Abstract
The precise role of the human primary motor cortex in walking is unknown. Our previous study showed that the primary motor cortex may contribute to specific requirements of walking (i.e., maintaining a constant movement frequency and bilaterally coordinating the feet). Because aging can impair (i) the ability to fulfill the aforementioned requirements and (ii) corticomuscular communication, we hypothesized that aging would impair the motoneuronal recruitment by the primary motor cortex during bilateral cyclical movements. Here, we used corticomuscular coherence (i.e., coherence between the primary motor cortex and the active muscles) to examine whether corticomuscular communication is affected in older individuals during cyclical movements that shared some functional requirements with walking. Fifteen young men and 9 older men performed cyclical, anti-phasic dorsiflexion and plantarflexion of the feet while seated. Coherence between the midline primary motor cortex and contracting leg muscles cyclically increased in both age groups. However, the coherence of older participants was characterized by (i) lower magnitude and (ii) mediolaterally broader and more rostrally centered cortical distributions. These characteristics suggest that aging changes how the primary motor cortex participates in the cyclical movements, and such change may extend to walking.
Similar content being viewed by others
Introduction
In humans, the primary motor cortex participates in the control of the basic patterns of walking1,2,3,4. However, the precise nature of its participation is not yet known. In our previous study (unpublished), we had observed that coherence between the midline primary motor cortex and the active leg muscles (i.e., corticomuscular coherence) increased dynamically during bilateral cyclical ankle movements. This finding suggested that the primary motor cortex contributed to maintaining a constant cyclical movement frequency and bilaterally coordinating the feet: functional requirements that are also present in walking.
Aging is associated with deficits in meeting the above requirements (i.e., increased movement variability and impaired bilateral coordination) during walking and other bilateral cyclical movements5,6,7,8. Aging is also associated with neuromuscular changes that can impair corticomuscular communication. These changes include decrease in the gray matter volume of the primary motor cortex9,10,11,12,13; decrease in the white matter volume of the posterior limbs of the internal capsule13,14, which contain the corticospinal tracts15,16; decrease in the number of motor neurons17,18,19; structural abnormalities of the neuromuscular junctions20; and re-organization of motor units that results in more fibers per neuron21,22,23. Indeed, previous studies have reported age-related reduction in (i) the amplitude of motor evoked potentials (i.e., corticospinal excitability)24 and (ii) corticomuscular coherence during sustained contractions of upper limb muscles25,26.
The purpose of this study was to examine how aging affected corticomuscular communication during movements that shared specific functional requirements with walking (i.e., bilateral cyclical ankle movements). In this study, corticomuscular communication was quantified by corticomuscular coherence. Based on the age-related impairment of motor performance and alteration of corticomuscular communication, we hypothesized that aging would be associated with lower magnitudes of corticomuscular coherence. To our knowledge, no study has examined how aging affects corticomuscular coherence during cyclical, anti-phasic movements. Several studies have examined the effects of aging on corticomuscular coherence25,26,27,28, but these studies are limited to sustained contractions.
Results
Motor performance
On average, young participants completed 170 ± 8 and 171 ± 6 cycles of self-paced and externally-paced movements, respectively. Older participants completed 165 ± 10 and 170 ± 2 cycles of self-paced and externally-paced movements, respectively. Among the participants, the minimum number of movement cycles was 139. Thus, for each participant, the first 139 of the recorded cycles were used to calculate the ensemble average of corticomuscular coherence. The inter-run rests ranged from 61 to 154 seconds for young participants and 60 to 156 seconds for older participants.
Table 1 summarizes the kinematics of the ankle movements. The mean cycle duration was significantly affected by aging (F1,87 = 4.66, p = 0.336 × 10−1) and the type of pacing (F1,87 = 6.44, p = 0.129 × 10−1), but not by the side of the body (F1,87 = 1.54 × 10−5, p = 0.997). Post hoc analysis showed that the mean cycle duration was significantly longer for older participants with self-pacing, compared to young participants with external pacing. The standard deviation of cycle durations was significantly affected by the type of pacing (F1,87 = 7.73, p = 0.666 × 10−2), but not by aging (F1,87 = 1.67, p = 0.200) or the side of the body (F1,87 = 2.86, p = 0.945 × 10−1). Post hoc analysis showed that, for both groups, the standard deviation was significantly greater with self-pacing. On the mean range of motion, the effects of aging (F1,87 = 3.90, p = 0.516 × 10−1), type of pacing (F1,87 = 0.0255, p = 0.873), and side of the body (F1,87 = 0.107, p = 0.744) were insignificant. On the standard deviation of the range of motion, the effects of aging (F1,87 = 0.312, p = 0.578), type of pacing (F1,87 = 1.63, p = 0.206), and side of the body (F1,87 = 0.253, p = 0.616) were also insignificant. The mean relative phase (x of ϕ in Table 1), which indicated the bilateral coordination of limbs, was significantly affected by aging (F1,43 = 4.24, p = 0.0456) but not by the type of pacing (F1,43 = 0.282, p = 0.598). Post hoc analysis showed that, although the movements were asymmetrical for both groups (with left dorsiflexion occurring slightly earlier than it should), young participants showed greater asymmetry than older participants. The standard deviation of the relative phase (s of ϕ in Table 1) was not significantly affected by aging (F1,43 = 1.19, p = 0.281) or the type of pacing (F1,43 = 0.537, p = 0.468). None of the parameters of motor performance was associated with a significant interaction between the factors (Supplementary Table S1).
