Abstract
Similar land-use types usually have similar soil properties, and, most likely, similar microbial communities. Here, we assessed whether land-use types or soil chemical properties are the primary drivers of soil microbial community composition, and how changes in one part of the ecosystem affect another. We applied Ion Torrent sequencing to the bacterial and fungal communities of five different land-use (vegetation) types in the Loess Plateau of China. We found that the overall trend of soil quality was natural forest > plantation > bare land. Dominant bacterial phyla consisted of Proteobacteria (42.35%), Actinobacteria (15.61%), Acidobacteria (13.32%), Bacteroidetes (8.43%), and Gemmatimonadetes (6.0%). The dominant fungi phyla were Ascomycota (40.39%), Basidiomycota (38.01%), and Zygomycota (16.86%). The results of Canonical Correspondence Analysis (CCA) and Redundancy Analysis (RDA) based on land-use types displayed groups according to the land-use types. Furthermore, the bacterial communities were mainly organized by soil organic carbon (SOC). The fungal communities were mainly related to available phosphorus (P). The results suggested that the changes of land use type generated changes in soil chemical properties, controlling the composition of microbial community in the semiarid Loess Plateau region. The microbial community could be an indicator for soil quality with respect to ecological restoration.
Similar content being viewed by others
Introduction
Land-use change is one of the main factors affecting the biodiversity and functioning of terrestrial ecosystems1. Soil-residing microorganisms are living parts of soil ecosystems and have complex interactions with their environment2. At an ecosystem scale, they can directly influence soil ecological processes and maintain soil stability3,4. Meanwhile, the properties of microbes are manipulated by environment conditions5. Diverse systems with dissimilar microbial communities are expected.
To remedy adverse impacts to ecological environments, vegetation restoration has been conducted on the Loess Plateau of China, leading to changes in land-use patterns and above- and below-ground environments. These fluctuations, in turn, have profound indirect and direct effects on soil microorganisms6,7. The differences of microbe community composition could signify that biogeography is driven by special soil chemical properties that fluctuate among land-use types8. Studies have indicated that changes in land-use type have great effects on microbial communities9,10. A growing body of evidence not only supports this finding but also identifies land-use change as one of the most important drivers affecting biodiversity in terrestrial ecosystems1,11. However, in some ecosystems, soil chemical properties outweigh the effects of land-use type12. Furthermore, soil can release considerable CO2, even in the winter13 because of microbial activity. Although there have been several reports on soil microbial composition in the Loess Plateau of China14,15,16, the effects of land-use types and soil chemical properties on bacteria and fungi community composition during both growing and dormant seasons are still poorly understood. The main drivers of microbial community structure remain unknown because of the complex ecosystems in this region. There is an obvious need to understand the consequences of land-use changes in order to predict ecosystem stability and ecological services in the Loess Plateau of China.
In this study, we used the Ion Torrent sequencing approach to explore the vast uncultivated soil microbes that are directly related to the effects of land-use change on soil chemical properties. This study investigated the effects of (1) land-use types, (2) seasonality, and (3) soil chemical properties on fungal and bacterial communities in five different land-use types including natural and plantation forests, shrub land, and bare land. We aimed to understand whether land-use types and/or soil chemical properties are the primary drivers of soil microbial community structure.
Materials and Methods
Study area and experimental site
The study was conducted at Mt. Gonglushan in the southern suburb of Yan’an City, Shaanxi Province, in the central part of Loess Plateau (36°25.40′N, 109°31.53′E, 1353 m a.s.l.). This area is a fragile semiarid ecosystem and it contains one of the largest global loess areas. The site is also in the ecological transition zone between forest and forest-steppe ecosystems. Indigenous forests are present in sites with relatively little human disturbance. The mean annual precipitation and air temperature were 504.7 mm and 10.1 °C, respectively, based on a 40-year average (1971–2010) recorded by the city meteorological station13. In this region, the growing season for deciduous species occurs from April to October17. Five land-use types representing natural and planted forests, and non-forest lands were selected for this study: (1) Liaodong oak (Quercus liaotungensis), the typical climax forest type, (2) oriental arborvitae (Platycladus orientalis) which occurs as a small stand, (3) shrub land including species of early lilac (Syringa oblata), rose (Rosa hugonis), and Caragana microphylla, (4) black locust (Robinia pseudoacacia), a fast-growing introduced species which contributes to major plantations in the region14, and (5) bare land which was abandoned decades ago. These different land-use types are separated by approximately hundreds of meters and have the same climate13. More details about the sampling sites were displayed in Table 1 and the supplementary figure.
