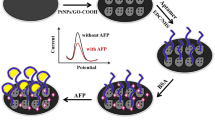
Abstract
A portable, affordable and simple detector is requested in a “Point-of-Care-Testing” (POCT) system. In this study, we exploited the potentialities of Differential Pressure Gauge (DPG) to the orientation of POCT technology. Alpha fetoprotein (AFP) was chosen as a model analyte that could specifically recognized by its antigen, and a tiny outfits equipped with a DPG was employed as the signal readout. Pt/SiO2 nanospheres were synthesized and modified with the detection antibody. In the presence of target, a sandwich of immunocomplex specifically formed and the Pt/SiO2 had been modified on the capture antibody. Which then can be dissolved to release plenty of Pt and the suspensions were transferred into a closed vial filled with appropriated amount of hydrogen peroxide. Subsequently, hydrogen peroxide was decomposed to produce oxygen, resulting in the enhancement of pressure in the closed vial and which can be detected by DPG easily. Under the optimized conditions, the read out signal from DPG had a direct relationship with AFP concentrations in the range of 10~200 ng/mL, and the detection limit was as low as 3.4 ng/mL. The proposed portable sensor had been successfully applied to detect AFP in serum samples with satisfactory results. This strategy holds a great promising in biological analysis as its convenient operations, reliable results and flexible apparatus.
Similar content being viewed by others
Introduction
Developing the technology of point-of-care testing (POCT) is meaningful for improving healthcare, maintaining sanitation and ensuring food safety due to the characteristic of “pocket” size, cost effectiveness, reliable quantitative results and easy operation1,2,3,4. Nowadays, more and more portable devices have been widespread used in daily life for analysis5, such as thermometer gauges for temperature, hygrometers for humidity, sphygmomanometer for blood pressure, personal glucose meter (PGM) for glucose and so on. In order to extend the application of portable devices, some groups have attempted to ameliorate them to be signal readout6. For examples, a common thermometer can related with ELISA to fabricate a biomolecular quantitation method based on the nanoparticle-mediated photothermal effect7; a portable infrared thermometer basing on an infrared detector has been commercialized, and a portable alcohol detector is widely used for alcohol testing based on a multimeter. The representative case is the personal glucose meter (PGM), used by diabetic patients themselves to monitor the concentration of blood glucose, has been transformed into a component of a sensor combining with biochemical techniques for quantifying non-glucose targets, such as DNA, enzyme and proteins8.
However, most of portable devices can only provide one signal which corresponds to a change of physical parameter and thereby restricted the application of those portable devices. In this study, we report a strategy exploiting the potentialities of Differential Pressure Gauge (DPG) to the orientation of POCT technology. In fact, pressure gauge is a useful device but has not been taken full advantages. Many chemical or biological reactions accompanied with a change of pressure, which can act as a signal reflecting the specific situation9,10. In hospital, a pressure gauge or a flow meter is used to monitor the service condition of an oxygen cylinder. Besides, a pressure gauge is essential to a pneumatic tire. However, little research about the detection of biochemical targets based on the pressure change has ever been reported. The main obstacle may stem from that the pressure change induced by biomedical targets is comparatively weak. Some groups attempted to design a capillary tube or volumetric bar-chart chip with a floating ink for indicating the micro-pressure change, which was caused by the decomposition of hydrogen peroxide to generate oxygen in a closed container. However, using the height of ink in channel as the data readout is apparently short of veracity and repeatability, because the results were susceptible to the device position located, the apparatus configuration designed and the individual proficiency of operations11,12. Furthermore, results just recorded on the paper were difficult to connecting with digital devices (i.e. iPads, computers, or phones with appropriate application programs), which emerged their increasing importance in personal health care13. In an early study, our group successfully employed a commercially available differential pressure gauge to set up an analytic method for thrombin detection13. However, it was found that catalase for catalysing the decomposition of hydrogen peroxide was inefficient and easily inactivated. It is necessary to find some way to address this problem.
