Abstract
Stress-induced alterations in neuronal plasticity and in hippocampal functions have been suggested to be involved in the development of mood disorders. In this context, we investigated in the hippocampus the activation of intracellular signaling cascades, the expression of epigenetic markers and plasticity-related genes in a mouse model of stress-induced hyperactivity and of mixed affective disorders. We also determined whether the antidepressant drug agomelatine, a MT1/MT2 melatonergic receptor agonist/5-HT2C receptor antagonist, could prevent some neurobiological and behavioral alterations produced by stress. C57BL/6J mice, exposed for 3 weeks to daily unpredictable socio-environmental stressors of mild intensity, were treated during the whole procedure with agomelatine (50 mg kg−1 per day, intraperitoneal). Stressed mice displayed robust increases in emotional arousal, vigilance and motor activity, together with a reward deficit and a reduction in anxiety-like behavior. Neurobiological investigations showed an increased phosphorylation of intracellular signaling proteins, including Atf1, Creb and p38, in the hippocampus of stressed mice. Decreased hippocampal level of the repressive epigenetic marks HDAC2 and H3K9me2, as well as increased level of the permissive mark H3K9/14ac suggested that chronic mild stress was associated with increased gene transcription, and clear-cut evidence was further indicated by changes in neuroplasticity-related genes, including Arc, Bcl2, Bdnf, Gdnf, Igf1 and Neurod1. Together with other findings, the present data suggest that chronic ultra-mild stress can model the hyperactivity or psychomotor agitation, as well as the mixed affective behaviors often observed during the manic state of bipolar disorder patients. Interestingly, agomelatine could normalize both the behavioral and the molecular alterations induced by stress, providing further insights into the mechanism of action of this new generation antidepressant drug.
Similar content being viewed by others
Introduction
Stress is a predisposing factor for a broad range of behavioral and pathophysiological disturbances, among which mood disorders are most frequent.1 More specifically, the hippocampus—a key region in emotional and cognitive processes—is known to be highly sensitive to glucocorticoids in line with the important role of this limbic structure in the integration of the stress response.2, 3 Although the timing and intensity of stressors seem to be critical for the neuroendocrine and behavioral outcomes, the exact mechanism by which stress modulates functional and structural plasticity in the hippocampus and other brain areas remains incompletely known.4 To date, it is well accepted that severe and long-lasting traumatic stress can lead to neuronal loss and synaptic dysfunction in the hippocampus, in association with low mood, anxiety and impaired cognition, which constitute core symptoms of major depression.5, 6 Furthermore, stressful life events can also precipitate manic episodes in bipolar disorder patients characterized by elevated mood, hyperactivity, impulsivity and increased vigilance.7, 8
At the molecular level, the sustained elevation of glucocorticoids observed during stressful situations might interfere with various intracellular signaling and transcriptional processes. In particular, chromatin remodeling via histone modifications occurs in response to diverse environmental variations and represents a key regulator of nuclear integration promoting structural and functional adaptations necessary for neuronal plasticity.9 Hence, during the past decade, epigenetic mechanisms have emerged as a key process by which environmental variations could leave persistent imprints on gene expression increasing the risk of mood disorders.10 In addition, antidepressants and mood stabilizers have been shown to mediate their therapeutic effects, at least in part, through epigenetic remodeling.10, 11
More recently, agomelatine, an agonist at melatonergic receptor 1 (MT1) and 2 (MT2) and a 5-HT2C receptor antagonist, has emerged as an efficient treatment for major depressive episodes.12 Several studies have shown that these receptors are present in brain areas involved on mood as the hippocampus, the cortex and the amygdala. Indeed, MT1 and MT2 melatonin receptors are present in the dentate gyrus, CA3 and CA1 regions, and the subiculum of the hippocampal formation.13 Similarly, in this area, 5-HT2C receptors have high expression in the rostral CA3 pyramidal field layer, in the strata oriens and radiatum of the caudal CA1 area, and in the ventral subiculum.14 At the preclinical level, most of the data supported the fact that the antidepressant-like effects of agomelatine depend on the synergy between melatonergic agonist and 5-HT2C antagonist properties.15, 16 Agomelatine is also thought to exert many of its effects via a re-synchronization of circadian rhythms.12 Disruption of circadian periodicity being a major factor for the onset of bipolar disorders,17, 18 agomelatine might be endorsed with therapeutic potentialities for bipolar disorders beside unipolar depression.
We previously reported that the chronic ultra-mild stress (CUMS) procedure (that consists of prolonged repetition of low-intensity stressors, including social isolation, perturbations of circadian rhythms and alteration of daily life habits) induces affective-like disorders associated with impulsive choice in the decision making task in adult mice.19, 20 In the present study, we aimed at further characterizing the manic-like phenotype, as well as exploring intracellular signaling, epigenetic regulations and genes transcription profile of CUMS-exposed mice. In addition, we tested the ability of agomelatine to restore a normal phenotype and reverse the stress-induced cellular and molecular alterations of these mice.
Materials and methods
Animals
Experiments were performed using 10- to 12-week-old C57BL/6J male mice (Charles River Laboratories, l′Arbresle, France) of 25–30 g body weight. Upon arrival in the laboratory, mice were housed in groups of four per cage under standard conditions (22±1 °C, 60% humidity, 12-h light–dark cycle with lights on at 0700 h, food and water ad libitum) for 1 week before the beginning of experiments. Procedures involving animals and their care were conducted in conformity with the institutional guidelines that are in compliance with national and international laws and policies (Directive 2010/63/UE, 22/09/2010, protocol ID 00966.01).
Stress, treatments
The CUMS procedure in mice is detailed elsewhere.19, 20 It is a modified version of the chronic mild stress protocol originally designed for rats.21 Briefly, mice (three batches of 16 stressed mice, CUMS) were subjected to daily socio-environmental stressors of low intensity, in an unpredictable manner (See Supplementary Table S1 and Supplementary Figure S1) during a 25-day period. Administration of compounds was initiated at the beginning of this stress procedure, every day at 1800 h (that is, 1 h before the onset of dark period), up to the 25th day. Agomelatine (50 mg kg−1, Servier, Suresnes, France) and its vehicle (hydroxyethylcellulose; HEC 1%, Servier) were administered intraperitoneally as described in previous studies.22 The control, non-stressed mice (three batches of 16 mice, NS) groups received the same treatments (HEC versus agomelatine) in the same conditions.
Behavioral tests
Behavioral tests were performed within the fourth week of stress, with a delay of at least 18 h after the last stressor, so as to avoid an eventual effect of acute stress. Animals were kept in the testing room at least 2 h before the test to allow habituation to the new environment.