Collection and preparation of soil samples
Soil sampling was conducted in August 2013 and February 2014, capturing the growing season and the dormant period, respectively. In this region, the plant growing period contains months from April to October, with June–August being the summer and December–February being the winter. The land-use types and their seasonal abbreviations in this study are as follows: oak forest (WO and SO), oriental arborvitae forest (WA and SA), black locust plantation (WL and SL), shrub land (WS and SS), and bare land (WB and SB) in winter and summer, respectively. In each land-use type, three soil samples were collected from locations approximately 50 m apart, generally on upper, middle, and lower locations of the land slope. At each location, a mixture of the top 20 cm of soil was collected from five sampling points along a “z”-type curve, and a 1 kg sample was obtained by the quartering method. After the roots and debris were removed, the samples were brought to the laboratory and preserved in two conditions: (1) stored at −40 °C in the refrigerator for molecular analysis and (2) sieved through 2 mm mesh and kept field-moist in a cooler at 4 °C18 until analyses for soil biological properties were conducted shortly thereafter.
DNA extraction
For each location, genomic DNA was extracted from a 0.3 g soil sample using the PowerSoil® DNA Isolation Kit (MOBIO Laboratories, Carlsbad, CA, USA), according to the supplier’s recommended protocol.
Depth-dependent sequence-based analysis of DNA
For bacteria, the V4 region in 16 S rRNA gene was examined after the Polymerase Chain Reaction (PCR) with primers of S-D-Arch-0519-a-S-15 (CAGCMGCCGCGGTAA) and S-D-Bact-0785-a-A-21 (GACTACHVGGGTATCTAATCC)19. For fungi, the ITS1 region was examined with the PCR product amplified by the nested PCR approach20 with the first PCR using the primers of ITS9mum (TGTACACACCGCCCGTCG)21 and LR3 (CCGTGTTTCAAGACGGG)22 and the second PCR using the primers of ITS1-F_KYO2 (TAGAGGAAGTAAAAGTCGTAA) and ITS2_KYO2 (TTYRCTRCGTTCTTCATC)23. The forward primers to amplify the final target region for Ion Torrent sequencing (S-D-Arch-0519-a-S-15 for bacteria and ITS1-F_KYO2 for fungi) were linked with the Ion Torrent specific adapters and Ion-Xpress barcode to distinguish the origin samples for each sequence. PCR amplification was performed in a 25.5 μL cocktail including 22.5 μL of Platinum PCR Super Mix High Fidelity (Invitrogen, CA, USA), 2.0 μL of template DNA, and 0.5 μL (10 μM) of forward and reverse primers.
The PCR for bacteria was performed using a Veriti thermal cycler (Applied Biosystems) with initial denaturation at 94 °C for 3 min, 35 cycles of 30 s at 94 °C for denaturation, 30 s at 50 °C for annealing, and 1 min at 68 °C for elongation. The thermal profile of the first PCR for fungi was initial denaturation at 94 °C for 3 min, 20 cycles of 30 s at 94 °C for denaturation, 30 s at 48 °C for annealing, and 1 min at 68 °C for elongation. The second PCR was performed using 2.0 μL of the first PCR product diluted 10 times as the temperate DNA with the following thermal conditions: initial denaturation at 94 °C for 3 min, 35 cycles of 30 s at 94 °C for denaturation, 30 s at 50 °C for annealing, and 1 min at 68 °C for elongation. The PCR was performed twice for each sample and the amplicons were visualized on 0.7% agarose gels stained with SYBR Green I (Molecular Probes, Eugene, OR, USA).
The successful amplicons were pooled, precipitated, resuspended with isopropanol, and then cleaned using the QIAquick Gel Extraction (Qiagen) and the Agencourt AMPure XP (Beckman Coulter, Inc., Brea, CA), according to the manufacturer’s instructions. Quantitate purified each PCR product with the Qubit dsDNA HS Assay Kit on a Qubit fluorometer 2.0 (Invitrogen, Carlsbad, CA, USA). The library mix was then prepared with an equal amount of DNA from each barcode sample. Size distributions and DNA concentrations were measured by Agilent 2100 (Agilent Technologies, Inc.) and diluted to 12 pM before the amplification and purification using Ion OneTouch 2 system with Ion PGM temperate OT2 400 Kit (Life Technology, Inc.). Sequencing was performed using Ion PGM sequencer with Ion PGM sequencing 400 Kit and a 316 V2 tip (Life Technology, Inc.).
