Interestingly, both WT and BDNF+/− OVX mice replaced with E2 showed a significant reduction in motor activity compared with intact controls, as measured by the distance traveled during the open-field test. Prior studies suggest that E2 replacement in OVX mice may result in lower general activity in anxiety-provoking situations, whereas they display increased activity in the safer home-cage environment.72 Importantly, however, these changes in activity do not appear to influence the animals’ memory performance in the present experiments when compared with their intact counterparts.
In contrast to E2-treated WT mice, those that received P4 showed no significant difference in BDNF expression compared with their OVX counterparts. Consistent with our findings, in cultured rat hippocampal slices, treatment with E2 at 10 nM resulted in an increase in BDNF protein levels whereas the same dose of P4 had no effect.30 While PV expression was significantly higher in P4-treated mice relative to OVX mice, both PV cell number as well as spatial memory performance were statistically indistinguishable from OVX mice. These results suggest again that the efficacy of hormone-based compounds may depend critically on BDNF expression. Likewise, BDNF expression in the hippocampus has been shown to be significantly affected by physiological changes in the levels of circulating E2.73, 74 The interaction between BDNF and E2 thus appears to be complex and bidirectional. It should be noted that the estrous cycle of sham-operated mice were not controlled for in the present study and this is a limitation which should be addressed in future investigations. Previous studies suggest that E2 may also regulate another major class of inhibitory interneurons, neuropeptide Y (NPY), via BDNF.75 One study by Nakamura and McEwen76 showed that ER-α is primarily located on hippocampal neurons which co-expressed GABA and NPY. Conversely, almost all ER-β immunoreactive cells within the subiculum of the hippocampus colocalize with PV in the adult female rat.77 PV- and NPY-expressing interneurons are not identical albeit there are some overlapping features.8, 78 In the dentate gyrus, PV-positive interneurons form a basket-like plexus around granule cells while NPY-positive interneurons form a population that innervates the outer molecular layer. However, some PV-positive basket cell populations also express NPY.75 Hippocampal GABAergic interneurons modulate neuronal activity79 and network oscillations,80 control size and propagation of neuronal assemblies,81 and neuronal plasticity.82 Hence, the expression profile of ERs on these interneurons needs to be fully examined in future experiments to further understand the mechanism by which E2 regulates the GABAergic system. To date, only a limited number of reports have addressed the impact of ovarian steroids on the GABAergic system in the adult hippocampus and even less is known about their role during adolescent development. The present study provides further support for an estrogenic effect on PV expression in female WT mice, in line with our previous findings.56 Moreover, our data suggest that E2 specifically regulates PV cell number in the dorsal CA1 while it appears to have no significant influence on SST, CR and GAD67 protein expression under the current experimental paradigm.
BDNF null mice do not usually survive past 2–4 weeks after birth due to the development of severe sensory deficits.83 BDNF heterozygous (+/−) mice expressing only one allele have approximately half the level of BDNF compared with WT controls and are viable, fertile and have a normal life span.84 Hence, compared with the BDNF null mice, BDNF+/− mice more closely resemble the human schizophrenia condition.85 These constitutive mutants show no significant changes in ER-α/β protein expression in the hippocampus (Supplementary Figure 2). However, in their early adulthood, BDNF+/− mice exhibit enhanced inter-male aggressiveness and hyperphagia with significant weight gain.84 Prior work from our laboratory have found increased activation of tropomyosin-related kinase B in the hippocampus of both male and female BDNF+/− mice relative to their WT counterparts.86 Further, a marked region-specific increase in neurotrophin-4 expression was found in the striatum of BDNF+/− mice in both sexes.86 Together, these findings suggest possible developmental, central and peripheral compensatory mechanisms in BDNF+/− mice which should be considered with data interpretation.