The behavioral tests (see Supplementary Methods) were adapted from existing protocols. Classical tests for measuring behaviors relevant to depression (tail suspension test (TST); forced swim test (FST); splash test; saccharin intake), locomotor activity (rotarod; open field (OF); actimetry) and anxiety-like behaviors (elevated plus maze (EPM); OF), were performed between day 21 and day 25.
Finally, animals were killed on day 26, and their brains were kept at −80 °C until use.
Real-time quantitative reverse transcriptase-PCR
Total mRNA was extracted from the entire hippocampus using the NucleoSpin RNA II kit (Macherey-Nagel, Hoerdt, France), and subsequent cDNA synthesis was performed using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Courtaboeuf, France) according to the manufacturer’s protocol. One part of the amplification was made with Absolute SYBR Green ROX Mix (Thermo Scientific, Illkirch, France) using the 7300 real-time PCR System (Applied Biosystems), and the other part using TaqMan (Applied Biosystems). The sequences of primers used are indicated in Supplementary Tables S2 and S3. The 2ΔΔCT (delta-delta comparative threshold) method was used to normalize the fold change in gene expression determined with both quantitative PCR technologies.
Western blots
Total hippocampi were lysed in ice-cold lysis buffer (1% Triton X-100, 150 mM NaCl, 1 mM EDTA, 10 mM Tris, pH 8.0, 10%, 10 mM NaF, 1 mM sodium orthovanadate and protease inhibitors cocktail). Protein extracts were loaded on a SDS–PAGE gel for electrophoresis migration and separated bands were transferred to a polyvinylidene difluoride membrane for 7 min using the iBlot gel transfer system (Life Technologies, St Aubin, France). Nonspecific binding of antibodies was prevented by preincubating the membranes in a blocking buffer (phosphate-buffered saline-Tween 0.01% supplemented with BSA 5%, w/v) for 1 h at room temperature. Then, membranes were incubated overnight at 4 °C in the same blocking buffer supplemented with the primary antibody (see Supplementary Table S4). After extensive washing, membranes were subsequently incubated with appropriate fluorescent secondary antibodies (Alexa Fluor 488 goat anti-rabbit or Alexa Fluor 488 donkey anti-mouse; Molecular Probes, Eugene, OR, USA) for 1 h at room temperature. Finally, membranes were washed, dried and scanned using the Odyssey infrared imaging system (Westburg, Leusden, The Netherlands). The intensity of bands of interest was quantified using ImageJ software. The quantity of proteins loaded on gel was controlled using the housekeeping protein β-tubulin (Cell Signaling Technology, Saint-Quentin-en-Yvelines, France).
Immunohistochemistry
Brain coronal sections (20 μm thick) were collected at the level of the hippocampus (according to Franklin and Paxinos’ atlas),23 using a cryostat, directly mounted on Superfrost Plus slides (Thermo Scientific) and stored at −80 °C until use. On the labeling day, sections were first fixed with 4% paraformaldehyde at 4 °C for 10 min. After blocking nonspecific binding with BSA 0.4 and 5% of the appropriate serum, and permeabilizing cell membranes with 0.05% of Tween 20, the sections were incubated overnight at 4 °C with the primary antibodies (see Supplementary Table S4). On the following day, sections were incubated for 1 h at room temperature in the presence of the appropriate biotinylated secondary antibodies, and the reaction was amplified using the ABC staining system (Vectastain ABC Elite Kit, Vector Laboratories, Les Ulis, France) for one additional hour. Finally, sections were treated with 3,3′-diaminobenzidine (Sigma FAST, D4293, Sigma-Aldrich, Saint Quentin-Fallavier, France) for revealing specifically bound antibodies. After dehydrating the sections and mounting with cover slip, pictures were taken with an optic microscope (Olympus BX21, zoom 4 and 10) and optical densities were measured and then normalized using the ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analyses
Data are represented as mean±s.e.m. The Student’s t-test was used for comparison between two groups (Prism 5.0, GraphPad software, USA). When more than two groups were compared, the two-way analysis of variance (stress × treatment) was used, followed by the Bonferroni post hoc test. When necessary, a planned pairwise comparison was made using Dunn’s t-test. The critical level of significance was set at P<0.05.
Results
At the end of the chronic stress period, a battery of well-established behavioral tests aiming at measuring locomotor behavior (actimetry, rotarod, OF), anxiety-related behaviors (EPM, OF) and depression-related behaviors (Splash test, saccharin intake, TST, FST) was used to assess CUMS-induced behavioral alterations. Behavioral investigations showed that stressed mice exhibit robust increases in emotional arousal, vigilance and motor activity, together with a reduction in anxiety and a reward deficit. Most of these behavioral impairments could be normalized by chronic agomelatine treatment. To further elucidate the molecular and cellular mechanisms associated with CUMS, we investigated the activation of intracellular signaling cascades by western blots, the expression of epigenetic markers by immunohistochemistry and the expression of plasticity-related genes by reverse transcriptase-PCR in the hippocampus. Marked increase in some intracellular signaling pathways, together with a permissive chromatin state and an increased gene expression were observed in the hippocampus of stressed mice. Chronic treatment with agomelatine corrected most of these neurochemical alterations.
Agomelatine prevents stress-induced behavioral alterations
At the end of the stress period, animals displayed a pronounced hyperactivity, as shown by the increased exploration in the OF arena (Figure 1a, +30.0±6.4%), especially during the first minutes of the test (Figure 1a, upper panel). This increased motor activity was confirmed by measuring spontaneous locomotion in an actimetry box. The ambulation, corresponding to the number of crossings in the center of the actimetry box (Figure 1b), was significantly increased in stressed mice (+82.0±11.5%). The number of rearings, corresponding to a vertical activity where the mouse had the forefeet up, was also increased after exposure to stress (+95.0±12.5%, Figure 1b). Similarly, the distance covered in the EPM test was increased in mice subjected to chronic mild stress (+20.0±7.6%, Supplementary Figure S2). In addition, the performance in the rotarod test was altered in stressed mice, as shown as a decrease in the latency to fall (−39.0±9.5%, Supplementary Figure S3) indicating impairment of movement coordination.