Data analysis
Sequence data was generated in a standard flowgram format (sff) of each barcode sample. First, the primer sequence was eliminated using Cutadapt ver.1.324. The subsequent sequence analysis was performed with a quantitative insight into microbial ecology (QIIME) pipeline25. The sequences shorter than 200 bp for bacteria and 160 bp for fungi and the sequences with an error rate greater than 0.2 were removed. Quality sequences were then clustered into operational taxonomic units (OTUs) with USERCH26 at a 97% similarity level. For the representative sequences of OTUs, a chimera check was performed by UCHIME27. Then, each OTU was classified into a taxonomic group with the databases of Greengenes for bacteria28 and UNITE for fungi29.
To assess microbial diversity among samples in a comparable manner, a normalized dataset was used for subsequent analyses, and the lowest sequence number for all sample subsets (23,261 for bacterial and 4,579 for fungi) was randomly selected. The diversity and evenness indices (Shannon and Simpson) were calculated at genetic distances (D) of 0.03. Shannon emphasizes the richness component of diversity, and Simpson emphasizes the evenness component. For both bacteria and fungi, there were only a few sequences that could not be identified to the phylum level. However, rarefaction analysis showed that all rarefaction curves were approaching smoothness with the increase of read number. It is, therefore, reasonable to infer that the obtained sequences covered the majority of the entire microbial community.
A non-parametric (Kruskal-Wallis) test was applied to determine whether there were significant differences in diversity indices among land use types (P < 0.05). To determine the relationships between soil chemical properties and diversity indices, Pearson correlations were calculated. In order to test a significant shift in microbial community composition based on land-use types, Adonis was used. The length of gradient was calculated firstly with the Detrended Correspondence Analysis (DCA) to determine which model would be most suitable for correlation analysis30. According to the results of DCA, a liner model Redundancy Analysis (RDA) was used for bacterial community as direct gradient analysis; while a unimodal model Canonical Correspondence Analysis (CCA) was used for fungal community. Besides the application of RDA and CCA31 which showed general relationships between each microbial community and soil chemical properties, the envfit was applied to the significance test between soil chemical properties and microbial community composition using the vegan package of R v.3.1.3 project (R Development Core Team. 2015).
Results
Land-use and soil characteristics
Soil chemical properties varied with land-use type (Table 2). The alkaline soil in this area has a pH value ranging from 8.18 to 8.48. The bare land had higher pH and lower soil nutrient than did the vegetated types. The black locust had higher pH value, highest available K and lower nutrient concentrations than did the other vegetated types. The natural climax oak forest had the highest soil organic carbon (SOC) and total nitrogen (TN), available P.
Soil bacterial community composition and abundance
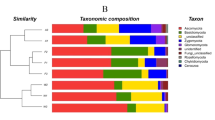
The dominant bacterial phyla contained Proteobacteria (42.35%), Actinobacteria (15.61%), Acidobacteria (13.32%), Bacteroidetes (8.43%), and Gemmatimonadetes (6.0%) (Fig. 1). In addition, Planctomycetes, Verrucomicrobia, Thaumarchaeota, Chloroflexi, and Nitrospirae with low abundances (<5%) existed in most soils, and 29 other rare phyla were also distinguished. Alphaproteobacteria was the most abundant Proteobacteria, followed by Deltaproteobacteria, Betaproteobacteria, and Gammaproteobacteria. Thermoleophilia, Actinobacteria, and Acidimicrobiia were the most abundant groups of Actinobacteria. The relative abundance of Proteobacteria was greater in natural vegetation (oak forest, oriental arborvitae forest, and shrub land) than it was in the bare land and black locust; Acidobacteria showed a reverse trend. The dominant phyla were the same across land-use types, but their relative abundances were different.
Relative abundances are based on the proportional frequencies of bacterial DNA sequences that could be defined at the phylum. Site abbreviations starting with S and W indicate sampling in summer and winter, respectively. The land use types and their abbreviations are: oak forest (SO and WO), oriental arborvitae forest (SA and WA), black locust plantation (SL and WL), shrub land (SS and WS), and bare land (SB and WB) respectively. The figure is generated by using the R v.3.1.3 project (R Development Core Team. 2015).
Relationships between soil chemical properties and bacteria distributions
The results of the RDA of soil chemical properties and bacterial community composition showed that groups according to land-use types (Fig. 2). Bare land and black locust were grouped into clusters distinct from others along the first axis comprising 24.78% of the total variation of site-environment relation. The second axes comprised 6.37% of the total variation and separated black locust from bare land and oak forest. Thus the bare land, black locust as well as others were grouped into distinct clusters, concurring with the Adonis results (P < 0.01). Soil chemical properties account for variation in bacterial community. Each index of soil chemical properties showed significant association with bacterial community composition (P < 0.001). The main factor for grouping the bare land sites was pH with higher abundance. SOC, TN, available P, and C/N tended to have lower values and were negatively associated with the bare land but positively associated with the oak forest sites, while the black locust sites were grouped with higher available K. In total, SOC exerted the largest effect on the distribution of bacterial communities.