Current antipsychotic drugs are at least partially effective against the positive symptoms of schizophrenia but they have little effect on the negative symptoms and cognitive deficits of the illness.87 In light of our data, future studies should compare E2 and antipsychotics as cognitive mediators, potentially via BDNF. Atypical antipsychotics would be particularly interesting to investigate due to some reports of their efficacy on certain domains of cognitive function88, 89 albeit these results have not been consistently replicated.90, 91 Reduced BDNF may be a biomarker of impaired memory and general cognition in women.92 Our data and those of others show only subtle or no effects of BDNF heterozygosity on spatial and nonspatial memory in BDNF+/− mice,59, 60, 93,94 albeit other behavioral anomalies have been noted.95, 96 An intriguing finding here is that these constitutive mutants showed either no response or an opposite response to hormone manipulation in both behavioral and molecular indices. A strong positive correlation between plasma BDNF and serum E2 concentrations has been demonstrated in fertile women but this was not found in women diagnosed with functional hypothalamic amenorrhea.97, 98 Women with functional hypothalamic amenorrhea have decreased plasma BDNF levels and an impaired pulsatile secretion of gonadotropin-releasing hormone, which subsequently affects the pituitary gland leading to decreased gonadotropin production and secretion and ultimately hypoestrogenism.98 Several studies in humans and rodents suggest an important role for ovarian BDNF in the formation and initiation of ovarian follicular growth.40 An altered hypothalamic–pituitary–gonadal axis may thus be evident in BDNF-deficient mice. Researchers have speculated that reduced gonadal hormone levels found in psychotic and clinical high-risk individuals reflect decreased hypothalamic–pituitary–gonadal axis activity.99 In fact, selective-estrogen receptor modulators, particularly raloxifene, which has recently emerged as a promising adjuvant in women with schizophrenia, modulates the hypothalamic–pituitary–gonadal axis and appears to change circulating levels of gonadotropin-releasing hormone, E2 and other estrogens. We propose that a reduction in BDNF, the important intermediary of positive estrogen effects as well as a regulator of hypothalamic–pituitary–gonadal axis functions, fundamentally upsets the estrogen signaling cascade and may underlie the lack of response to hormone manipulation seen in the BDNF+/− mice. This area of research deserves more in-depth study, which may help improve current therapeutic options for treatment-resistant schizophrenia patients with genetic variations in BDNF.
In conclusion, our data suggest that E2 is involved in the regulation of dorsal hippocampal PV expression and cell number in the CA1 subfield, corresponding to their positive effects on spatial memory. This may be a novel mechanism by which the higher circulating levels of E2 in female patients with schizophrenia compared with male patients preserves cognition and delays the onset of psychosis. These effects of E2 seem to depend critically on BDNF expression as BDNF heterozygosity diminished the response of female mice to hormone manipulation on both molecular and behavioral indices. Our results raise the intriguing possibility that the treatment response to estrogenic compounds such as selective-estrogen receptor modulators will depend on the genetic status of females with schizophrenia, namely on variants of the BDNF gene.
References
McClellan J, Stock S . Practice parameter for the assessment and treatment of children and adolescents with schizophrenia. J Am Acad Child Adolesc Psychiatry 2013; 52: 976–990.
Carlisle LL, McClellan J . Psychopharmacology of schizophrenia in children and adolescents. Pediatr Clin North Am 2011; 58: 205–218.
Castle D, Sham P, Murray R . Differences in distribution of ages of onset in males and females with schizophrenia. Schizophr Res 1998; 33: 179–183.
Häfner H, Behrens S, De Vry J, Gattaz WF . Oestradiol enhances the vulnerability threshold for schizophrenia in women by an early effect on dopaminergic neurotransmission. Evidence from an epidemiological study and from animal experiments. Eur Arch Psychiatry Clin Neurosci 1991; 241: 65–68.
Riecher-Rössler A, Loffler W, Munk-Jorgensen P . What do we really know about late-onset schizophrenia? Eur Arch Psychiatry Clin Neurosci 1997; 247: 195–208.