Effects of chronic agomelatine treatment on stress-induced locomotor dysfunction. (a) Stressed (CUMS) and non-stressed (NS) mice were placed in an open field test, and the distance covered in the arena was measured over time (top panel). In the bottom panel, the total distance covered for the 20-min test period is represented. Two-way analysis of variance (ANOVA) indicated a significant effect of stress (F(1,28)=5.78; P<0.05), but no effect of treatment (F(1,28)=2.95; P>0.05). However, there was a significant interaction between the stress and the treatment factors (F(1,28)=6.89; P<0.05), and the Bonferroni post hoc test revealed a significant increase in the distance covered by stressed mice (P<0.01), prevented by agomelatine (AGO, P<0.01). (b) Mice were placed in actimetry boxes, and the numbers of crossings in the center of the apparatus, as well as the numbers of rearings were measured with an infrared device over 1 h (top panel). In the bottom panel, the total numbers of crossings and total numbers of rearings over the 1-h test period are indicated. Two-way ANOVA indicated a significant effect of stress on both crossing activity (F(1,28)=15.21; P<0.01) and rearing activity (F(1,28)=9.12; P<0.001). A significant interaction between the stress and the treatment factors on crossing (F(1,28)=7.30; P<0.05) and rearing behaviors (F(1,28)=12.43; P<0.01) was observed. Bonferroni post hoc test revealed a significant increase in the crossings and rearings in stressed mice (P<0.001), which could be prevented by agomelatine (P<0.01 and P<0.001, respectively). Data are expressed as mean±s.e.m of n=8 mice per group. **,§§P<0.01; ***,§§§P<0.001. CUMS, chronic ultra-mild stress; HEC, hydroxyethylcellulose.
Interestingly, stress-induced alterations in the distance covered in the OF, as well as the numbers of crossings and rearings in actimetry boxes were significantly reduced by chronic administration of agomelatine (Figures 1a and b).
Furthermore, we assessed a reward-related behavior by measuring the consumption of saccharin solution and the time spent in self-grooming in CUMS versus non-stressed mice. Data in Figure 2 indicate that CUMS significantly decreased both saccharin intake and grooming behavior (−35.0±6.5% and −43.0±7.6%, respectively, Figures 2a and b), and here too, chronic treatment with agomelatine brought these behaviors back to those displayed by non-stressed mice (Figures 2a and b). It is noteworthy that the CUMS procedure did not modify water consumption and body weight (Supplementary Figure S4).
Effects of chronic agomelatine treatment on stress-induced affective behaviors. (a) Saccharin consumption was measured for an overnight period. There was a significant interaction between the stress and the treatment factors (F(1,39)=8.29; P<0.01), and the Bonferroni post hoc test revealed a significant decrease in the saccharin intake of stressed mice (P<0.05), prevented by agomelatine (P<0.001). (b) The time spent in grooming behavior over a total period of 5 min was measured in mice subjected to the splash test. Two-way analysis of variance (ANOVA) indicated a significant effect of stress (F(1,70)=13.9; P<0.01), as well as treatment (F(1,70)=7.62; P<0.05) on grooming behavior. The Bonferroni post hoc test revealed a significant decrease in the self-grooming of stressed mice (P<0.01) prevented by agomelatine (P<0.01). (c) The immobility time in the tail suspension test paradigm was measured in both control (NS) and CUMS-exposed mice. Two-way ANOVA indicated a significant effect of stress (F(1,30)=7.69; P<0.01) and treatment (F(1,30)=16.59; P<0.001) on immobility time. There was a significant interaction between the stress and the treatment (F(1,30)=4.55; P<0.05), and the Bonferroni post hoc test revealed a significant decrease in the immobility time of stressed mice (P<0.01) prevented by agomelatine (P<0.001). (d, e) Mice were subjected to the forced swim test, and the time spent in the swimming activity (mobility) and the struggling activity (number of bursts) over a 6-min period are represented. There was a significant interaction between the stress and the treatment factors (F(1,26)=4.25; P<0.05) for the mobility time, and the Bonferroni post hoc test revealed a significant decrease in the mobility time of stressed mice treated with agomelatine as compared with stressed mice treated with vehicle (P<0.05). A tendency toward an interaction between the stress and the treatment factors for the number of bursts (F(1,26)=3.12; P=0.089) was observed, and a planned pairwise comparison using Dunn’s t-test indicated a significant decrease in the number of bursts of stressed mice treated with agomelatine as compared with stressed mice treated with vehicle (P<0.05). (f) Mice were subjected to an open field test, and the number of visits to the center of the arena was measured over a 20-min period. There was a significant interaction between the stress and the treatment (F(1,26)=5.94; P<0.05), and the Bonferroni post hoc test revealed a significant increase in the number of entries into the center of the arena in stressed mice (P<0.05). Data are expressed as mean±s.e.m. of n=7–12 mice. *,§P<0.05; **,§§P<0.01; ***,§§§P<0.001. AGO, agomelatine; CUMS, chronic ultra-mild stress; HEC, hydroxyethylcellulose; NS, non-stressed.
Finally, we also performed validated tests to measure the defense-related behaviors and anxiety levels using the TST, the FST, the OF test and the EPM test (Figure 2). CUMS significantly reduced the immobility time in the TST (−52.0±11.7%, Figure 2c), and this effect was prevented by chronic agomelatine. In contrast, the immobility time in the FST did not differ in CUMS versus non-stressed mice (Figure 2d). However, small increases in the overall mobility and the number of activity bursts were observed in the FST after the chronic stress exposure (+9.0±4.8% and+16.0±7.2%, respectively, Figures 2d and e). The mobility duration and the number of activity bursts were significantly different in stressed mice treated with agomelatine versus those treated with vehicle (Figures 2d and e), indicating an effect of agomelatine on escape-like behaviors in this paradigm. In the OF, CUMS mice were apparently more prone to explore the center than non-stressed mice as shown by a higher number of entries of the former group in the center (+46.0±12.4%, Figure 2f). Here too, chronic administration of agomelatine tended to prevent this CUMS-induced behavioral change, although the difference was not significant (Figure 2f).
Altogether, these behavioral analyses indicated that CUMS caused a marked hyperactivity, together with increase in defense mechanisms and a decrease in feeding-related behavior. All these behavioral alterations were prevented when mice were treated with agomelatine (50 mg kg−1 per day) during the whole stress procedure.
Agomelatine prevents stress-induced alterations in intracellular signaling cascades
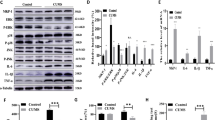
To further elucidate the cellular mechanisms associated with CUMS, we investigated the hippocampal intracellular signaling pathways involved in cell survival, proliferation and differentiation in stressed versus non-stressed mice. As shown by the western blot analyses (Figure 3), CUMS altered several hippocampal intracellular signaling pathways.