To examine the effects of motion artifacts due to head movements, we quantified the cyclical linear movements of the markers at the electroencephalographic (EEG) electrode locations, AF7 and AF8. For both groups of participants, regardless of the type of pacing, the markers were within a volume of approximately 1 cm3 during each movement cycle. For young participants, the average cyclical linear head movements were no more than 7 mm, 6 mm, and 4 mm, in the rostrocaudal, mediolateral, and longitudinal directions, respectively. This was true for both self-paced and externally-paced movements. The equivalent measures for the older participants were no more than 8 mm, 7 mm, and 4 mm. Because the head movements were small, we assumed that the effects of motion artifacts due to head movements on EEG signals were negligible.
Cyclical patterns of corticomuscular coherence
Figure 1 shows brief time courses of all collected signals from representative young and older participants during externally-paced movements. During dorsiflexion, both participants showed increased activation of the tibialis anterior muscle, with no obvious discrepancy in the electroencephalographic (EMG) patterns. However, the young participant also showed co-contractions of the medial gastrocnemius muscles during dorsiflexion while such co-contraction was indiscernible in the older participant (Fig. 1). The above observations were also true for self-paced movements (Supplementary Fig. S1).
Ankle angles have been centered and normalized to its range. EMG signals have also been normalized to its range. For the older participant, the maximum values of the shown EMG signals were 0.482 and 0.277 mV for the right and left TA muscles, respectively, and 0.0112 and 0.0138 mV for the right and left MG muscles, respectively. For the young participant, the maximum values of the shown EMG signals were 0.738 and 1.26 mV for the right and left TA muscles, respectively, and 0.0512 and 0.0569 mV for the right and left MG muscles, respectively.
Figure 2 shows the cyclical corticomuscular coherence of the representative young and older participants during externally-paced movements. For both participants, the coherence between Cz and the tibialis anterior muscles increased cyclically, approximately coinciding with dorsiflexion (cf. Fig. 1). Furthermore, the cyclical increase occurred below 50 Hz, particularly between β to low-γ range, and exceeded the threshold of significance. The two participants differed in their coherence between Cz and the medial gastrocnemius muscles: the young participant showed a cyclical increase in coherence during dorsiflexion while the older participant showed no such pattern (Fig. 2). The above observations were also true for the self-paced movements (Supplementary Fig. S2).
Validating corticomuscular coherence
Figure 3 shows the significant portions of experimental and surrogate corticomuscular coherence for a representative older participant during externally-paced movements. Unlike experimental coherence, surrogate coherence did not show a cyclical increase at higher frequencies. Thus, shuffling the pairing between EEG and EMG signals abolished the cyclical increase in their coherence. However, shuffled pairing did not abolish their coherence at lower frequencies, typically below 6 Hz (Fig. 3). According to 2-way analysis of variance (ANOVA), the volume of significant coherence was significantly affected by the shuffled pairing above (F1,372 = 70.2, p = 0.112 × 10−14) and below 6 Hz (F1,372 = 40.2, p = 0.674 × 10−9). Post hoc analysis revealed that, after shuffling, the volume of significant coherence was (i) significantly smaller (and almost negligible) above 6 Hz and (ii) significantly larger below 6 Hz. Above 6 Hz, the volume of significant coherence was also significantly affected by aging (F1,372 = 4.86, p = 0.281 × 10−1), and post-hoc analysis revealed that the volume was significantly larger for young participants. Furthermore, above 6 Hz, aging and the type of coherence (i.e., surrogate or experimental) interacted significantly (F1,372 = 4.58, p = 0.329 × 10−1), probably indicating that young participants experienced greater reductions in the volume of significant coherence due to the shuffled pairing. Below 6 Hz, the volume of significant coherence was not significantly affected by aging (F1,372 = 0.0922, p = 0.762), and aging and the type of pacing did not interact significantly (F1,372 = 1.26, p = 0.262).
Magnitude and frequency of corticomuscular coherence
For all participants, the threshold of significance was 0.0847. The magnitude of significant coherence was significantly affected by aging (F1,177 = 4.72, p = 0.311 × 10−1), and post hoc analysis revealed that the magnitude was smaller for older participants (Fig. 4). The magnitude was not significantly affected by the type of pacing (F1,177 = 0.113, p = 0.737), muscle (F1,177 = 0.0815, p = 0.776), or side of the body (F1,177 = 0.286, p = 0.593).