Enzyme-linked immunosorbent assay (ELISA) is an important molecular recognition technology, which has been substantially used for biomarker quantification with high specificity14,15,16,17,18,19,20,21,22. In mostly of these biosensors, the signal readout relies heavily on optical-based measurements, which request measuring instrument such as ultraviolet spectrophotometer or fluorospectrophotometer. This is so inconvenient in on-pot determination or resource limited area. Though colorimetric immunoassays was proposed to be a simple, sensitive, and fast approach, it was still difficult to realize quantitative analysis since the naked eyes is insensitive to the slight color changing. Recently, ELISA had been coupled with glucose meter to develop new sensing systems for multiple bio-molecules detection. For examples, Yang group had reported a method that integrated ELISA with magnetic mesoporous silica and used glucose meter as signal readout for PbTx-2 detection23. Liu group combined ELISA and ring-oven washing technique for cancer biomarker detection24. But to the best of our knowledge, little literature using DPG as readout had been reported. Only Yang group currently had attempted this combined technique25. In theory, the decomposition of 1 mmol H2O2 generates 11.2 mL oxygen gas under standard conditions, which increases the pressure as high as 2.27 × 105 Pa in a closed container of 10 mL, and these change caused by a trace of target would be detected by DPG easily. Therefore, it holds a great promise in combining ELISA with DPG to develop a POCT sensor for a fast, accurate and reliable assay.
As mentioned early, there were some drawbacks about the decomposition of H2O2 by the catalase. It has been reported that the present of Pt can decompose H2O2 with high efficiency and this reaction is inert to surrounding environments26. In this study, an immunosorbent-based assay equipped with DPG as signal readout has been developed for AFP determination; Pt has been embed in the SiO2 to prepare Pt/SiO2 nanoparticles firstly, which then be further modified with antibody through its amino groups on the surface. Then which has been used as the catalyst to decompose H2O2 to produce oxygen gas in a closed vial and causing the increasing of pressure in the vial, which can be used to present the AFP concentration. The proposed biosensor has been applied to detect AFP in serum samples with satisfied results.
Methods
Materials and Instruments
A commercially available differential pressure gauge (DPG) was purchased from BENETECH (GM511). Fourier Transform Infrared (FT-IR) spectra were recorded on a Nicolet 6700 (Thermo Fisher Scientific, USA) spectrometer in transmission mode. The High-Resolution Transmission Electron Microscopy (TEM) images were obtained with Tecnai G2 F20 S-TWIN TEM (FEI, USA).
Tetraethylorthosilicate (TEOS), 3-aminopropyltriethoxysilane (APTES) were purchased from Sigma-Aldrich. Hexadecyltrimethyl ammonium bromide (CTAB), Poly (vinylpyrrolidone) (PVP) (Mw = 29000), Dihydrogen hexachloroplatinate (H2PtCl6■6H2O) were purchased from Sinopharm Chemical Reagent (Shanghai, China), a Diagnostic Kit for Alpha fetal protein (ELISA) was purchased from Biocell Biotechnology (Zhengzhou China).
Mouse-derived antihuman antibody pairs for capturing AFP and human AFP antigen were obtained from BiosPacific (Emeryville, CA) and severed as detection antibody.
Synthesis of Silicon dioxide Nanospheres
Amine-group functionalized silicon dioxide nanospheres were firstly synthesized by hydrothermally method according to the early reports27,28. Briefly, 0.25 g CTAB, 0.08 g NaOH was dissolved in water to a volume of 120 mL and heat to 353 K. Then 2.5 mL TEOS was drop wised to the surfactant solution followed by 245 μL APTES. After stirring for 2 h to give rise white precipitates, the solid product was filtered, washed three times with deionized water and methanol to completely remove CTAB and excessive MPTMS, then dried in a vacuum oven overnight.
Doping Silica Nanospheres with Pt (Pt/SiO2)
The loading of Pt into silica nanospheres was performed by refluxing a mixture of 0.5 g silica nanospheres, 26.6 mg PVP and 124.3 mg H2PtCl6●6H2O in 400 mL water/methanol solution for 3 h. It was found that the color of the mixture was turn into black from pale yellow29. Then the solution was cooled down to room temperature slowly, and centrifuged at 9000 rpm for 7 min. The precipitates were washed three times with deionized water to get the Pt doping silica nanospheres functionalized with amine-group (Pt/SiO2).