Effects of chronic agomelatine treatment on intracellular signaling in the hippocampus of stressed mice. Western blot analyses of phospho-Akt, phospho-Erk, phospho-p38, phospho-Creb, phospho-Atf1 and phospho-TrkB were performed on hippocampal extracts. Two-way analysis of variance revealed a significant interaction between stress and treatment factors for p-p38 (F(1,12)=16.22; P<0.001), p-Creb (F(1,28)=10.63; P<0.01) and p-Atf1 (F(1,11)=5.19; P<0.05). There was a significant effect of treatment on p-Akt (F(1,26)=13.72; P<0.001), p-Erk (F(1,14)=23.82; P<0.001), p-p38 (F(1,12)=18.35; P<0.01) and p-Creb (F(1,28)=6.49; P<0.05), and an effect of stress on p-p38 (F(1,12)=11.77; P<0.01). A trend for a stress effect was observed for p-Akt (F(1,26)=4.09; P=0.053), p-Erk (F(1,14)=4.55; P=0.051) and p-Atf1 (F(1,11)=3.55; P=0.086). The Bonferroni post hoc analyses revealed a significant difference between non-stressed mice treated with vehicle (NS HEC) and stressed mice treated with vehicle (CMS HEC) for the levels of p-p38 (P<0.001), p-Creb (P<0.05) and p-Atf1 (P<0.05). Agomelatine treatment (50 mg kg−1 per day) prevented the phosphorylation of p38 (P<0001) and Creb (P<0.001) induced by stress (CMS HEC versus CMS AGO). In addition, agomelatine decreased p-Akt (P<0.05) and p-Erk (P<0.05) in stressed mice (CMS HEC versus CMS AGO). Data are expressed as percentage of non-stressed mice treated with vehicle (NS HEC), and each bar represents the mean±s.e.m. (n=4–8 mice). *,§P<0.05; **,§§P<0.01; ***,§§§P<0.001. AGO, agomelatine; CUMS, chronic ultra-mild stress; HEC, hydroxyethylcellulose; NS, non-stressed.
Phosphorylation of the protein kinase B (Akt), the extracellular signal-regulated kinase (Erk), the p38 mitogen-activated protein kinase (p38), the cAMP response element-binding protein (Creb), and the activating transcription factor 1 (Atf1) were all increased in CUMS-exposed mice (+16.7±6.2%, +22.1±8.0%, +71.4±14.6%, +41.9±10.5% and +54.6±14.8%, respectively, Figure 3). In contrast, TrkB phosphorylation was not significantly changed by CUMS. As shown in Figure 3, the CUMS-induced increases in phospho-Akt, phospho-Erk, phospho-p38 and phospho-Creb were completely suppressed by chronic treatment with agomelatine.
Altogether, western blot investigations showed that specific effectors of the mitogen-activated protein kinases and PI3-K pathways, involving Akt, Erk, p38, Creb and Atf1, were increased after CUMS, and that agomelatine could prevent most of these changes.
Agomelatine prevents stress-induced changes in the expression of neuroplasticity-related genes and epigenetic regulations
The expression of various genes involved in neuronal plasticity, and genes implicated in the regulation of transcription or epigenetic regulation were also measured (Supplementary Tables S5–S7). First, qPCR screening (Supplementary Table S5) revealed that chronic stress promoted hippocampal expression of several plasticity-related genes including the brain-derived neurotrophic factor (Bdnf) gene (+28±7%), the neurogenic differentiation 1 (Neurod1) gene (+69±10%), the insulin growth factor 1 (Igf1) gene (+41±7%), the activity-regulated cytoskeleton-associated protein (Arc) gene (+56±10%), the glial cell-derived neurotrophic factor (Gdnf) gene (+58±15%) and the b-cell lymphoma 2 (Bcl2) gene (+46±9%). In addition, the expression of the nuclear receptor subfamily 1, group D, member 2 (Nr1d2), known as a transcription activator, was also higher (+40±5%, Supplementary Table S6), whereas the expression of the nuclear receptor co-repressor 1 (Ncor1), known as a transcription repressor, was lower (−37±6%, Supplementary Table S6) in CUMS versus non-stressed mice. This general increase in all these plasticity-related genes occurred in conjunction with an increase in hippocampal cell proliferation within the gyrus dentus, at day 21 of the CUMS session (Supplementary Figure S5).
A screening for modulations of epigenetic markers involved in synaptic plasticity including histone deacetylases, DNA methyltransferases, methyl binding domain protein and histone acetyltransferase was also performed (Supplementary Table S7). A significant CUMS-induced reduction of the epigenetic repressor Hdac2 (−22±4%, Supplementary Table S7)—a key player in hippocampal plasticity—was observed. Hence, on one hand, we decided to further investigate the expression of epigenetic markers, and measured the expression of HDACs and posttranslational modifications at histone tails (Supplementary Figures S6–S8). As shown by immunostaining analyses, CUMS decreased HDAC2 protein levels, but not those of HDAC1, in hippocampal CA1 and CA3 subfields (−18.0±1.2% and −14.0±4.3%, respectively, Supplementary Figure S5A). On the other hand, the expression of histone 3 lysine 9 di-methylated (H3K9me2) was specifically decreased in the CA3 region (−23.0±4.3%, Supplementary Figure S6A), whereas the expression of histone 3 lysine 9 and 14 acetylated (H3K9/14ac) was increased in the CA1 and CA3 subfields (+63.0±12.5% and +32.0±12.5%, respectively, Supplementary Figure S7B) in mice that had been exposed to CUMS. In contrast, the expression of histone 4 acetylated (H4ac) and that of histone 3 lysine 4 tri-methylated (H3K4me3) were not affected by CUMS (Supplementary Figures S7C and S7D).
The ability of agomelatine to correct these important CUMS-induced epigenetic modifications in the hippocampus was investigated. Data in Figure 4 show that chronic treatment with agomelatine significantly prevented the stress-mediated decrease in HDAC2 and H3K9me2, as well as the stress-induced increase in H3K9/14ac in the CA3 region of the hippocampus. The action of agomelatine was rather specific of the CA3 subfield, as no significant effects were observed in the CA1 and DG subfields (Supplementary Figure S8). In a similar manner, some of the alterations in plasticity-related gene expression observed after CUMS exposure were normalized by the chronic agomelatine treatment (Figure 5). In particular, agomelatine significantly restored expression of the Bdnf gene, the Neurod1 gene, the Igf1 gene and the Arc gene to the levels observed in non-stressed mice. CUMS-induced expressions of the Gdnf gene and the Bcl2 gene were not significantly affected by agomelatine treatment.