Krysta K, Murawiec S, Klasik A, Wiglusz MS, Krupka-Matuszczyk I . Sex-specific differences in cognitive functioning among schizophrenic patients. Psychiatr Danub 2013; 25: S244–S246.
Cohen RZ, Seeman MV, Gotowiec A, Kopala L . Earlier puberty as a predictor of later onset of schizophrenia in women. Am J Psychiatry 1999; 156: 1059–1064.
Nitsch R, Soriano E, Frotscher M . The parvalbumin-containing nonpyramidal neurons in the rat hippocampus. Anat Embryol (Berl) 1990; 181: 413–425.
Chang SS, Renshaw DC . Psychosis and pregnancy. Compr Ther 1986; 12: 36–41.
Häfner H . Gender differences in schizophrenia. Psychoneuroendocrinology 2003; 28: 17–54.
Mahe V, Montagnon F, Nartowski J, Dumane A . Post-abortion mania. Br J Psychiatry 1999; 175: 389–390.
Cotton SM, Lambert M, Schimmelmann BG, Foley DL, Morley KI, McGorry PD et al. Gender differences in premorbid, entry, treatment, and outcome characteristics in a treated epidemiological sample of 661 patients with first episode psychosis. Schizophr Res 2009; 114: 17–24.
Morgan VA, Castle DJ, Jablensky AV . Do women express and experience psychosis differently from men? Epidemiological evidence from the Australian National Study of Low Prevalence (Psychotic) Disorders. Aust N Z J Psychiatry 2008; 42: 74–82.
Seeman MV . Interaction of sex, age, and neuroleptic dose. Compr Psychiatry 1983; 24: 125–128.
Brinton RD . Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends Pharmacol Sci 2009; 30: 212–222.
Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS . Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol 2008; 29: 219–237.
Hampson E . Estrogen-related variations in human spatial and articulatory-motor skills. Psychoneuroendocrinology 1990; 15: 97–111.
Hoff AL, Kremen WS, Wieneke MH, Lauriello J, Blankfeld HM, Faustman WO et al. Association of estrogen levels with neuropsychological performance in women with schizophrenia. Am J Psychiatry 2001; 158: 1134–1139.
Ko YH, Joe SH, Cho W, Park JH, Lee JJ, Jung IK et al. Estrogen, cognitive function and negative symptoms in female schizophrenia. Neuropsychobiology 2006; 53: 169–175.
Riecher-Rossler A, Hafner H, Stumbaum M, Maurer K, Schmidt R . Can estradiol modulate schizophrenic symptomatology? Schizophr Bull 1994; 20: 203–214.
Huber TJ, Rollnik J, Wilhelms J, von zur Muhlen A, Emrich HM, Schneider U . Estradiol levels in psychotic disorders. Psychoneuroendocrinology 2001; 26: 27–35.
Kulkarni J, de Castella A, Smith D, Taffe J, Keks N, Copolov D . A clinical trial of the effects of estrogen in acutely psychotic women. Schizophr Res 1996; 20: 247–252.
Oades RD, Schepker R . Serum gonadal steroid hormones in young schizophrenic patients. Psychoneuroendocrinology 1994; 19: 373–385.
Ghafari E, Fararouie M, Shirazi HG, Farhangfar A, Ghaderi F, Mohammadi A . Combination of estrogen and antipsychotics in the treatment of women with chronic schizophrenia: a double-blind, randomized, placebo-controlled clinical trial. Clin Schizophr Relat Psychoses 2013; 6: 172–176.
Kulkarni J, de Castella A, Fitzgerald PB, Gurvich CT, Bailey M, Bartholomeusz C et al. Estrogen in severe mental illness: a potential new treatment approach. Arch Gen Psychiatry 2008; 65: 955–960.
Kulkarni J, Gavrilidis E, Wang W, Worsley R, Fitzgerald PB, Gurvich C et al. Estradiol for treatment-resistant schizophrenia: a large-scale randomized-controlled trial in women of child-bearing age. Mol Psychiatry 2014.