Modification of Pt/SiO2 with Detection Antibody
The as-synthesized Pt/SiO2 nanoparticle was labelled with detection antibody by cross-linker of glutaraldehyde. 1.5 mg of Pt/SiO2 was dissolved into 1 mL sodium bicarbonate buffer (100 mM, pH 8). Then 1 mL 5% glutaraldehyde stock solution (w/v) was added and slight stirring for 30 min at room temperature. The mixture was centrifuged at 9000 rpm to remove excess glutaraldehyde, and the nanoparticles were re-suspended in 2 mL PBS (100 mM, pH 7.3). Next, 50 μL detection antibody of 10 mg/mL was added to the suspension drop by drop with stirring. After incubation at room temperature for 1 h, the suspension was centrifuged at 9000 rpm to remove the excess antibody. At last, the unsaturated binding was blocked by bovine serum albumin (BSA) (10% w/v in deionized water). The detection antibody functionalized Pt/SiO2 was re-suspended in PBS and stored at 4 °C for further usage.
Procedures of Immunosorbent Assay with DPG
Firstly, 100 μL at various concentrations (10~200 ng/ml) of AFP antigen solution with BSA blocking were added into the 96-wells get from the diagnostic kit and incubated on a shaker (500 rpm) for 30 min. Then the plate was drained and washed by wash buffer (100 mM PBS with 0.05% Tweeen-20) three times. After that, 100 μL detection antibody functionalized Pt/SiO2 was added into each well, sealed and incubated at room temperature on a shaker (500 rpm), washed three times by washing buffer and tapped dry. Finally, the Pt/SiO2 was dissolved by 100 μLNaOH (0.10 M) and incubated on a shaker (100 rpm) for 10 min, 100 μL of HCl (0.01 M) was added to the well to regulate the pH value. Then the released Pt was collected and injected into a tiny outfit fabricated by ourselves. And the pressure in the outfit was monitored by the DPG.
Results and Discussion
Principle of the portable sensor for AFP
Figure 1 shows the details of the proposed approach for AFP detection, which consists of the imunosorbent-based target recognition and the signal-converted micro-pressure monitoring by a tiny outfits fabricated by ourselves. As the linker of the two parts, silica nanospheres doped with Pt (Pt/SiO2) was firstly synthesized and then modified on AFP antibody. In the presence of AFP, a sandwich-liked immunocomplex of detection antibody-AFP-capture antibody (coated in the well) specifically formed. After removing the excess detection antibody, the immunocomplex was destroyed by the addition of NaOH solution to dissolve SiO2, resulting in a plenty of released Pt. The suspensions containing Pt were subsequently transferred into a vial filled with 4 mL H2O2. It was found that a lot of bubble generated since the H2O2 was decomposed to H2O and O2 by Pt nanoparticles. And the enhancement of pressure in an airtight vial caused by the accumulation of O2 was recorded by DPG during the whole process. Since the pressure detected has a relationship with the AFP concentration, a simple portable biosensor for AFP detection can be developed.
Characterizations of the Pt/SiO2
To deposit Pt nanoparticles directly on the surface of SiO2, a template strategy was employed here according to the early reported method30,31. Primary amine was found to be an effective functional group to combine with metal ions. So the NH2- SiO2 can promote the reduction of Pt4+ located on the surface of SiO2 to produce Pt nanoparticles. High-Resolution Transmission Electron Microscopy has been applied to characterize the morphology of SiO2 and Pt/SiO2. As shown in Fig. 2(A), the diameter of SiO2 was about 400 nm. And high density of Pt nanoparticles with diameter of 2 nm were observed on the surface of the SiO2 (Fig. 2(B)), which confirmed that Pt had loaded in the silicon nanospheres effectively.