For young participants, the center frequency of significant coherence during externally-paced movements were 20.3 ± 4.2 and 20.5 ± 5.5 Hz for the left and right tibialis anterior muscles, respectively, and 20.7 ± 7.3 and 20.1 ± 4.0 Hz for the left and right medial gastrocnemius muscles, respectively. The equivalent values during self-paced movements were 18.1 ± 3.7 and 17.0 ± 5.1 Hz for the left and right tibialis anterior muscles, respectively, and 18.3 ± 4.1 and 18.7 ± 5.2 Hz for the left and right medial gastrocnemius muscles, respectively. For older participants, the center frequency of significant coherence during externally-paced movements were 19.9 ± 3.2 and 20.3 ± 4.1 Hz for the left and right tibialis anterior muscles, respectively, and 18.2 ± 5.5 and 20.3 ± 4.1 Hz for the left and right medial gastrocnemius muscles, respectively. The equivalent values during self-paced movements were 17.3 ± 3.5 and 19.0 ± 3.9 Hz for the left and right tibialis anterior muscles, respectively, and 19.6 ± 6.8 and 18.8 ± 4.0 Hz for the left and right medial gastrocnemius muscles, respectively. The frequency was not significantly affected by aging (F1,177 = 0.800, p = 0.372), type of pacing (F1,177 = 2.97, p = 0.866 × 10−1), muscle (F1,177 = 0.175, p = 0.676), or side of the body (F1,177 = 0.00747, p = 0.931). For neither the magnitude nor the frequency, did the factors of 4-way ANOVA interact significantly (Supplementary Table S2).
Cortical distribution of corticomuscular coherence
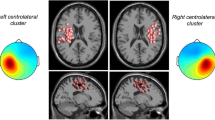
Figure 5 shows the cortical distributions of significant coherence for representative young and older participants during externally-paced movements. The representative young participant generally showed cortical distributions that centered around Cz for both muscles. The representative older participant also showed such distributions for the tibialis anterior muscles, but not for the medial gastrocnemius muscles (Fig. 5). These observations were also true for self-paced movements (Supplementary Fig. S3).
Tables 2 and 3 summarize the results of fitting a bivariate normal distribution to the cortical distributions of significant coherence for older and young participants, respectively. Supplementary Table S3 and Supplementary Table S4 summarize the results of applying 4-way ANOVA on the parameters in Tables 2 and 3. For optimally fitted normal distributions, the root-mean square deviation (RMSD), coefficient of determination (COD), and peak value (A) were not significantly affected by any of the factors: aging, type of pacing, muscle, or side of the body (Supplementary Table S3).
The standard deviation in the rostrocaudal direction (σRC) was also not significantly affected by any of the factors, but the standard deviation in the mediolateral direction (σML) was significantly affected by aging (F1,113 = 4.03, p = 0.0471). Post hoc analysis showed that σML was slightly but significantly smaller for younger participants. The mean of the optimally fitted normal distributions in the rostrocaudal direction (μRC) was significantly affected by aging (F1,113 = 4.63, p = 0.0335) and muscle (F1,113 = 4.75, p = 0.0314). Post hoc analysis showed that the fitted normal distributions were located more rostrally for older participant and the medial gastrocnemius muscles. The mean of the optimally fitted normal distributions in the mediolateral direction (μMC) was not significantly affected by any of the factors. For σRC, the type of pacing and muscle interacted significantly (F1,113 = 6.27, p = 0.0137). This interaction probably indicated that, with external pacing, σRC tended to increase for the tibialis anterior muscles and decrease for the medial gastrocnemius muscles. For other parameters in Tables 2 and 3, the factors of the 4-way ANOVA did not interact significantly (Supplementary Table S4).
Discussion
Between young and older participants, we observed discrepancies in several aspects of corticomuscular coherence during bilateral cyclical ankle movements. As we hypothesized, the magnitude of cyclical corticomuscular coherence was lower for older participants than for younger participants (Fig. 4). The lower magnitude of coherence suggests that the primary motor cortex participates differently in older individuals during simple cyclical movements: the participation is either (i) less overall or (ii) less linear, as coherence only quantifies the liner aspect of corticomuscular communication.
Our result agreed with previous studies that observed decreased magnitude of corticomuscular coherence in older individuals during sustained contractions of upper limb muscles25,26. However, during sustained contractions of upper limb muscles, some studies reported age-related increase in the magnitude of coherence27,28. Kamp et al.28 had attributed the age-related increase to greater cortical involvement that occurred as compensation against the effects of aging. Although such phenomenon complies with the compensation hypothesis29, it may be task-specific to sustained isometric contraction with continuous visual monitoring of the level of muscle activation27,28. With greater conscious control of muscle activation than what the ankle movements required in this study, sustained contractions are more likely to depend on the primary motor cortex to directly activate the muscles. In this case, compensation by increasing the cortical involvement is plausible. Furthermore, the ankle movements in this study may have also depended on subcortical and spinal neuronal networks to generate the cyclical movements. If so, age-related increase in activity could have occurred outside the corticomuscular communication.