The land-use types and their icons are as follows: black locust plantation (⦁ and ⚬), oriental arborvitae forest (◆ and ♢), oak forest (◼ and ◻), shrub land (▴ and ▵), and bare land (★ and ☆), in summer and winter respectively. The figure is generated by using the R v.3.1.3 project (R Development Core Team. 2015).
For bacterial, there were diversity (Shannon) and evenness (Simpson) differences between black locust and bare land in the summer, but others has no difference (P < 0.05) (Table 3). The diversity indices showed significant correlation with soil K (R2 = 0.73, P < 0.001; R2 = 0.59, P < 0.001, respectively).
Soil fungi community composition and abundance
The majority of dominant fungi phyla (Fig. 3) belonged to Ascomycota (40.39%), Basidiomycota (38.01%), and Zygomycota (16.86%). Sordariomycetes, Dothideomycetes, and Lecanoromycetes were the most abundant groups of Ascomycota. Agaricomycetes and Tremellomycetes were the most abundant groups of Basidiomycota. Most of the Zygomycota were incertae. Ascomycota was the most abundant in black locust, and bare land (but summer bare land samples), but was least abundant in the oak forest. The oak forest had the most Basidiomycota, while the black locust had the least. There were no differences in fungi diversity indices between land-use types (Table 3) and no correlations with soil chemical properties.
The land use types and their abbreviations are: oak forest (SO and WO), oriental arborvitae forest (SA and WA), black locust plantation (SL and WL), shrub land (SS and WS), and bare land (SB and WB) respectively. The figure is performed in R v.3.1.3 project (R Development Core Team. 2015), using the R packages vegan.
Relationships between soil properties and fungi community distribution
From the CCA with all the soil chemical properties, the fungal community under the similar land use types typically clustered together (Fig. 4). The bare land type was clustered far away from other land-use types along the first axis comprising 8.38% of the total variation. The oak forest type and bare land type clustered in different groups from the black locust type and oriental arborvitae forest type along the second axes comprising 6.01% of the total variation. The Adonis results also demonstrated those appearances (P < 0.01). Meanwhile, soil chemical properties exerted a significant effect on the composition of fungal community. SOC, total N, available K and P showed significant association with fungal community composition (P < 0.001), and the significance of soil pH and C/N is 0.01. The main grouping factor for the bare land type was pH, and the oak forest type was clustered in the area with high SOC, TN, P, and C/N. Available K showed a positive association and higher abundance in the black locust. In total, available P content was the main factor influencing the fungi community distribution.
The land-use types and their icons are as follows: black locust plantation (⦁ and ⚬), oriental arborvitae forest (◆ and ♢), oak forest (◼ and ◻), shrub land (▴ and ▵), and bare land (★ and ☆), in summer and winter respectively. The figure is performed in R v.3.1.3 project (R Development Core Team. 2015), using the R packages vegan.
Discussion
A previous study showed that similar land-use types have similar soil chemical properties9. We found that vegetated land had advantages in soil chemical properties over bare land and that the natural forest land had advantages over the other land-use types. These findings demonstrated the potential positive effects of vegetation restoration, especially with natural vegetation types, on soil nutrient conditions. The bare land and black locust types had higher pH values than did the other land-use types, illustrating that plantations do not improve soil conditions to the same extent as did natural vegetation types. Therefore, reforestation projects, and the resulting differences in land-use type, might contribute to dissimilar microenvironments, which are closely associated with soil-borne microbes.
Similar to the results of early surveys6,32, the dominant bacterial phyla in Loess Plateau samples were Proteobacteria, Acidobacteria, Actinobacteria, and Bacteroidetes. This is different from the results of Xiong, et al.33 who showed that Bacteroidetes and Firmicutes were dominant in alkaline lake sediments.
Microbial community structure was driven by both land-use type and soil chemical properties. The importance of soil chemical properties in shaping microbial communities has been established by a number of studies. As general rule, soil pH plays a key role in controlling the distribution of microbial communities34,35,36. In our study, although pH can significantly structure bacterial communities, the main factor that influenced bacterial community structure was SOC. Our results agreed with the results of other studies that showed that SOC had the greatest effect on soil bacterial community structure37,38. This may be reflected by the fact that the high pH soils in the bare land and black locust forest types were nutrient-poor and therefore grouped distinctly. Variances in soil quality properties and the copiotrophic–oligotrophic classification of soil bacterial phyla might also explain this inconsistency37. Similar to Zhang, et al.39, we found that the relative abundance of Acidobacteria increased with soil pH. However, other studies showed that the relative abundance of Acidobacteria was typically negatively correlated with soil pH35,36,40. Other studies indicated that the relative abundance of Acidobacteria only started to decrease markedly when the pH was below 5.535,36,41. In this study, soil pH had a narrow alkaline range (8.18 to 8.48).