Huerta-Ramos E, Iniesta R, Ochoa S, Cobo J, Miquel E, Roca M et al. Effects of raloxifene on cognition in postmenopausal women with schizophrenia: a double-blind, randomized, placebo-controlled trial. Eur Neuropsychopharmacol 2014; 24: 223–231.
Raveendranathan D, Shivakumar V, Jayaram N, Rao NP, Venkatasubramanian G . Beneficial effects of add-on raloxifene in schizophrenia. Arch Womens Ment Health 2012; 15: 147–148.
Shivakumar V, Venkatasubramanian G . Successful use of adjuvant raloxifene treatment in clozapine-resistant schizophrenia. Indian J Psychiatry 2012; 54: 394.
Aguirre CC, Baudry M . Progesterone reverses 17beta-estradiol-mediated neuroprotection and BDNF induction in cultured hippocampal slices. Eur J Neurosci 2009; 29: 447–454.
Hill RA . Interaction of sex steroid hormones and brain-derived neurotrophic factor-tyrosine kinase B signalling: relevance to schizophrenia and depression. J Neuroendocrinol 2012; 24: 1553–1561.
Wu YC, Hill RA, Gogos A, van den Buuse M . Sex differences and the role of estrogen in animal models of schizophrenia: interaction with BDNF. Neuroscience 2013; 239: 67–83.
Singh M, Meyer EM, Simpkins JW . The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague-Dawley rats. Endocrinology 1995; 136: 2320–2324.
Miranda RC, Sohrabji F, Toran-Allerand CD . Presumptive estrogen target neurons express mRNAs for both the neurotrophins and neurotrophin receptors: a basis for potential developmental interactions of estrogen with the neurotrophins. Mol Cell Neurosci 1993; 4: 510–525.
Sohrabji F, Miranda RC, Toran-Allerand CD . Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci USA 1995; 92: 11110–11114.
Scharfman HE, Maclusky NJ . Similarities between actions of estrogen and BDNF in the hippocampus: coincidence or clue? Trends Neurosci 2005; 28: 79–85.
Blurton-Jones M, Tuszynski MH . Estradiol-induced modulation of estrogen receptor-beta and GABA within the adult neocortex: a potential transsynaptic mechanism for estrogen modulation of BDNF. J Comp Neurol 2006; 499: 603–612.
Solum DT, Handa RJ . Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. J Neurosci 2002; 22: 2650–2659.
Berchtold NC, Kesslak JP, Pike CJ, Adlard PA, Cotman CW . Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. Eur J Neurosci 2001; 14: 1992–2002.
Dissen GA, Garcia-Rudaz C, Ojeda SR . Role of neurotrophic factors in early ovarian development. Semin Reprod Med 2009; 27: 24–31.
Durany N, Michel T, Zochling R, Boissl KW, Cruz-Sanchez FF, Riederer P et al. Brain-derived neurotrophic factor and neurotrophin 3 in schizophrenic psychoses. Schizophr Res 2001; 52: 79–86.
Thompson Ray M, Weickert CS, Wyatt E, Webster MJ . Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. J Psychiatry Neurosci 2011; 36: 195–203.
Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL et al. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci 2004; 24: 4401–4411.
Zai GC, Zai CC, Chowdhury NI, Tiwari AK, Souza RP, Lieberman JA et al. The role of brain-derived neurotrophic factor (BDNF) gene variants in antipsychotic response and antipsychotic-induced weight gain. Prog Neuropsychopharmacol Biol Psychiatry 2012; 39: 96–101.
Zhang JP, Lencz T, Geisler S, DeRosse P, Bromet EJ, Malhotra AK . Genetic variation in BDNF is associated with antipsychotic treatment resistance in patients with schizophrenia. Schizophr Res 2013; 146: 285–288.
Rybakowski JK . BDNF gene: functional Val66Met polymorphism in mood disorders and schizophrenia. Pharmacogenomics 2008; 9: 1589–1593.