Unlike the magnitude of corticomuscular coherence, the parameters of motor performance did not indicate known age-related deteriorations (i.e., increased movement variability and impaired bilateral coordination). Thus, despite slower movements with self-pacing, the ability to perform the ankle movements was generally preserved among older participants (Table 1).
Despite the lack of age-related discrepancy in motor performance, aging affected the magnitude of corticomuscular coherence. This finding suggests several possibilities: (i) the cyclical increase in corticomuscular coherence was irrelevant to the functional requirements of the ankle movements; (ii) the observed age-related discrepancy indicated pre-symptomatic changes in motor control, in a similar fashion to the pre-symptomatic pathology of neurodegenerative diseases such as Parkinson’s disease30; and (iii) there was a floor effect (i.e., magnitude of coherence would have shown greater age-related discrepancy had the task been more demanding). To confirm the functional relevance of corticomuscular coherence, future research should consider tasks that are either (i) challenging enough to induce a difference in performance between young and older individuals or (ii) varied in difficulty to examine how the coherence relates to difficulty (e.g., inclusion of in-phasic movements or multiple movement frequencies). Alternatively, future studies could target older individuals, whose performance of a particular movement is known to be impaired.
The cortical distribution of corticomuscular coherence was slightly but significantly broader in the mediolateral direction for older participants (indicated by σML in Tables 2 and 3). Functional neuroimaging studies have shown that older individuals recruit additional cortical, subcortical, or cerebellar areas to perform various isolated movements of the fingers, wrists, and ankles31,32,33,34,35,36,37,38. If older participants engaged a broader area of the primary motor cortex, its activity could have propagated to EEG electrodes that abut Cz in the mediolateral directions, thereby broadening the cortical distribution of coherence. Aging was also associated with a more rostrally centered cortical distribution of corticomuscular coherence (illustrated in Fig. 5 and Supplementary Fig. S3). The sub-division of the primary motor cortex into rostral and caudal regions has been suggested by several studies39,40,41. For example, using retrograde transneuronal transmission of the rabies virus in rhesus monkeys, Rathelot and Strick39 have shown that approximately 70 to 90% of corticospinal neurons, which project monosynaptically to the motoneurons of proximal and distal forelimb muscles, were located in the caudal region of the primary motor cortex. The rostral region contained approximately 5 to 10% of such corticospinal neurons, with the remainder located in rostral region of the post-central gyrus39. It is possible that the caudal region of the primary motor cortex is more vulnerable to age-related deterioration, thereby causing older individuals to engage the rostral region. Similarly, Plow et al.42 have reported that the cortical distribution of motor evoked potentials is more rostrally centered for older individuals (i.e., the center of corticospinal excitability is shifted rostrally).
There were several limitations in this study. First, our older participants included two individuals aged 51 and 61 years, who are younger than individuals normally considered old (e.g., 65 years old). Also, without imaging, we could not determine whether the older participants had experienced neural changes that would affect corticomuscular communication. However, out of such neural changes, (i) the gray matter volume declines more or less steadily from the age of 20 years9,10,11,12,13, (ii) the white matter volume either starts to decline around the age of 40 years13 or declines steadily from the age of 20 years14, (iii) the number of motor neurons decreases steadily from the age of 20 years old17,18 though the decrease may accelerate around the age of 60 years19, and (iv) the apparent re-organization of the motor units occurs more or less steadily from the age of 20 years21,22. Furthermore, the reduction in corticospinal excitability24 and corticomuscular coherence26 has been observed in individuals older than 55 years of age. Therefore, despite the inclusion of two younger individuals, we assumed that our older participants had undergone at least some change in their corticomuscular communication. The second limitation is the uncertainty regarding the levels of muscle and central fatigue due to the ankle movements. Particularly, young participants may have experienced some muscle fatigue during the second run of externally-paced movements (i.e., the mean power frequency of the EMG signal from the right tibialis anterior muscle significantly decreased by 8.35 ± 7.81 Hz during the last three cycles, compared to the first three cycles), and the fatigue could have enhanced corticomuscular coherence43 though we suspect the effects to be modest44. Third, we did not consider a sedentary lifestyle as a significant confounder. Although the task that we chose for this study was not demanding in terms of power output, cardiopulmonary stress, precision, or complexity (i.e., the movements were unfamiliar to the participants yet simple enough to learn in a short amount of time and sustain for prolonged periods), a sedentary lifestyle could have affected corticomuscular communication independently from age-related changes. Fourth, the power of our statistical analysis may be limited due to the multi-factorial design and the small sample size. Lastly, coherence between surface EEG and EMG signals is only a gross measure of corticomuscular communication as a scalp EEG signal is the sum of all electrical field potentials in the vicinity of the measuring electrode, and a surface EMG signal is the sum of all motor unit action potentials in the vicinity of the measuring electrode.