Effects of chronic agomelatine treatment on epigenetic marks in the hippocampus of stressed mice. Representative photomicrographs of HDAC2 (a), H3K9me (b) and H3K9ac (c) immunolabelings in the CA3 subfield of the hippocampus are shown in the left panels, and the quantification of respective optical density values in the right panel. (a) Two-way analysis of variance (ANOVA) indicated a significant effect of stress (F(1,16)=5.76; P<0.05), but no effect of treatment (F(1,16)=2.02; P>0.05) for the repressive mark HDAC2. There was a significant interaction between the stress and the treatment (F(1,16)=5.17; P<0.05), and the Bonferroni post hoc test revealed a significant decrease in HDAC2 immunolabeling in the CA3 subfield in CUMS mice (P<0.01), prevented by agomelatine (P<0.05). (b) Two-way ANOVA analysis indicated a significant effect of treatment (F(1,17)=4.75; P<0.05), but no effect of stress (F(1,17)=3.46; P>0.05) for the repressive mark H3K9me. There was a significant interaction between the stress and the treatment (F(1,17)=6.83; P<0.05), and the Bonferroni post hoc test revealed a significant decrease in H3K9me immunolabeling in the CA3 subfield in CUMS mice (P<0.05), prevented by agomelatine (P<0.01). (c) Two-way ANOVA analysis indicated a significant effect of stress (F(1,16)=5.25; P<0.05) and a tendency for an effect of treatment (F(1,16)=3.71; P=0.072) for the repressive mark H3K9ac. There was a significant interaction between the stress and the treatment (F(1,16)=9.86; P<0.01), and the Bonferroni post hoc test revealed a significant increase in H3K9ac labeling in the CA3 subfield in CUMS mice (P<0.01), prevented by agomelatine (P<0.01). Quantifications of immunolabelings are expressed as arbitrary units and each bar is the mean±s.e.m. of n=5–6 mice. *,§P<0.05; **,§§P<0.01. AGO, agomelatine; CUMS, chronic ultra-mild stress; HEC, hydroxyethylcellulose; NS, non-stressed.
Effects of chronic agomelatine treatment on plasticity-related gene expression in the hippocampus of stressed mice. mRNA levels of plasticity-related genes, measured by quantitative real-time reverse transcriptase-PCR, are expressed as fold changes compared with non-stressed mice treated with hydroxyethylcellulose (NS HEC). Two-way analysis of variance indicated a significant interaction between stress and treatment factors for Neurod1 (F(1,32)=4.80; P<0.05), Igf1 (F(1,34)=6.15; P<0.05) and Arc (F(1,29)=7.05; P<0.05). In addition, there was also an effect of stress on Neurod1 (F(1,32)=14.41; P<0.001), Igf1 (F(1,34)=13.30; P<0.001), Gdnf (F(1,25)=56.71; P<0.001) and Bcl2 (F(1,26)=12.47; P<0.01) and a treatment effect on Bdnf (F(1,31)=17.16; P<0.001), Neurod1 (F(1,32)=21.05; P<0.001), Arc (F(1,29)=4.50; P<0.05) and Gdnf (F(1,25)=5.57; P<0.05). The Bonferroni post hoc tests indicated a significant increase of Bdnf (P<0.05), Neurod1 (P<0.001), Igf1 (P<0.001), Arc (P<0.05), Gdnf (P<0.001) and Bcl2 (P<0.05) in stressed mice treated with vehicle (CUMS HEC) compared with non-stressed mice treated with vehicle (NS HEC). Similarly, there was a significant difference between stressed mice treated with vehicle (CUMS HEC) and stressed mice treated with agomelatine (CUMS AGO) for Bdnf (P<0.001), Neurod1 (P<0.001), Igf1 (P<0.05) and Arc (P<0.01). Each bar is the mean±s.e.m. of n=7–12 mice. *P<0.05; §§P<0.01; ***,§§§P<0.001. CUMS, chronic ultra-mild stress.
Altogether, these data indicate that CUMS triggered marked histone posttranslational changes, favoring chromatin open state. Interestingly, this permissive state of chromatin was associated with increased transcription of genes involved in neuroplasticity, and concomitant treatment with agomelatine could abolish most of these CUMS-induced changes.
Discussion
Substantial evidence has been accumulated to suggest that stressful life events might trigger mood disorders and that successful antidepressant treatments might involve a normalization of stress-related alterations in cortico-limbic structures.24, 25 In this context, the aim of the present study was to investigate the effect of prolonged socio-environmental stressors of low intensity on depression-related behaviors and hippocampal function in mice, and explore the biological mechanisms and therapeutic potentialities of agomelatine in this stress model.
Effects of stress on neuroplasticity and behavior
The data reported herein clearly show that a CUMS induces a decrease in saccharin consumption, reflecting anhedonia, a core symptom of depression, as observed after other chronic stresses.21 A decrease in grooming was also claimed to be relevant to depression as it is decreased by chronic stress, an effect reversed by classical antidepressant drugs.26 Here, we show that both saccharin consumption and grooming are also reversed by chronic agomelatine (Figure 2). We also found that CUMS caused a robust decrease of immobility in the TST, and an increased mobility and a greater number of burst activity in the FST. Many behavioral reactions in the TST and the FST can be seen as the expression of active defense mechanisms in reaction to the aversive challenge of the environment.27, 28 Hence, in our experiments, CUMS exposure facilitated the flight response as shown by tonic/energetic burst activity, which might be interpreted as an excessive emotional arousal and a high vigilance state.29 In addition, stressed mice displayed a reduced anxiety-like behavior in the open field test and an increased locomotor and motor activity, reflecting a hyperactive state and risk-taking behaviors. Earlier reports, including those using the similar CUMS procedure in mice,19, 20, 29 also mentioned an increased locomotor activity following chronic mild stress exposure in rodents, which might be equally interpreted as psychomotor agitation relevant to mood disorders see also Gronli et al.30 Because convergent studies demonstrated that physical activity could significantly enhance hippocampal neurogenesis and plasticity,31 the increased locomotor activity observed in our study after chronic stress might be tentatively related to the enhancement of hippocampal plasticity, as illustrated by the enhanced expression of neuroplasticity markers (p-Akt, p-Erk, p38, p-Creb, p-Atf1) and increased cell proliferation. Nevertheless, stressed mice also displayed a pronounced impairment in motor coordination probably due to CUMS-induced distractibility and reduced ability to focus on the task.29
At the neurobiological level, chronic mild stress activates hippocampal intracellular signaling pathways, such as mitogen-activated protein kinases known to be involved in neuronal survival, differentiation and proliferation.32 In addition, we found robust changes in epigenetic regulation and gene transcription. CUMS-induced increase in chromatin permissive state and plasticity-related gene expression might correspond to the nuclear events downstream of activated intracellular signaling cascades. Among CUMS-associated epigenetic modifications, we observed a significant decrease in both mRNA and protein levels of the transcriptional repressor HDAC2. This is in line with previous studies showing that low levels of HDAC2 in the hippocampus is associated with enhanced synaptic plasticity.33 Changes of histone-modifying enzymes in the hippocampus have been observed following exposure to severe stressors, for example, social defeat stress, or exposure to environmental adversity during development, for example, prenatal stress and maternal separation in rodents.34, 35, 36 Interestingly, the present study is the first to demonstrate that prolonged exposure to low-intensity stressors at adulthood can trigger sustained alterations of histone posttranslational regulation in mice. In addition, postmortem brain analysis also revealed a decreased expression of HDAC2 and H3K9 methylation, pointing toward a permissive state of chromatin, in the nucleus accumbens of depressed patients.37 In the periphery, mRNA profiling in white blood cells revealed a downregulation of HDAC2 and HDAC5 in the depressive state of major depressive disorder. These alterations at HDAC2 were normalized in the remitted state,38 indicating that HDAC2 could represent a suitable biomarker for mood disorders.