The dominant bacteria (Proteobacteria, Acidobacteria, and Actinobacteria) were greatly responsive to SOC content. The results obtained in this study are in line with the general framework that uses the terms copiotroph and oligotroph to describe microbial communities with ecological attributes typical of r- and K- strategists, respectively37. Copiotrophs require high nutrition and can utilize labile SOC to keep growth high rates if resource conditions are sufficient. For example, in this study, Proteobacteria increased with SOC and were the most abundant in SOC-rich oak forest soils. On the contrary, there were many more oligotrophic groups of Acidobacteria, which is expected to be the first bacteria to colonize nutrient-poor substrates in areas with low resource availability37, such as bare land and black locust forests. Other studies have shown similar results in that Acidobacteria are less abundant and Proteobacteria are more abundant in soils with high resource availability31,42. Oligotrophs are probably able to outcompete copiotrophs under stress conditions of low resource concentrations37. Our results further confirmed this and extended evidence for these ecological classifications for direct or indirect bacterial responses to soil nutrient availability. Several other studies have also showed that the Proteobacteria/Acidobacteria ratio reflected the soil chemical properties, with lower ratios occurring in oligotrophic soils. Our study further supports this finding because we found the highest ratios in the oak forest site and the lowest ratios in bare land and black locust forest. Therefore, it is likely that land-use and soil nutrient conditions are the major explanatory variables of bacterial community structure. In addition, bacterial community structure can also reflect the soil nutrient status in Loess Plateau, China.
We found that the fungal taxa mainly belonged to Ascomycota, Basidiomycota, and Zygomycota, consistent with previous studies43,44. However, different results were reported for Arctic soils where Zygomycota and Chytridiomycota dominated45,46. Ascomycota and Basidiomycota tended to reside in cooler and arid environments because of their evolutionary histories47.
Fungal communities had lower diversity than did bacterial communities, and there was no correlation with the soil chemical properties. In contrast to the bacterial communities, the fungal communities were mainly driven by soil P content, consistent with results from Lauber et al.10, which demonstrated that extractable P concentrations were often correlated with fungal communities. This further suggests that bacterial and fungal communities respond to different soil chemical properties factors.
Leckie et al.48 and Kasel et al.48,49 determined that land-use played a role in modulating the soil fungal community structure. Lauber et al.10 observed that soil chemical properties more strongly influenced fungal communities than did land-use type. In this study, the CCA showed that the bare land, black locust forest and oak forest were grouped into distinct clusters according to land-use types, and the primary controlling factor was available P. Sites with similar available P content tended to cluster together. This may be related to the corresponding different microclimates for each land-use type, or a function of the fungi. Distribution of fungi was related to soil P. Basidiomycota abundance increased with increasing soil P, but Ascomycota abundance decreased. Oak trees serve as host species and the relatively P-rich oak forest sites contained more Basidiomycota. Basidiomycota is the dominant ectomycorrhizal species and is conducive to the success of reforestation projects. In contrast, Lauber10 found that P-rich soils contained a higher content of Ascomycota than Basidiomycota. The reason for this discrepancy may be related to the range of soil P (1.8–17 mg·kg−1 in Lauber10 and 1.65–3.31 mg·kg−1 in our study). Zygomycota, as saprotrophic species, are responsible for decomposing plant litter, alter soil chemical properties43,50. This was mirrored by most of the differences between the dystrophic bare land site and the other forest sites. There was much more litter in forests than there was in the bare land area. There were more Ascomycota in the bare land and black locust, corresponding with the report that approximately 46% Ascomycota can form lichen which can grow and persist in deserts and mountaintops51. New microbial communities were forced to form so as to adapt to the hazardous environment52. This was confirmed by our study results, which showed that bare land and black locust have a large number of lichen. Without vegetative cover, bare land becomes more reflective and is often subjected to direct sunlight and subsequent drought. Lichens form poikilohydric biomes, meaning they desiccate and remain dormant and highly tolerant of dry conditions, but can rehydrate after rain. We can therefore infer that different land-use types effect the selection of fungal types in terms of those that are the most fit for survival in the given conditions. This can explain the finding that vegetated land and natural forests had advantages in soil quality than did bare land and planted forests, respectively.