In this study, young and older participants performed cyclical, anti-phasic ankle movements. During this movement, we observed discrepancies in the magnitude and cortical distributions of corticomuscular coherence between young and older participants. The coherence of older participants was characterized by (i) lower magnitude and (ii) mediolaterally broader and more rostrally centered cortical distributions. The lower magnitude suggests that the primary motor cortex either participates less in the control of the movement or in a less linear fashion (e.g., polysynaptically via spinal circuits). The broader and rostrally shifted cortical contributions may indicate compensation against age-related neuromuscular changes. Thus, we have shown that corticomuscular communication is affected in older individuals during bilateral cyclical movements, which share specific functional requirements with walking. Aging may similarly affect corticomuscular communication during walking.
Methods
Participants
By convenience sampling, we recruited 16 young men and 11 older men. One young participant and two older participants were excluded from data analysis because artifacts could not be removed sufficiently from their EEG signals. The remaining 15 young participants were 27 ± 7 years old, 177 ± 7 cm tall, and 75 ± 11 kg in weight. The remaining 9 older participants were 66 ± 7 years old, 176 ± 6 cm tall, and 86 ± 8 kg in weight. All participants were able to walk unassisted and reported no neurological disorders or dementia. Before participating in this study, all participants provided their written informed consent. All experimental protocols, which were performed according to the relevant guidelines, had been approved by the University Health Network Research Ethics Board, Toronto, Canada.
Experimental task
Each participant performed 6 one-minute runs of cyclical ankle movements while sitting. The first run was always externally paced by the sound of a metronome, and subsequent runs alternated between self- and externally-paced movements. Between runs, the participants rested briefly. The self- and externally-paced conditions have been included because age-related discrepancies have been observed with both types of pacing5,6,31,34,37,38. During externally-paced runs, the participants were instructed to dorsiflex and plantarflex their feet in an anti-phasic manner: at each beat of the metronome, which had been set to 108 beats per minute, one foot was maximally dorsiflexed and the other foot was maximally plantarflexed. During self-paced runs, the participants were instructed to maintain the same rhythm as the externally-paced runs. Before the first run, the participants practiced the movement until they felt comfortable with the rhythm and anti-phasic coordination of the limbs. During each run, the participants were instructed to gaze forward and look at a bullseye. They were also instructed to relax their upper body and to refrain from moving their head, talking, swallowing, coughing, clenching their jaw, and excessively blinking. Given the simplicity of movement, we assumed that it was easy to retain the necessary motor skills during inter-run rests.
Data collection
All signals were recorded in one-minute epochs. Each epoch (i) began several cycles after the participant had started the movement and (ii) ended after approximately one minute, before the participant was told to stop the movement. The sampling of kinematic data, EEG signals, and EMG signals were synchronized by an analogic switch.
To record kinematic data, we used an optical motion capture system. The system comprised a data acquisition device (MX Giganet, Vicon Motion Systems Ltd., United Kingdom), nine optical cameras (Bonita, Vicon Motion Systems Ltd., United Kingdom), data acquisition software (Nexus 1.8.5, Vicon Motion Systems Ltd., United Kingdom), and 14 mm retroreflective markers. The participants wore socks and a tight-fitting outfit. To track head movements, markers were placed over the EEG electrode locations, AF7 and AF8. To track lower body movements, markers were placed bilaterally over the greater trochanters, lateral epicondyles of the femur, lateral malleoli, and second metatarsal heads. The marker positions were sampled at 100 Hz.
To record EEG signals, we used an active electrode system (g.GAMMAsys, g.tec medical engineering GmbH, Austria) with signal amplifiers (g.USBamp, g.tec medical engineering GmbH, Austria) and recording software (g.Recorder, g.tec medical engineering GmbH, Austria). We used a monopolar montage to record EEG signals from 20 locations, which covered the midline primary motor cortex (Cz) and its vicinity: AFz, Fz, F1, F2, F3, F4, FCz, FC1, FC2, FC3, FC4, Cz, C1, C2, C3, C4, CPz, CP1, CP2, and Pz45. The reference electrode was placed on the left ear lobe and the ground electrode over the right zygomatic process of the temporal bone. The signals were sampled at 1.2 kHz without filtering.
To record EMG signals from the tibialis anterior muscle and the medial head of the gastrocnemius muscle on both sides, we measured the muscle activities using a wireless EMG system (Trigno™ Wireless EMG System, Delsys Inc., United States), which had a bandwidth of 20 to 450 Hz and the common mode rejection ratio of over 80 dB. All EMG signals were sampled at 2 kHz.
Data analysis
All calculations were performed in a commercial numerical computing environment (MATLAB R2014b, The MathWorks, Inc., United States).