Further, we showed that transcripts encoding trophic factors (Bdnf, Gdnf, Igf1), pro-survival factor (Bcl2), differentiation factor (Neurod1) and cell/dendritic growth factor (Arc), known to be critical in neuronal plasticity, were increased after chronic mild stress, when compared with non-stressed mice. Hence, our neurobiological investigations demonstrated the occurrence of marked molecular and cellular changes underlying increased hippocampal plasticity in CUMS-exposed mice. These results are rather provocative because it is generally admitted that chronic stress paradigms in rodents is associated with impaired synaptic integrity, reduced dendritic arborization and decreased neurogenesis in the hippocampus, as well as depressive- and anxiety-like behaviors.39, 40 However, few studies reported that chronic stress could also enhance neurogenesis, as well as dendritic spines and excrescence in CA3 neurons of the hippocampus.40, 41 In our CUMS conditions—in contrast to other protocols using longer and/or stronger stressors—we used a repetition of low-intensity socio-environmental stressors for a total period of 3 weeks suggesting that the timing, duration and intensity of the stressors might be critical for triggering adaptive neuroendocrine and behavioral outcomes. Furthermore, using the same stress procedure, we previously reported a reduced expression of hippocampal GR mRNA in CUMS mice.19 Interestingly, other reports, using different models of exposure to chronic stress, showed that GR downregulation either via genetic knockdown or pharmacological blockade, might be associated with increased synaptic plasticity.42, 43
Effect of agomelatine treatment on stress-related alterations
Agomelatine is an antidepressant that acts as a melatonergic (MT1/MT2) agonist and a 5-HT2C antagonist.12 Like most antidepressant drugs, agomelatine was shown to positively modulate neurogenesis, hippocampal plasticity and neurotrophic signaling in various rodent models of depression or after stress.16, 22, 44, 45 Although in the present study, we did not identify the cell types that expressed the genes modified either by stress and/or agomelatine treatment, it can be noticed that both the glucocorticoid receptors, GRs, and the agomelatine targets, MT1/MT2 and 5-HT2C, are present on CA1, CA3 and DG hippocampal neurons, which also expressed the genes regulated by stress and antidepressant treatments.46 However, previous studies have pointed out the granule cells of the ventral part of the DG as a main target for agomelatine effects,16 although our data also show CA1 and CA3 to be regulated by stress and/or agomelatine in line in part with Mairesse et al.47 Further studies should be addressed to carefully characterize the cell type(s) involved in these effects.
Interestingly, in our studies, we did not observe any effect of chronic agomelatine treatment on the expression of hippocampal plasticity markers in non-stressed mice. Interestingly, agomelatine specifically normalized the hippocampal alterations induced by chronic mild stress, indicating that this drug exerts a disease-dependent action on neuronal activity. MT2 receptors, one of agomelatine’s targets, are abundantly expressed in various hippocampal cell populations.13 Agomelatine could normalize neuronal activity, as specifically indicated with c-Fos levels in the prenatal stress model.47 Such effects might account, at least partly, for agomelatine-induced normalization of the increased intracellular signaling and associated epigenetic and neuroplastic changes observed in CUMS-exposed mice. Such normalizing effects have not been reported elsewhere, probably because only few studies have investigated the action of antidepressants or mood-stabilizing agents in rodent models exhibiting enhanced hippocampal plasticity. Nevertheless, in line with our present data, a recent study showed that fluoxetine antagonizes the increased locomotor activity and the changes in p-Creb, p-Erk and Bdnf in the hippocampus after olfactory bulbectomy in mice.48 Similar effects have been observed with lithium that reversed the upregulation of Creb signaling induced by chronic stress.49 Furthermore, selective serotonin reuptake inhibitors or tricyclic antidepressants were shown to modulate brain HDAC activity and histone posttranslational modifications in animal models of depression or stress.11 Here, we showed that agomelatine could similarly correct the altered HDAC2 levels, as well as posttranslational modifications at histone tails (for example, H3K27me3 and H3K9/14ac) in adult mice subjected to prolonged exposure to stress, an action that was causally related to behavioral remission. Altogether, these observations support the hypothesis that the reversal of chromatin-related alterations is required for the therapeutic action of antidepressant drugs, as already suggested.11
At the behavioral level, we know that chronic mild stress causes reward, motivation and attention deficits, symptoms related to decreased mesolimbic dopaminergic activity. The behavioral effects of agomelatine after chronic stress, which resulted in the normalization of CUMS-induced hyperactivity and of affective and cognitive defects, might be attributed to its antagonist action at 5-HT2C receptors, as the 5-HT2C receptor blockade property of agomelatine enhances dopamine release in the frontal cortex.50 In line with the frequent association between catecholamines and motor activity and the previous studies on agomelatine with distinct models of mood disorder,47, 51 agomelatine treatment was also found here to normalize the CUMS-induced enhancement in motor activity. Importantly, MT receptor-mediated control of emotional reactivity and vigilance might also be involved in the effects of agomelatine because the melatonergic receptor antagonist S22153 antagonized the antidepressant-like effect of agomelatine in animal models of depression.52
Altogether, our data suggest that the normalizing effects of agomelatine on the neurobiological and behavioral dysfunctions observed after chronic mild stress is attributed to its synergistic action via the melatonergic agonist and 5-HT2C antagonist properties, as already reported.16
Conclusions and perspectives
In summary, our neurobiological and behavioral investigations provided clear-cut evidence that prolonged exposure of C57Bl/6 mice to socio-environmental stressors of low intensity can induce marked molecular and cellular changes leading to increased hippocampal plasticity, together with increased locomotor activity, hyperarousal, anhedonia, distractibility and increased risk-taking behaviors. Agomelatine effectively prevented both neurochemical and behavioral alterations triggered by stress. In addition, the observed epigenetic changes in the hippocampus suggest that chromatin remodeling via histone modifications might increase susceptibility to mood disorders, and that the reversal of such changes by agomelatine is associated with its therapeutic action.