Fourier transform infrared (FTIR) spectroscopy of the Pt/SiO2 was also been tested. Although the stretching vibration peak of –NH2 is overlapped with –OH (3100~3600 cm−1), and the deformation vibration peak of –NH2 is overlapped with characteristic peak of Si-O-Si. (15600~1650 cm−1). The peaks appear at 3078, 2924, 1528 cm−1 is the characteristic peaks of C-H in APTES. (Figure 2(C)) These evidences indicated that amine groups had been modified the silica nanospheres. And energy-dispersive X-ray spectroscopy (EDX) had been used to further confirm the presence of platinum in the silicon nanospheres. As shown in Fig. 2(D), it was found that the Pt characteristic peak and the percentage of platinum was calculated to be about 5.3%.
Optimized of the reaction conditions
First of all, in order to verify the feasibility of our tiny outfits on the detection of micro-pressure change, Pt/SiO2 without any modifications was chosen as the test sample. Figure 3(A) shows the time-dependent changes of pressure with different amount of Pt/SiO2. It is found that the pressure of the system linearly increased with the extension of the reaction time before 30 min. Accordingly, there has a linear relationship between pressure and the amount of Pt/SiO2, 15 min (a) and 30 min (b), respectively (shown in Fig. 3(B)). These results indicate that there has relationship between the detected pressure and the amount of Pt/SiO2, and we may change the reaction time to meet the requirement of the detection limit. In this study, 15 min was selected as the reaction time without special mentioned.
(A) Time-dependent changes in pressure with different volume of Pt/SiO2 (1.00 mg/mL):Blank 0, (a) 3, (b) 6, (c) 9, (d) 12,(e) 15 μL in the tiny outfit containing 4 mL of 1.6 mol●L−1 H2O2. (B): the linear relationship between pressure signal and the volume of Pt/SiO2 at different reaction time, 15 min (a) and 30 min (b).
The concentration of H2O2 is another key role in this detection system. As shown in Fig. 4, with the increasing concentration of the H2O2 from 0.4 to 1.6 M (4 mL), the trend of pressure increases tardily, while a sharply increases is observed when the concentration of H2O2 is 2.0 M (the red curve). The reason for this phenomenon mainly stems from the unstable feature of H2O2, which implies a higher concentration of H2O2 over 1.6 M would decompose automatically and cause corresponding background. Thus, 1.6 M was chosen as the suitable concentration of H2O2.
Quantificational detection of AFP
Figure 5 shows the relationship between the pressure detected and the AFP concentration. The pressured signal recorded by DGP increased with the increasing of AFP concentration. And there is a good linear relationship between the enhanced signal and the concentration of AFP in linear range of 10~200 ng/mL, the regression equation can be expressed as follow:

where ΔP is the difference of pressure signal recorded by PDG at present and absent of target, C is the concentration of AFP, while R is the regression coefficient. The detection limit is calculated to be 3.4 ng/mL (S/N = 3). This result meets the need of practical application since the average level of AFP in healthy human serum is lesser than 20 ng/mL.
It should be noted that although some conventional ELISA method gives out a lower detection limit, it is actually difficult to distinguish the subtle change in color only by naked eye without any assistant of instruments. As regards of the portable assay, DPG equipped ELISA assay provides a promising approach for point-of-care testing with an accurate and reliable result. Besides, there are many strategies characterized by portable or rapid detection also show deficiency in LOD, Table 1 shows several examples.
Selectivity of the sensor
The specificity of this assay for AFP detection was investigated by testing several interferences that commonly meet during the detection of AFP, such as glucose, thrombin and CEA. The concentration of AFP was fixed at 40 ng/mL while the other interferences were all 100 folds. Experiments were conducted under the same conditions and repeated three times for each situation. As shown in Fig. 6, the pressure enhanced greatly at present of target but nearly no signal changing had been detected if we added interferences only. These results clearly demonstrated the high specificity of the developed assay.
Serum sample detection
The proposed biosensor had been applied to detect the AFP concentration in the real samples (Three volunteers from the first affiliated hospital of Fujian Medical University). As shown in Table 2, the results detected by the proposed method had good agreements with the reference values provided by the hospital. Different levels of AFP had been added into the samples and were tested by the presented method, and results showed that recoveries of AFP were in the range of 96.6~103.3% with RSDs of 1.2~4.3%. These indicate the proposed method can be applied to detect AFP in the clinic samples with satisfactory results.