For each participant, we calculated three measures of performance: the range of motion at the ankle, movement cycle durations, and the relative phase between the left and right ankles. For each measure, the intra-individual mean and standard deviation were calculated across all movement cycles. Each cycle was defined by two consecutive local maxima in the vertical elevation of the motion-capture marker over the second metatarsal head of the right foot. The relative phase was calculated according to the method described by Abe et al.46. On the intra-individual mean and standard deviation of cycle durations and range of motion, we performed 3-way ANOVA with (i) aging (i.e., young or older), (ii) type of pacing (i.e., self- or externally-paced), and (iii) sides of the body (i.e., left or right) as factors. On the intra-individual mean and standard deviation of relative phase (with right dorsiflexion leading the cycle), we performed 2-way ANOVA with (i) aging and (ii) type of pacing as factors.
Using zero-phase digital filtering, the EEG signals were notch-filtered at 60 Hz and band-pass filtered between 0.5 and 100 Hz. Then, the filtered EEG signals were decomposed by independent component analysis47,48. According to the principles described by Libenson49, the filtered EEG signals and their independent components were visually inspected for artifacts. The contributions of independent components that contained an artifact were subtracted from the filtered EEG signals to produce noise-reduced EEG signals. This subtraction was restricted to the observed duration of the artifactual waveform. The EMG signals were centered and then full-wave rectified. In corticomuscular coherence, the assumption is that rectification would enhance the power spectral density of the EMG signal at the frequency of common input that recruits the constituent motor units. This assumption is supported by experimental evidence50 and computational modeling51.
For each participant, corticomuscular coherence was calculated between all EEG electrode locations and the two muscles on both sides. The noise-reduced EEG signals and rectified EMG signals were synchronized by down-sampling them at 400 Hz, and their wavelet coherence was calculated using the complex Morlet wavelet. The resultant corticomuscular coherence (i.e., approximately one-minute long) was segmented into individual movement cycles and ensemble-averaged to yield a pattern of corticomuscular coherence over one movement cycle).
For each participant, the magnitude of cyclical coherence was examined at all EEG electrode locations. Cyclical corticomuscular coherence was binned across frequency and time: binning across frequency resulted in one pixel per Hz between 1 and 100 Hz; binning across time resulted in one pixel per percent of the movement cycle. Then, above 6 Hz, we calculated the integral of coherence with all pixels that exceeded the threshold of significance (i.e., the volume of significant coherence). The volume of significant coherence was calculated in units of Hz multiplied by the percent of movement cycle duration (Hz%Movement Cycle). The threshold of significance was calculated for the range of 1 to 100 Hz, using the equation by Ushiyama et al.52. At the EEG electrode position, Cz, we also calculated the center frequency (fc) for the volume of significant coherence (i.e., geometric centroid along frequency). On the volume and center frequency of significant coherence at Cz, we performed 4-way ANOVA with (i) aging, (ii) type of pacing, (iii) side of the body, and iv) muscle (i.e., tibialis anterior or medial gastrocnemius muscles) as factors. All of these factors were relevant to aging and corticomuscular communication: both types of pacing have been associated with age-related discrepancies in motor performance and brain activation5,6,31,34,37,38, aging is known to affect bilateral coordination6,7,8, and corticospinal connection differs between the tibialis anterior and gastrocnemius muscles53. The number of movement cycles could affect the magnitude of coherence in an ensemble average. Therefore, for the above ANOVA, the ensemble average for each participant was calculated with the minimum number of cycles completed among the participants.
For each participant, a cortical distribution was formed with volumes of significant coherence between 13 and 30 Hz (i.e., the β band). These cortical distributions were quantified using surface fitting: we fitted a bivariate normal distribution to each participant’s cortical distribution of the volume of significant coherence. To compare the cortical distributions, 4-way ANOVA was performed on the following parameters of surface fitting: the mean (i.e., location of peak value) and standard deviation of the fitted normal distribution, root-mean-square deviation, and coefficient of determination. The factors of the 4-way ANOVA were (i) aging, (ii) type of pacing, (iii) side of the body, and (iv) muscle. The fitted distributions, whose coefficient of determination was below 0.5 or whose peak was located outside the studied cortical area, were excluded from the analysis.
If any factor in ANOVA showed a significant main effect, we performed post hoc analysis with Tukey’s honestly significant difference procedure. The significant level was set to 5% for all tests.
To validate the cyclical patterns of corticomuscular coherence, we calculated surrogate coherence at Cz. For each participant, an ensemble average of coherence was calculated by pairing the ith cycle of an EEG signal with the jth cycle of an EMG signal, such that i ≠ j and none of the original pairing was preserved. For each participant, 100 such ensemble averages were calculated with differently permutated pairing of EEG and EMG signals, and the average magnitude of the 100 ensemble averages was used as surrogate coherence. In preliminary analysis, we observed that surrogate coherence generally showed relatively high magnitude below 6 Hz. Thus, we also examined how surrogate and experimental coherence at Cz differed in magnitude above and below 6 Hz. This comparison was performed by 2-way ANOVA with (i) aging and (ii) type of coherence as factors. The ANOVA was performed separately above and below 6 Hz.