The fact that the behavioral changes induced by CUMS closely resembled symptoms usually observed in the manic state of bipolar disorders is also interesting (see Supplementary Table S8). The CUMS model as described in the present study might be of particular interest to further investigate the possible implication of stress, alterations in circadian activity rhythms and hippocampal dysfunctions in the development of bipolar disorders. Although no compelling evidence for an increased hippocampal intracellular signaling was reported in humans, validated models of manic-like behaviors showed neurochemical changes, including hippocampal upregulation of BDNF protein and increased PI3-K and mitogen-activated protein kinases signaling,53, 54 as found here in CUMS mice. Future clinical studies, more particularly postmortem brain analyses, should address whether changes of the above-mentioned biomarkers occur in the hippocampus of bipolar patients.
References
Gunnar M, Quevedo K . The neurobiology of stress and development. Annu Rev Psychol 2007; 58: 145–173.
McEwen BS, Gould EA, Sakai RR . The vulnerability of the hippocampus to protective and destructive effects of glucocorticoids in relation to stress. Br J Psychiatry Suppl 1992; 15: 18–23.
Sala M, Perez J, Soloff P, Ucelli di Nemi S, Caverzasi E, Soares JC et al. Stress and hippocampal abnormalities in psychiatric disorders. Eur Neuropsychopharmacol 2004; 14: 393–405.
McEwen BS . Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 2007; 87: 873–904.
Apfel BA, Ross J, Hlavin J, Meyerhoff DJ, Metzler TJ, Marmar CR et al. Hippocampal volume differences in Gulf War veterans with current versus lifetime posttraumatic stress disorder symptoms. Biol Psychiatry 2011; 69: 541–548.
Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY et al. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry 2004; 56: 640–650.
Malkoff-Schwartz S, Frank E, Anderson B, Sherrill JT, Siegel L, Patterson D et al. Stressful life events and social rhythm disruption in the onset of manic and depressive bipolar episodes: a preliminary investigation. Arch Gen Psychiatry 1998; 55: 702–707.
Proudfoot J, Doran J, Manicavasagar V, Parker G . The precipitants of manic/hypomanic episodes in the context of bipolar disorder: a review. J Affect Disord 2011; 133: 381–387.
Maze I, Noh KM, Allis CD . Histone regulation in the CNS: basic principles of epigenetic plasticity. Neuropsychopharmacology 2013; 38: 3–22.
Boulle F, van den Hove DL, Jakob SB, Rutten BP, Hamon M, van Os J et al. Epigenetic regulation of the BDNF gene: implications for psychiatric disorders. Mol Psychiatry 2012; 17: 584–596.
Vialou V, Feng J, Robison AJ, Nestler EJ . Epigenetic mechanisms of depression and antidepressant action. Annu Rev Pharmacol Toxicol 2013; 53: 59–87.
de Bodinat C, Guardiola-Lemaitre B, Mocaer E, Renard P, Munoz C, Millan MJ . Agomelatine, the first melatonergic antidepressant: discovery, characterization and development. Nat Rev Drug Discov 2010; 9: 628–642.
Musshoff U, Riewenherm D, Berger E, Fauteck JD, Speckmann EJ . Melatonin receptors in rat hippocampus: molecular and functional investigations. Hippocampus 2002; 12: 165–173.
Berumen LC, Rodriguez A, Miledi R, Garcia-Alcocer G . Serotonin receptors in hippocampus. ScientificWorldJournal 2012; 2012: 823493.
Bertaina-Anglade V, la Rochelle CD, Boyer PA, Mocaer E . Antidepressant-like effects of agomelatine (S 20098) in the learned helplessness model. Behav Pharmacol 2006; 17: 703–713.
Soumier A, Banasr M, Lortet S, Masmejean F, Bernard N, Kerkerian-Le-Goff L et al. Mechanisms contributing to the phase-dependent regulation of neurogenesis by the novel antidepressant, agomelatine, in the adult rat hippocampus. Neuropsychopharmacology 2009; 34: 2390–2403.
Harvey AG . Sleep and circadian rhythms in bipolar disorder: seeking synchrony, harmony, and regulation. Am J Psychiatry 2008; 165: 820–829.
Lanfumey L, Mongeau R, Hamon M . Biological rhythms and melatonin in mood disorders and their treatments. Pharmacol Ther 2013; 138: 176–184.
Froger N, Palazzo E, Boni C, Hanoun N, Saurini F, Joubert C et al. Neurochemical and behavioral alterations in glucocorticoid receptor-impaired transgenic mice after chronic mild stress. J Neurosci 2004; 24: 2787–2796.
Pardon MC, Perez-Diaz F, Joubert C, Cohen-Salmon C . Influence of a chronic ultramild stress procedure on decision-making in mice. J Psychiatry Neurosci 2000; 25: 167–177.
Willner P . Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 2005; 52: 90–110.
Paizanis E, Renoir T, Lelievre V, Saurini F, Melfort M, Gabriel C et al. Behavioural and neuroplastic effects of the new-generation antidepressant agomelatine compared to fluoxetine in glucocorticoid receptor-impaired mice. Int J Neuropsychopharmacol 2010; 13: 759–774.
Franklin KBJ, Paxinos G . The Mouse Brain in Stereotaxic Coordinates, 3rd edn. Elsevier Academic Press: San Diego, CA, USA, 2007.
Calabrese F, Molteni R, Racagni G, Riva MA . Neuronal plasticity: a link between stress and mood disorders. Psychoneuroendocrinology 2009; 34 (Suppl 1): S208–S216.
Touma C . Stress and affective disorders: animal models elucidating the molecular basis of neuroendocrine-behavior interactions. Pharmacopsychiatry 2011; 44 (Suppl 1): S15–S26.
Yalcin I, Belzung C, Surget A . Mouse strain differences in the unpredictable chronic mild stress: a four-antidepressant survey. Behav Brain Res 2008; 193: 140–143.
Cryan JF, Mombereau C, Vassout A . The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev 2005; 29: 571–625.
Narboux-Neme N, Sagne C, Doly S, Diaz SL, Martin CB, Angenard G et al. Severe serotonin depletion after conditional deletion of the vesicular monoamine transporter 2 gene in serotonin neurons: neural and behavioral consequences. Neuropsychopharmacology 2011; 36: 2538–2550.
Negroni J, Venault P, Pardon MC, Perez-Diaz F, Chapouthier G, Cohen-Salmon C . Chronic ultra-mild stress improves locomotor performance of B6D2F1 mice in a motor risk situation. Behav Brain Res 2004; 155: 265–273.