Niitsu T, Shirayama Y, Matsuzawa D, Hasegawa T, Kanahara N, Hashimoto T et al. Associations of serum brain-derived neurotrophic factor with cognitive impairments and negative symptoms in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35: 1836–1840.
Glorioso C, Sabatini M, Unger T, Hashimoto T, Monteggia LM, Lewis DA et al. Specificity and timing of neocortical transcriptome changes in response to BDNF gene ablation during embryogenesis or adulthood. Mol Psychiatry 2006; 11: 633–648.
Grosse G, Djalali S, Deng DR, Holtje M, Hinz B, Schwartzkopff K et al. Area-specific effects of brain-derived neurotrophic factor (BDNF) genetic ablation on various neuronal subtypes of the mouse brain. Brain Res Dev Brain Res 2005; 156: 111–126.
Marin O . Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci 2012; 13: 107–120.
Volman V, Behrens MM, Sejnowski TJ . Downregulation of parvalbumin at cortical GABA synapses reduces network gamma oscillatory activity. J Neurosci 2011; 31: 18137–18148.
Benes FM, Kwok EW, Vincent SL, Todtenkopf MS . A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry 1998; 44: 88–97.
Konradi C, Yang CK, Zimmerman EI, Lohmann KM, Gresch P, Pantazopoulos H et al. Hippocampal interneurons are abnormal in schizophrenia. Schizophr Res 2011; 131: 165–173.
Zhang ZJ, Reynolds GP . A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res 2002; 55: 1–10.
Lin LC, Sibille E . Reduced brain somatostatin in mood disorders: a common pathophysiological substrate and drug target? Front Pharmacol 2013; 4: 110.
Wu YC, Du X, van den Buuse M, Hill RA . Sex differences in the adolescent developmental trajectory of parvalbumin interneurons in the hippocampus: a role for estradiol. Psychoneuroendocrinology 2014; 45: 167–178.
Fanselow MS, Dong HW . Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 2010; 65: 7–19.
Caruso D, Pesaresi M, Abbiati F, Calabrese D, Giatti S, Garcia-Segura LM et al. Comparison of plasma and cerebrospinal fluid levels of neuroactive steroids with their brain, spinal cord and peripheral nerve levels in male and female rats. Psychoneuroendocrinology 2013; 38: 2278–2290.
Klug M, Hill RA, Choy KH, Kyrios M, Hannan AJ, van den Buuse M . Long-term behavioral and NMDA receptor effects of young-adult corticosterone treatment in BDNF heterozygous mice. Neurobiol Dis 2012; 46: 722–731.
Klug M, van den Buuse M . An investigation into "two hit" effects of BDNF deficiency and young-adult cannabinoid receptor stimulation on prepulse inhibition regulation and memory in mice. Front Behav Neurosci 2013; 7: 149.
Usall J, Huerta-Ramos E, Iniesta R, Cobo J, Araya S, Roca M et al. Raloxifene as an adjunctive treatment for postmenopausal women with schizophrenia: a double-blind, randomized, placebo-controlled trial. J Clin Psychiatry 2011; 72: 1552–1557.
Cenquizca LA, Swanson LW . Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev 2007; 56: 1–26.
Han CJ, O'Tuathaigh CM, van Trigt L, Quinn JJ, Fanselow MS, Mongeau R et al. Trace but not delay fear conditioning requires attention and the anterior cingulate cortex. Proc Natl Acad Sci USA 2003; 100: 13087–13092.
Jones MW, Wilson MA . Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol 2005; 3: e402.
Lavenex PB, Amaral DG, Lavenex P . Hippocampal lesion prevents spatial relational learning in adult macaque monkeys. J Neurosci 2006; 26: 4546–4558.
Sultan KT, Brown KN, Shi SH . Production and organization of neocortical interneurons. Front Cell Neurosci 2013; 7: 221.