Additional Information
How to cite this article: Yoshida, T. et al. Dynamic cortical participation during bilateral, cyclical ankle movements: effects of aging. Sci. Rep. 7, 44658; doi: 10.1038/srep44658 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Fukuyama, H. et al. Brain functional activity during gait in normal subjects: a SPECT study. Neurosci. Lett. 228, 183–186 (1997).
Hanakawa, T. et al. Mechanisms underlying gait disturbance in Parkinson’s disease. A single photon emission computed tomography study. Brain 122, 1271–1282 (1999).
Miyai, I. et al. Cortical mapping of gait in humans: a near-infrared spectroscopic topography study. Neuroimage 14, 1186–1192 (2001).
Petersen, T. H., Willerslev-Olsen, M., Conway, B. A. & Nielsen, J. B. The motor cortex drives the muscles during walking in human subjects. J. Physiol. 590, 2443–2452 (2012).
Ogaya, S., Higuchi, Y., Tanaka, M. & Fuchioka, S. Effect of aging on seated stepping variability. J. Phys. Ther. Sci. 25, 901–903 (2013).
Plotnik, M., Giladi, N. & Hausdorff, J. M. A new measure for quantifying the bilateral coordination of human gait: Effects of aging and Parkinson’s disease. Exp. Brain Res. 181, 561–570 (2007).
Wishart, L. R., Lee, T. D., Murdoch, J. E. & Hodges, N. J. Effects of aging on automatic and effortful processes in bimanual coordination. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 55, 85–94 (2000).
Temprado, J. J., Vercruysse, S., Salesse, R. & Berton, E. A dynamic systems approach to the effects of aging on bimanual coordination. Gerontology 56, 335–344 (2010).
Salat, D. H. et al. Thinning of the cerebral cortex in aging. Cereb. Cortex 14, 721–730 (2004).
Taki, Y. et al. Correlations among brain gray matter volumes, age, gender, and hemisphere in healthy individuals. PLoS ONE 6 (2011).
Sowell, E. R. et al. Mapping cortical change across the human life span. Nat. Neurosci. 6, 309–315 (2003).
Grieve, S. M., Clark, C. R., Williams, L. M., Peduto, A. J. & Gordon, E. Preservation of limbic and paralimbic structures in aging. Hum. Brain Mapp. 25, 391–401 (2005).
Good, C. D. et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14, 21–36 (2001).
Salat, D. H. et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol. Aging 26, 1215–1227 (2005).
Amaral, D. G. In Principles of Neural Science 5th, 356–369 (McGraw-Hill, 2013).
Guyton, A. C. & Hall, J. E. In Textbook of Medical Physiology 11th, 685–697 (Saunders, 2006).
Kawamura, Y., Okazaki, H., O’brien, P. C. & Dyck, P. J. Lumbar motoneurons of man: (I) number and diameter histogram of alpha and gamma axons of ventral root. J. Neuropathol. Exp. Neurol. 36, 853–860 (1977).
Kawamura, Y., O’brien, P., Okazaki, H. & Dyck, P. J. Lumbar motoneurons of man II: the number and diameter distribution of large- and intermediate- diameter cytons in ‘motoneuron columns’ of spinal cord of man. J. Neuropathol. Exp. Neurol. 36, 861–870 (1977).
Tomlinson, B. E. & Irving, D. The numbers of limb motor neurons in the human lumbosacral cord throughout life. J. Neurol. Sci. 34, 213–219 (1977).
Valdez, G. et al. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc. Natl. Acad. Sci. USA. 107, 14863–14868 (2010).
Stalberg, E. & Fawcett, P. R. W. Macro EMG in healthy subjects of different ages. J. Neurol. Neurosurg. Psychiatry 45, 870–878 (1982).
De Koning, P. et al. Estimation of the number of motor units based on macro-EMG. J. Neurol. Neurosurg. Psychiatry 51, 403–411 (1988).
Stalberg, E. et al. The quadriceps femoris muscle in 20–70-year-old subjects: relationship between knee extension torque, electrophysiological parameters, and muscle fiber characteristics. Muscle Nerve 12, 382–389 (1989).
Eisen, A., Entezari-Taher, M. & Stewart, H. Cortical projections to spinal motoneurons: changes with aging and amyotrophic lateral sclerosis. Neurology 46, 1396–1404 (1996).
Bayram, M. B., Siemionow, V. & Yue, G. H. Weakening of corticomuscular signal coupling during voluntary motor action in aging. J Gerontol A Biol Sci Med Sci 70, 1037–1043 (2015).
Graziadio, S. et al. Developmental tuning and decay in senescence of oscillations linking the corticospinal system. J. Neurosci. 30, 3663–3674 (2010).
Johnson, A. N. & Shinohara, M. Corticomuscular coherence with and without additional task in the elderly. J. Appl. Physiol. 112, 970–981 (2012).
Kamp, D., Krause, V., Butz, M., Schnitzler, A. & Pollok, B. Changes of cortico-muscular coherence: an early marker of healthy aging? Age 35, 49–58 (2013).
Seidler, R. D. et al. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci. Biobehav. Rev. 34, 721–733 (2010).