Gronli J, Murison R, Fiske E, Bjorvatn B, Sorensen E, Portas CM et al. Effects of chronic mild stress on sexual behavior, locomotor activity and consumption of sucrose and saccharine solutions. Physiol Behav 2005; 84: 571–577.
van Praag H, Christie BR, Sejnowski TJ, Gage FH . Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA 1999; 96: 13427–13431.
Thomas GM, Huganir RL . MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci 2004; 5: 173–183.
Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature 2009; 459: 55–60.
Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ . Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci 2006; 9: 519–525.
Van den Hove DL, Kenis G, Brass A, Opstelten R, Rutten BP, Bruschettini M et al. Vulnerability versus resilience to prenatal stress in male and female rats; implications from gene expression profiles in the hippocampus and frontal cortex. Eur Neuropsychopharmacol 2013; 23: 1226–1246.
Suri D, Bhattacharya A, Vaidya VA . Early stress evokes temporally distinct consequences on the hippocampal transcriptome, anxiety and cognitive behaviour. Int J Neuropsychopharmacol 2014; 17: 289–301.
Covington HE 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O et al. Antidepressant actions of histone deacetylase inhibitors. J Neurosci 2009; 29: 11451–11460.
Hobara T, Uchida S, Otsuki K, Matsubara T, Funato H, Matsuo K et al. Altered gene expression of histone deacetylases in mood disorder patients. J Psychiatr Res 2010; 44: 263–270.
Alonso R, Griebel G, Pavone G, Stemmelin J, Le Fur G, Soubrie P . Blockade of CRF(1) or V(1b) receptors reverses stress-induced suppression of neurogenesis in a mouse model of depression. Mol Psychiatry 2004; 9: 278–286.
Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T et al. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry 2008; 13: 717–728.
Sunanda, Rao MS, Raju TR . Effect of chronic restraint stress on dendritic spines and excrescences of hippocampal CA3 pyramidal neurons–a quantitative study. Brain Res 1995; 694: 312–317.
Fitzsimons CP, van Hooijdonk LW, Schouten M, Zalachoras I, Brinks V, Zheng T et al. Knockdown of the glucocorticoid receptor alters functional integration of newborn neurons in the adult hippocampus and impairs fear-motivated behavior. Mol Psychiatry 2013; 18: 993–1005.
Krugers HJ, Goltstein PM, van der Linden S, Joels M . Blockade of glucocorticoid receptors rapidly restores hippocampal CA1 synaptic plasticity after exposure to chronic stress. Eur J Neurosci 2006; 23: 3051–3055.
Banasr M, Soumier A, Hery M, Mocaer E, Daszuta A . Agomelatine, a new antidepressant, induces regional changes in hippocampal neurogenesis. Biol Psychiatry 2006; 59: 1087–1096.
Gumuslu E, Mutlu O, Sunnetci D, Ulak G, Celikyurt IK, Cine N et al. The antidepressant agomelatine improves memory deterioration and upregulates CREB and BDNF gene expression levels in unpredictable chronic mild stress (UCMS)-exposed mice. Drug Target Insights 2014; 8: 11–21.
Surget A, Wang Y, Leman S, Ibarguen-Vargas Y, Edgar N, Griebel G et al. Corticolimbic transcriptome changes are state-dependent and region-specific in a rodent model of depression and of antidepressant reversal. Neuropsychopharmacology 2009; 34: 1363–1380.
Mairesse J, Silletti V, Laloux C, Zuena AR, Giovine A, Consolazione M et al. Chronic agomelatine treatment corrects the abnormalities in the circadian rhythm of motor activity and sleep/wake cycle induced by prenatal restraint stress in adult rats. Int J Neuropsychopharmacol 2013; 16: 323–338.
Freitas AE, Machado DG, Budni J, Neis VB, Balen GO, Lopes MW et al. Fluoxetine modulates hippocampal cell signaling pathways implicated in neuroplasticity in olfactory bulbectomized mice. Behav Brain Res 2013; 237: 176–184.
Boer U, Cierny I, Krause D, Heinrich A, Lin H, Mayr G et al. Chronic lithium salt treatment reduces CRE/CREB-directed gene transcription and reverses its upregulation by chronic psychosocial stress in transgenic reporter gene mice. Neuropsychopharmacology 2008; 33: 2407–2415.
Millan MJ, Gobert A, Lejeune F, Dekeyne A, Newman-Tancredi A, Pasteau V et al. The novel melatonin agonist agomelatine (S20098) is an antagonist at 5-hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways. J Pharmacol Exp Ther 2003; 306: 954–964.
Norman TR, Cranston I, Irons JA, Gabriel C, Dekeyne A, Millan MJ et al. Agomelatine suppresses locomotor hyperactivity in olfactory bulbectomised rats: a comparison to melatonin and to the 5-HT(2c) antagonist, S32006. Eur J Pharmacol 2011; 674: 27–32.
Chenu F, El Mansari M, Blier P . Electrophysiological effects of repeated administration of agomelatine on the dopamine, norepinephrine, and serotonin systems in the rat brain. Neuropsychopharmacology 2013; 38: 275–284.
Kirshenbaum GS, Clapcote SJ, Duffy S, Burgess CR, Petersen J, Jarowek KJ et al. Mania-like behavior induced by genetic dysfunction of the neuron-specific Na+,K+-ATPase alpha3 sodium pump. Proc Natl Acad Sci USA 2011; 108: 18144–18149.
Prickaerts J, Moechars D, Cryns K, Lenaerts I, van Craenendonck H, Goris I et al. Transgenic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and mania. J Neurosci 2006; 26: 9022–9029.
Acknowledgements
This work was supported by grants from the Institut National de la Santé et de la Recherche Médicale (France), ANR (2011-BSV-017-01) and IRIS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Translational Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Boulle, F., Massart, R., Stragier, E. et al. Hippocampal and behavioral dysfunctions in a mouse model of environmental stress: normalization by agomelatine. Transl Psychiatry 4, e485 (2014). https://doi.org/10.1038/tp.2014.125
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2014.125
This article is cited by
-
Chronic mild stress paradigm as a rat model of depression: facts, artifacts, and future perspectives
Psychopharmacology (2022)
-
A new experimental design to study inflammation-related versus non-inflammation-related depression in mice
Journal of Neuroinflammation (2021)
-
Hydrogen Sulfide Reverses LPS-Induced Behavioral Deficits by Suppressing Microglial Activation and Promoting M2 Polarization
Journal of Neuroimmune Pharmacology (2021)
-
Features of the Social Behavior of Mice after Prolonged Exposure to Psychoemotional and Infective Factors
Neuroscience and Behavioral Physiology (2021)
-
Differential effects of chronic stress in young-adult and old female mice: cognitive-behavioral manifestations and neurobiological correlates
Molecular Psychiatry (2018)