While seasonal changes in microbial community structure have been noted53,54, we expected the seasonal differences in the microbial communities to reflect the vitality of the summer samples and the dormancy of the winter samples. However, we found that seasonal variations were actually relatively small. Independent of season, the microbial communities from the same site was clustered together. This is possibly because these communities were uniform in their responses to seasonal change with little change in cellular abundance from physiological adaptation55,56. Seasonal variations in many fungal dynamics are in large part tied to plant species57,58. In addition, under repeated stresses, microbial communities may become more resistant or possess conservative strategies59,60. This study region experienced repeated drought and heavy rainfall stresses. In addition, another possible reason was that our DNA-based analyses included a potentially huge dormant community or even dead microbial community61.
Jangid, et al.55 showed that nutrient input had a larger effect on microbial structure than did season. In this study, the fact that soil microbial community structure correlated with the soil resources suggests that the microbial community may be more responsive to changes in resource inputs. Although seasonal change can also affect C inputs into the soil, samples were taken from non-rhizosphere soil. Under different land-use types, soil properties might be relatively stable and be able to support microbial communities with similar resistance and restorative properties.
In conclusion, similar land-use types usually have similar soil chemical properties and, similar microbial communities in a specific area. Our results support previous studies that soil chemical properties and land use types have crucial influence on the composition and divertity of microbial community. The changes of land use type generated soil chemical properties, both of which played a significant role in controling the composition of microbial community. In turn, the microbial community composition might be an indicator of changes in soil chemical properties or responses to land use types after ecological restoration on the Loess Plateau.
Additional Information
How to cite this article: Tian, Q. et al. Land-use types and soil chemical properties influence soil microbial communities in the semiarid Loess Plateau region in China. Sci. Rep. 7, 45289; doi: 10.1038/srep45289 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Sala, O. E. et al. Global biodiversity scenarios for the year 2100. Science 287, 1770–1774 (2000).
Zhang, C. et al. Efficacy of carbonaceous nanocomposites for sorbing ionizable antibiotic sulfamethazine from aqueous solution. Water Res. 95, 103–112 (2016).
Bell, T., Newman, J. A., Silverman, B. W., Turner, S. L. & Lilley, A. K. The contribution of species richness and composition to bacterial services. Nature 436, 1157–1160 (2005).
Green, J. & Bohannan, B. J. M. Spatial scaling of microbial biodiversity. Trends Ecol. Evol. 21, 501–507 (2006).
Singh, B. K., Dawson, L. A., Macdonald, C. A. & Buckland, S. M. Impact of biotic and abiotic interaction on soil microbial communities and functions: A field study. Appl. Soil Ecol. 41, 239–248 (2009).
Fierer, N. & Jackson, R. B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 103, 626–631 (2006).
Frey, S. D., Knorr, M., Parrent, J. L. & Simpson, R. T. Chronic nitrogen enrichment affects the structure and function of the soil microbial community in temperate hardwood and pine forests. For. Ecol. Manage. 196, 159–171 (2004).
Wal, A. V. D. et al. Fungal biomass development in a chronosequence of land abandonment. Soil Biol. Biochem. 38, 51–60 (2006).
Johnson, M. J., Lee, K. Y. & Scow, K. M. DNA fingerprinting reveals links among agricultural crops, soil properties, and the composition of soil microbial communities. Geoderma 114, 279–303 (2003).
Lauber, C. L., Strickland, M. S., Bradford, M. A. & Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 40, 2407–2415 (2008).
Chapin, F. S. et al. Consequences of changing biodiversity. Nature 405, 234–242 (2000).
Kuramae, E. E. et al. Soil characteristics more strongly influence soil bacterial communities than land-use type. FEMS Microbiol. Ecol. 79, 12–24 (2012).
Shi, W. Y., Yan, M. J., Zhang, J. G., Guan, J. H. & Du, S. Soil CO2 emissions from five different types of land use on the semiarid Loess Plateau of China, with emphasis on the contribution of winter soil respiration. Atmos. Environ. 88, 74–82 (2014).
Wang, B., Liu, G. B. & Xue, S. Effect of black locust (Robinia pseudoacacia) on soil chemical and microbiological properties in the eroded hilly area of China’s Loess Plateau. Environ. Earth Sci. 65, 597–607 (2012).
Zhang, C., Liu, G., Xue, S. & Wang, G. Changes in rhizospheric microbial community structure and function during the natural recovery of abandoned cropland on the Loess Plateau, China. Ecol. Eng. 75, 161–171 (2015).
Zhang, J. et al. Ectomycorrhizal fungal communities of Quercus liaotungensis along local slopes in the temperate oak forests on the Loess Plateau, China. Ecol. Res. 28, 297–305 (2013).
Du, S. et al. Sapflow characteristics and climatic responses in three forest species in the semiarid Loess Plateau region of China. Agr. Forest Meteorol. 151, 1–10 (2011).