Murray AJ, Sauer JF, Riedel G, McClure C, Ansel L, Cheyne L et al. Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nat Neurosci 2011; 14: 297–299.
Morris RG, Garrud P, Rawlins JN, O'Keefe J . Place navigation impaired in rats with hippocampal lesions. Nature 1982; 297: 681–683.
Nyberg L, McIntosh AR, Cabeza R, Habib R, Houle S, Tulving E . General and specific brain regions involved in encoding and retrieval of events: what, where, and when. Proc Natl Acad Sci USA 1996; 93: 11280–11285.
Squire LR . Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev 1992; 99: 195–231.
Miller JF, Neufang M, Solway A, Brandt A, Trippel M, Mader I et al. Neural activity in human hippocampal formation reveals the spatial context of retrieved memories. Science 2013; 342: 1111–1114.
Morgan MA, Pfaff DW . Effects of estrogen on activity and fear-related behaviors in mice. Horm Behav 2001; 40: 472–482.
Gibbs RB . Levels of trkA and BDNF mRNA, but not NGF mRNA, fluctuate across the estrous cycle and increase in response to acute hormone replacement. Brain Res 1998; 810: 294.
Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ . Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J Neurosci 2003; 23: 11641–11652.
Scharfman HE, MacLusky NJ . Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: complexity of steroid hormone-growth factor interactions in the adult CNS. Front Neuroendocrinol 2006; 27: 415–435.
Nakamura NH, McEwen BS . Changes in interneuronal phenotypes regulated by estradiol in the adult rat hippocampus: a potential role for neuropeptide Y. Neuroscience 2005; 136: 357–369.
Blurton-Jones M, Tuszynski MH . Estrogen receptor-beta colocalizes extensively with parvalbumin-labeled inhibitory neurons in the cortex, amygdala, basal forebrain, and hippocampal formation of intact and ovariectomized adult rats. J Comp Neurol 2002; 452: 276–287.
Deller T, Leranth C . Synaptic connections of neuropeptide Y (NPY) immunoreactive neurons in the hilar area of the rat hippocampus. J Comp Neurol 1990; 300: 433–447.
Mody I, Pearce RA . Diversity of inhibitory neurotransmission through GABA(A) receptors. Trends Neurosci 2004; 27: 569–575.
Whittington MA, Traub RD . Interneuron diversity series: inhibitory interneurons and network oscillations in vitro. Trends Neurosci 2003; 26: 676–682.
Pouille F, Scanziani M . Routing of spike series by dynamic circuits in the hippocampus. Nature 2004; 429: 717–723.
Fagiolini M, Fritschy JM, Low K, Mohler H, Rudolph U, Hensch TK . Specific GABAA circuits for visual cortical plasticity. Science 2004; 303: 1681–1683.
Ernfors P, Lee KF, Jaenisch R . Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature 1994; 368: 147–150.
Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH et al. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci USA 1999; 96: 15239–15244.
Weickert CS, Ligons DL, Romanczyk T, Ungaro G, Hyde TM, Herman MM et al. Reductions in neurotrophin receptor mRNAs in the prefrontal cortex of patients with schizophrenia. Mol Psychiatry 2005; 10: 637–650.
Hill RA, van den Buuse M . Sex-dependent and region-specific changes in TrkB signaling in BDNF heterozygous mice. Brain Res 2011.
Lambert TJ, Castle DJ . Pharmacological approaches to the management of schizophrenia. Med J Aust 2003; 178: Suppl:S57–Suppl:S61.
Lindenmayer JP, Khan A, Iskander A, Abad MT, Parker B . A randomized controlled trial of olanzapine versus haloperidol in the treatment of primary negative symptoms and neurocognitive deficits in schizophrenia. J Clin Psychiatry 2007; 68: 368–379.
Woodward ND, Purdon SE, Meltzer HY, Zald DH . A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. Int J Neuropsychopharmacol 2005; 8: 457–472.