Fearnley, J. M. & Lees, A. J. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain 114, 2283–2301 (1991).
Sailer, A., Dichgans, J. & Gerloff, C. The influence of normal aging on the cortical processing of a simple motor task. Neurology 55, 979–985 (2000).
Mattay, V. S. et al. Neurophysiological correlates of age-related changes in human motor function. Neurology 58, 630–635 (2002).
Ward, N. S. & Frackowiak, R. S. J. Age-related changes in the neural correlates of motor performance. Brain 126, 873–888 (2003).
Heuninckx, S., Wenderoth, N., Debaere, F., Peeters, R. & Swinnen, S. P. Neural basis of aging: the penetration of cognition into action control. J. Neurosci. 25, 6787–6796 (2005).
Heuninckx, S., Wenderoth, N. & Swinnen, S. P. Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons. J. Neurosci. 28, 91–99 (2008).
Wu, T. & Hallett, M. The influence of normal human ageing on automatic movements. J. Physiol. 562, 605–615 (2005).
Riecker, A. et al. Functional significance of age-related differences in motor activation patterns. Neuroimage 32, 1345–1354 (2006).
Calautti, C., Serrati, C. & Baron, J. C. Effects of age on brain activation during auditory-cued thumb-to-index opposition: a positron emission tomography study. Stroke 32, 139–146 (2001).
Rathelot, J. A. & Strick, P. L. Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc. Natl. Acad. Sci. USA. 106, 918–923 (2009).
Binkofski, F. et al. Neural activity in human primary motor cortex areas 4a and 4p is modulated differentially by attention to action. J. Neurophysiol. 88, 514–519 (2002).
Geyer, S. et al. Two different areas within the primary motor cortex of man. Nature 382, 805–807 (1996).
Plow, E. B. et al. Age-related weakness of proximal muscle studied with motor cortical mapping: a TMS study. PLoS ONE 9 (2014).
Ushiyama, J. et al. Muscle fatigue-induced enhancement of corticomuscular coherence following sustained submaximal isometric contraction of the tibialis anterior muscle. J. Appl. Physiol. 110, 1233–1240 (2011).
Stirn, I., Jarm, T., Kapus, V. & Strojnik, V. Evaluation of muscle fatigue during 100 m front crawl. Eur. J. Appl. Physiol. 111, 101–113 (2011).
Society, A. E. American electroencephalographic society guidelines for standard electrode position nomenclature. J. Clin. Neurophysiol. 8, 200–202 (1991).
Abe, K. et al. Classifying lower limb dynamics in Parkinson’s disease. Brain Res. Bull. 61, 219–226 (2003).
Hyvärinen, A. Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans. Neural Networks 10, 626–634 (1999).
Hyvärinen, A. & Oja, E. Independent component analysis: algorithms and applications. Neural Netw. 13, 411–430 (2000).
Libenson, M. H. In Practical Approach to Electroencephalography 1st, 124–145 (Saunders, 2010).
Ward, N. J., Farmer, S. F., Berthouze, L. & Halliday, D. M. Rectification of EMG in low force contractions improves detection of motor unit coherence in the beta-frequency band. J. Neurophysiol. 110, 1744–1750 (2013).
Myers, L. J. et al. Rectification and non-linear pre-processing of EMG signals for cortico-muscular analysis. J. Neurosci. Methods 124, 157–165 (2003).
Ushiyama, J. et al. Contraction level-related modulation of corticomuscular coherence differs between the tibialis anterior and soleus muscles in humans. J. Appl. Physiol. 112, 1258–1267 (2012).
Brouwer, B. & Ashby, P. Corticospinal projections to lower limb motoneurons in man. Exp. Brain Res. 89, 649–654 (1992).
Acknowledgements
T.Y. was supported by the Toronto Rehabilitation Institute Student Scholarship from the University of Toronto and the following funding from the Natural Sciences and Engineering Research Council of Canada: CREATE Academic Rehabilitation Engineering Fellowship, Discovery Grant (#RGPIN-2016-06358), and Dean Connor and Maris Uffelmann private donation.
Author information
Authors and Affiliations
Contributions
T.Y., K.M., and K.Z. designed the experiment. K.Z. also provided technical consulting. T.Y. performed the experiments and analyzed the data. T.Y., K.M., R.C., and M.R.P. interpreted the data. T.Y. drafted the manuscript. K.Z., R.C., and M.R.P. edited the manuscript. T.Y. and K.M. revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yoshida, T., Masani, K., Zabjek, K. et al. Dynamic cortical participation during bilateral, cyclical ankle movements: effects of aging. Sci Rep 7, 44658 (2017). https://doi.org/10.1038/srep44658
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep44658
This article is cited by
-
Corticomuscular control of walking in older people and people with Parkinson’s disease
Scientific Reports (2020)
-
Effect of training status on beta-range corticomuscular coherence in agonist vs. antagonist muscles during isometric knee contractions
Experimental Brain Research (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.