Liu, Y. et al. Effect of rhamnolipid solubilization on hexadecane bioavailability: enhancement or reduction? J. Hazard. Mater. 322, 394–401 (2017).
Klindworth, A. et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 41, 1 (2013).
Porterjordan, K. et al. Nested polymerase chain reaction assay for the detection of cytomegalovirus overcomes false positives caused by contamination with fragmented DNA. J. Med. Virol. 30, 85–91 (1990).
Egger, K. N. Molecular analysis of ectomycorrhizal fungal communities. Can. J. Bot. 73, 1415–1422 (1995).
Vilgalys, R. & Gonzalez, D. Organization of ribosomal DNA in the basidiomycete Thanatephorus praticola . Curr. Genet. 18, 277–280 (1990).
Toju, H., Tanabe, A. S., Yamamoto, S. & Sato, H. High-coverage ITS primers for the DNA-based identification of ascomycetes and basidiomycetes in environmental samples. PloS ONE 7, e40863 (2012).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet Journal 17, 10–12 (2011).
Caporaso, J. G., Kuczynski, J. & Stombaugh, J. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336 (2010).
Edgar, R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461 (2010).
Edgar, R. C., Haas, B. J., Clemente, J. C., Quince, C. & Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27, 2194–2200 (2011).
DeSantis, T. Z. et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 72, 5069–5072 (2006).
Abarenkov, K. et al. The UNITE database for molecular identification of fungi - recent updates and future perspectives. New Phytol. 186, 281–285 (2010).
Lee, O. O. et al. In situ environment rather than substrate type dictates microbial community structure of biofilms in a cold seep system. Sc. Rep. 4, 3587 (2014).
McCaig, A. E., Glover, L. A. & Prosser, J. I. Molecular analysis of bacterial community structure and diversity in unimproved and improved upland grass pastures. Appl. Environ. Microbiol. 65, 1721–1730 (1999).
Urbanová, M., Šnajdr, J. & Baldrian, P. Composition of fungal and bacterial communities in forest litter and soil is largely determined by dominant trees. Soil Biol. Biochem. 84, 53–64 (2015).
Xiong, J. B. et al. Geographic distance and pH drive bacterial distribution in alkaline lake sediments across Tibetan Plateau. Environ. Microbiol. 14, 2457–2466 (2012).
Liu, J. J. et al. High throughput sequencing analysis of biogeographical distribution of bacterial communities in the black soils of northeast China. Soil Biol. Biochem. 70, 113–122 (2014).
Lauber, C. L., Hamady, M., Knight, R. & Fierer, N. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl. Environ. Microbiol. 75, 5111–5120 (2009).
Rousk, J. et al. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 4, 1340–1351 (2010).
Fierer, N., Bradford, M. A. & Jackson, R. B. Toward an ecological classification of soil bacteria. Ecology 88, 1354–1364 (2007).
Li, S. K., Xiao, X., Yin, X. B. & Wang, F. P. Bacterial community along a historic lake sediment core of Ardley Island, west Antarctica. Extremophiles 10, 461–467 (2006).
Zhang, X. et al. The soil carbon/nitrogen ratio and moisture affect microbial community structures in alkaline permafrost-affected soils with different vegetation types on the Tibetan plateau. Res. Microbiol. 165, 128–139 (2014).
Chu, H. Y. et al. Soil bacterial diversity in the Arctic is not fundamentally different from that found in other biomes. Environ. Microbiol. 12, 2998–3006 (2010).
Griffiths, R. I. et al. The bacterial biogeography of British soils. Environ. Microbiol. 13, 1642–1654 (2011).
Axelrood, P. E., Chow, M. L., Radomski, C. C., Mcdermott, J. M. & Davies, J. Molecular characterization of bacterial diversity from British Columbia forest soils subjected to disturbance. Can. J. Microbiol. 48, 655–674 (2002).
Bjorb.kmo, M. F. M. et al. High diversity of root associated fungi in both alpine and arctic Dryas octopetala . BMC Plant Biol. 10, 1–12 (2010).
Leff, J. W. et al. Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc. Natl. Acad. Sci. USA 112, 10967–10972 (2015).
Chu, H., Neufeld, J. D., Walker, V. K. & Grogan, P. The influence of vegetation type on the dominant soil bacteria, archaea, and fungi in a low arctic tundra landscape. Soil Sci. Soc. Am. J. 75, 1756–1765 (2011).
Lentendu, G. et al. Assessment of soil fungal diversity in different alpine tundra habitats by means of pyrosequencing. Fungal Divers. 49, 113–123 (2011).