Keefe RS, Seidman LJ, Christensen BK, Hamer RM, Sharma T, Sitskoorn MM et al. Comparative effect of atypical and conventional antipsychotic drugs on neurocognition in first-episode psychosis: a randomized, double-blind trial of olanzapine versus low doses of haloperidol. Am J Psychiatry 2004; 161: 985–995.
Swartz MS, Perkins DO, Stroup TS, Davis SM, Capuano G, Rosenheck RA et al. Effects of antipsychotic medications on psychosocial functioning in patients with chronic schizophrenia: findings from the NIMH CATIE study. Am J Psychiatry 2007; 164: 428–436.
Komulainen P, Pedersen M, Hanninen T, Bruunsgaard H, Lakka TA, Kivipelto M et al. BDNF is a novel marker of cognitive function in ageing women: the DR's EXTRA Study. Neurobiol Learn Mem 2008; 90: 596–603.
Carreton O, Giralt A, Torres-Peraza JF, Brito V, Lucas JJ, Gines S et al. Age-dependent decline of motor neocortex but not hippocampal performance in heterozygous BDNF mice correlates with a decrease of cortical PSD-95 but an increase of hippocampal TrkB levels. Exp Neurol 2012; 237: 335–345.
Linnarsson S, Bjorklund A, Ernfors P . Learning deficit in BDNF mutant mice. Eur J Neurosci 1997; 9: 2581–2587.
Liu IY, Lyons WE, Mamounas LA, Thompson RF . Brain-derived neurotrophic factor plays a critical role in contextual fear conditioning. J Neurosci 2004; 24: 7958–7963.
Manning EE, van den Buuse M . BDNF deficiency and young-adult methamphetamine induce sex-specific effects on prepulse inhibition regulation. Front Cell Neurosci 2013; 7: 92.
Begliuomini S, Casarosa E, Pluchino N, Lenzi E, Centofanti M, Freschi L et al. Influence of endogenous and exogenous sex hormones on plasma brain-derived neurotrophic factor. Hum Reprod 2007; 22: 995–1002.
Podfigurna-Stopa A, Casarosa E, Luisi M, Czyzyk A, Meczekalski B, Genazzani AR . Decreased plasma concentrations of brain-derived neurotrophic factor (BDNF) in patients with functional hypothalamic amenorrhea. Gynecol Endocrinol 2013; 29: 817–820.
Trotman HD, Holtzman CW, Ryan AT, Shapiro DI, MacDonald AN, Goulding SM et al. The development of psychotic disorders in adolescence: a potential role for hormones. Horm Behav 2013; 64: 411–419.
Acknowledgements
This project is supported by a National Health and Medical Research Council of Australia (NHMRC) postdoctoral training fellowship (RAH), an NHMRC senior research fellowship (MvdB) and an NHMRC Project grant (1044887) as well as operational infrastructure support from the State Government of Victoria.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Translational Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Wu, Y., Du, X., van den Buuse, M. et al. Analyzing the influence of BDNF heterozygosity on spatial memory response to 17β-estradiol. Transl Psychiatry 5, e498 (2015). https://doi.org/10.1038/tp.2014.143
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2014.143
This article is cited by
-
Kidins220/ARMS modulates brain morphology and anxiety-like traits in adult mice
Cell Death Discovery (2022)
-
The effects of interval training on peripheral brain derived neurotrophic factor (BDNF) in young adults: a systematic review and meta-analysis
Scientific Reports (2021)
-
Treadmill Exercise Reverses the Change of Dendritic Morphology and Activates BNDF-mTOR Signaling Pathway in the Hippocampus and Cerebral Cortex of Ovariectomized Mice
Journal of Molecular Neuroscience (2021)
-
Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders
Molecular Psychiatry (2020)
-
Brain-derived neurotrophic factor Val66Met genotype and ovarian steroids interactively modulate working memory-related hippocampal function in women: a multimodal neuroimaging study
Molecular Psychiatry (2018)