Conclusions
In summary, an immunesorbent-based assay using PDG as signal readout for AFP detection is developed. Pt/SiO2 nanoparticles were used as nanotags modified with detection antibody, as well as the catalyst of H2O2 solution to produce O2. In the presence of AFP, a sandwich immune complex of capture antibody-AFP-detection antibody specifically formed. After destroying the Pt/SiO2 nanospheres, the plenty of released Pt nanoparticles were subsequently transferred into an airtight vial. In consequence, the target-induced pressure change can be detected by DPG easily and give out a fine quantificational result. This presented approach provides a reliable option to quantitative analysis of AFP with good selectivity. By alter the corresponding antigen-antiboby, this assay would be extend to any bioassay using a catalyst as tags. What’s more, the features of pocket-size, low-cost and wide potential applications in biological analysis may become the new highlight of PDG.
Additional Information
How to cite this article: Wang, Q. et al. A Portable Immunosensor with Differential Pressure Gauges Readout for Alpha Fetoprotein Detection. Sci. Rep. 7, 45343; doi: 10.1038/srep45343 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Dou, M. et al. A Versatile PDMS/Paper Hybrid Microfluidic Platformfor Sensitive Infectious Disease Diagnosis. Anal. Chem. 86, 7978–86 (2014).
Sanjay, S. T., Dou, M., Sun, J. et al. A paper/polymer hybrid microfluidic microplate for rapid quantitative detection of multiple disease biomarkers. [J]. Scientific Reports 6, 30474 (2016).
Dou, M. et al. A fully battery-powered inexpensive spectrophotometric system for high-sensitivity point-of-care analysis on a microfluidic chip. Analyst 141, 3898–3903 (2016).
Dou, M. et al. Multiplexed instrument-free meningitis diagnosis on a polymer/paper hybrid microfluidic biochip. Biosen. Bioelectro 87, 865–873 (2016).
Yager, P., Domingo, G. J. & Gerdes, J. Point-of-care diagnostics for global health. Annu. Rev. Biomed. Eng 10, 107–144 (2008).
Luppa, P. B., Müller, C., Schlichtiger, A. & Schlebusch, H. Point-of-care testing (POCT): Current techniques and future perspectives. TrAC, Trends Anal. Chem 30, 887–898 (2011).
Fu, G., Sanjay, S. T., Dou, M. et al. Nanoparticle-mediated photothermal effect enables a new method for quantitative biochemical analysis using a thermometer. [J]. Nanoscale 8(10), 5422–5427 (2016).
Xiang, Y. & Lu, Y. Using personal glucose meters and functional DNA sensors to quantify a variety of analytical targets. Nature chemistry 3, 697–703 (2011).
Suladze, S., Cinar, S., Sperlich, B. & Winter, R. Pressure Modulation of the Enzymatic Activity of Phospholipase A2, A Putative Membrane-Associated Pressure Sensor. J. Am. Chem. Soc 137, 12588–12596 (2015).
Braun, C. et al. Ca3N2 and Mg3N2: Unpredicted High-Pressure Behavior of Binary Nitrides. J. Am. Chem. Soc 133, 4307–4315 (2011).
Lee, S., Kwon, D., Yim, C. & Jeon, S. Facile Detection of Troponin I Using Dendritic Platinum Nanoparticles and Capillary Tube Indicators. Anal. Chem 87, 5004–5008 (2015).
Song, Y. et al. Multiplexed volumetric bar-chart chip for point-of-care diagnostics. Nat. Commun 3, 1283 (2012).
Yang, W. et al. A micro-pressure sensor-based analytic platform and its application in thrombin quantification. Anal. Methods 7, 7985–7988 (2015).
Self, C. H. & Cook, D. B. Advances in immunoassay technology. Curr. Opin. Biotechnol 7, 60–65 (1996).
Ohkuma, H. et al. Development of a highly sensitive enzyme-linked immunosorbent assay for bisphenol A in serum. Analyst 127, 93–97 (2002).