Treseder, K. K. et al. Evolutionary histories of soil fungi are reflected in their large-scale biogeography. Ecol. Lett. 17, 1086–1093 (2014).
Leckie, S. E., Prescott, C. E., Grayston, S. J., Neufeld, J. D. & Mohn, W. W. Characterization of humus microbial communities in adjacent forest types that differ in nitrogen availability. Microb. Ecol. 48, 29–40 (2004).
Kasel, S., Bennett, L. T. & Tibbits, J. Land use influences soil fungal community composition across central Victoria, south-eastern Australia. Soil Biol. Biochem. 40, 1724–1732 (2008).
Kuramae, E. E. et al. Structural and functional variation in soil fungal communities associated with litter bags containing maize leaf. FEMS Microbiol. Ecol. 84, 519–531 (2013).
Honegger, R. Functional Aspects of the Lichen Symbiosis. Annu. Rev. Plant Biol. 42, 553–578 (2003).
Liu, S. H. et al. Bioremediation mechanisms of combined pollution of PAHs and heavy metals by bacteria and fungi: A mini review. Bioresour. Technol. 224, 25–33 (2017).
Griffiths, R. I., Whiteley, A. S., O’Donnell, A. G. & Bailey, M. J. Physiological and community responses of established grassland bacterial populations to water stress. Appl. Environ. Microbiol. 69, 6961–6968 (2003).
Kennedy, N. M., Gleeson, D. E., Connolly, J. & Clipson, N. J. W. Seasonal and management influences on bacterial community structure in an upland grassland soil. FEMS Microbiol. Ecol. 53, 329–337 (2005).
Jangid, K. et al. Relative impacts of land-use, management intensity and fertilization upon soil microbial community structure in agricultural systems. Soil Biol. Biochem. 40, 2843–2853 (2008).
Kieft, T. L., Soroker, E. & Firestone, M. K. Microbial biomass response to a rapid increase in water potential when dry soil is wetted. Soil Biol. Biochem. 19, 119–126 (1987).
Courty, P. E., Franc, A., Pierrat, J. C. & Garbaye, J. Temporal changes in the ectomycorrhizal community in two soil horizons of a temperate oak forest. Appl. Environ. Microbiol. 74, 5792–5801 (2008).
Dumbrell, A. J. et al. Distinct seasonal assemblages of arbuscular mycorrhizal fungi revealed by massively parallel pyrosequencing. New Phytol. 190, 794–804 (2011).
Placella, S. A ., Brodie, E. L & Firestone, M. K. Rainfall-induced carbon dioxide pulses result from sequential resuscitation of phylogenetically clustered microbial groups. Proc. Natl. Acad. Sci. USA 109, 10931–10936 (2012).
Barnard, R. L., Osborne, C. A. & Firestone, M. K. Responses of soil bacterial and fungal communities to extreme desiccation and rewetting. ISME Journal 7, 2229–2241 (2013).
Cangelosi, G. A. & Meschke, J. S. Dead or alive: molecular assessment of microbial viability. Appl. Environ. Microbiol. 80, 5884–5891 (2014).
Acknowledgements
This research was jointly supported by the National Natural Science Foundation of China (nos 41411140035, 41471440), the Strategic Priority Research Project of the Chinese Academy of Sciences (XDA05050202), and the Japan Society for the Promotion of Science for the NSFC-JSPS bilateral joint research project. We are grateful to Dr. Hongjiang Han who has kindly commented on the manuscript preparation.
Author information
Authors and Affiliations
Contributions
Q.T. and S.D. conceived the research and carried out the field investigations. Q.T. and T.T. performed laboratory experiments and analyzed the data. All members commented on the data analyses and manuscript preparation. Q.T. wrote the manuscript and all other authors reviewed it.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Tian, Q., Taniguchi, T., Shi, WY. et al. Land-use types and soil chemical properties influence soil microbial communities in the semiarid Loess Plateau region in China. Sci Rep 7, 45289 (2017). https://doi.org/10.1038/srep45289
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep45289
This article is cited by
-
Chemical attributes, bacterial community, and antibiotic resistance genes are affected by intensive use of soil in agro-ecosystems of the Atlantic Forest, Southeastern Brazil
Environmental Geochemistry and Health (2024)
-
Effects of land-use patterns on soil microbial diversity and composition in the Loess Plateau, China
Journal of Arid Land (2024)
-
Culture-dependent and culture-independent characterization of bacterial community diversity in different types of sandy lands: the case of Minqin County, China
BMC Microbiology (2021)
-
Variation of rhizosphere microbial community in continuous mono-maize seed production
Scientific Reports (2021)
-
Diversity of arbuscular mycorrhizal fungi and its chemical drivers across dryland habitats
Mycorrhiza (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.