Lu, H. et al. Screening for testosterone, methyltestosterone, 19-nortestosterone residues and their metabolites in bovine urine with enzyme-linked immunosorbent assay (ELISA). Anal. Chim. Acta 570, 116–123 (2006).
Cheng, C. M. et al. Paper‐Based ELISA. Angew. Chem., Int. Ed 49, 4771–4774 (2010).
Alberti, D. et al. A Quantitative relaxometric version of the ELISA test for the measurement of cell surface biomarkers. Angew. Chem., Int. Ed 53, 3488–3491 (2014).
Liu, Y. et al. Microchip-based ELISA strategy for the detection of low-level disease biomarker in serum. Anal. Chim. Acta 650, 77–82 (2009).
Hsu, C.-K. et al. Paper-Based ELISA for the Detection of Autoimmune Antibodies in Body FluidThe Case of Bullous Pemphigoid. Anal. Chem 86, 4605–4610 (2014).
Wu, J., Zhu, J., Yin, H., Buckanovich, R. J. & Lubman, D. M. Analysis of glycan variation on glycoproteins from serum by the reverse lectin-based ELISA assay. J. Proteome Res 13, 2197–2204 (2014).
Shih, C.-M. et al. Paper-based ELISA to rapidly detect Escherichia coli. Talanta 145, 2–5 (2015).
Gao, Z., Tang, D., Xu, M., Chen, G. & Yang, H. Nanoparticle-based pseudo hapten for target-responsive cargo release from a magnetic mesoporous silica nanocontainer. Chem. Commun 50, 6256–6258 (2014).
Liu, W., Guo, Y., Zhao, M., Li, H. & Zhang, Z. Ring-Oven Washing Technique Integrated Paper-based Immunodevice for Sensitive Detection of Cancer Biomarker. Anal. Chem 87, 7951–7957 (2015).
Zhu, Z. et al. Translating Molecular Recognition into a Pressure Signal to enable Rapid, Sensitive, and Portable Biomedical Analysis. Angew. Chem., Int. Ed 54, 10448–10453 (2015).
Zhu, Z. et al. Au@ Pt Nanoparticle Encapsulated Target‐Responsive Hydrogel with Volumetric Bar‐Chart Chip Readout for Quantitative Point‐of‐Care Testing. Angew. Chem., Int. Ed 53, 12503–12507 (2014).
Lai, C.-Y. et al. A mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules. J. Am. Chem. Soc 125, 4451–4459 (2003).
Song, H. et al. Hydrothermal growth of mesoporous SBA-15 silica in the presence of PVP-stabilized Pt nanoparticles: synthesis, characterization, and catalytic properties. J. Am. Chem. Soc 128, 3027–3037 (2006).
Wang, Y., Ren, J., Deng, K., Gui, L. & Tang, Y. Preparation of tractable platinum, rhodium, and ruthenium nanoclusters with small particle size in organic media. Chem. Mater 12, 1622–1627 (2000).
Li, A., Zhao, J. X. & Pierce, D. T. Silica nanoparticles for template synthesis of supported Pt and Pt-Ru electrocatalysts. Journal of Colloid & Interface Science 351, 365–373 (2010).
Zhu, X. et al. An ultrathin self-humidifying membrane for PEM fuel cell application: fabrication, characterization, and experimental analysis. Journal of Physical Chemistry B 110, 14240–14248 (2006).
Acknowledgements
This work was financially supported NSFC (21575025), Nature Sciences Funding of Fujian Province (2014J06005), and program for New Century Excellent Talents in University (NCET-12-0619).
Author information
Authors and Affiliations
Contributions
Z.-Y.L., R.-J.L. and Q.-P.W. designed the research; W.-Q.Y. and R.-J.L. performed the experiments; K.S., B.Q. and G.-N.C. contributed to the reagents/materials/analysis tools; R.-J.L., Z.-Y.L., Y.L., and L.-H.G. wrote the paper; all authors approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wang, Q., Li, R., Shao, K. et al. A Portable Immunosensor with Differential Pressure Gauges Readout for Alpha Fetoprotein Detection. Sci Rep 7, 45343 (2017). https://doi.org/10.1038/srep45343
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep45343
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.