Abstract
The study aimed to investigate the effects of silk fibroin in a mouse model of dry eye. The experimental dry eye mouse model was developed using more than twelve-weeks-old NOD.B10.H2b mice exposing them to 30–40% ambient humidity and injecting them with scopolamine hydrobromide for 10 days. Tear production and corneal irregularity score were measured by the instillation of phosphate buffered saline or silk fibroin. Corneal detachment and conjunctival goblet cell density were observed by hematoxylin and eosin or periodic acid Schiff staining in the cornea or conjunctiva. The expression of inflammatory markers was detected by immunohistochemistry in the lacrimal gland. The silk group tear production was increased, and corneal smoothness was improved. The corneal epithelial cells and conjunctival goblet cells were recovered in the silk groups. The expression of inflammatory factors was inhibited in the lacrimal gland of the silk group. These results show that silk fibroin improved the cornea, conjunctiva, and lacrimal gland in the mouse model of dry eye. These findings suggest that silk fibroin has anti-inflammatory effects in the experimental models of dry eye.
Similar content being viewed by others
Introduction
Dry eye is a chronic ocular surface disease, and a serious dry eye will cause a visual disorder that affects quality of life1. Dry eye is compounded by destabilization of the tear film that leads to a decrease in tear production, which results from immoderate vaporization of tears on the ocular surface2. The continued imbalance of the tear film and dry eye condition will cause damage of the corneal epithelial cells and loss of the conjunctival goblet cells3,4,5,6. Long-term dry eye has been reported with increased expression of inflammation factors such as tumor necrosis factor-alpha (TNF-α), matrix metalloproteinase (MMP)-2, MMP-9, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in the ocular surface7,8,9,10,11.
Dry eye is treated with artificial tears such as hyaluronic acid as well as with cyclosporine A, tetracyclines, macrolides, and omega-3 and omega-6 fatty acids12,13,14,15,16,17,18,19,20,21. However, this treatment is unsuitable for long-term therapy because it may induce critical side effects such as high blood pressure, glaucoma, cataract, and infection22. Hence, it is essential to discover new therapeutic agents with excellent efficacy for dry eye.
Silk fibroin is a natural protein produced by Bombyx mori. It has been widely used as a scaffold of biomaterials for tissue engineering and regeneration23,24,25,26. The main component of the silk fibroin protein has been reported to be the perfect substrate in a variety of cells for proliferation and adhesion. Silk fibroin has biodegradability, hemostatic properties, non-toxic low antigenic properties, and non-inflammatory properties in the biomedical field27,28,29,30. Silk fibroin is used as a surgical suture material, as well as wound dressing, drug delivery system, and contact lenses; it is used in various forms, such as a gel film and powder solution31,32,33. In addition, efficacy of silk fibroin was reported through the epithelial, limbal epithelial, limbal mesenchymal stromal, and endothelial in the cornea, and pigment epithelial cells in the retina34,35,36,37,38,39,40,41,42. However, there has been no report on the efficacy of silk fibroin in dry eye.
In this study, we investigated the effects of silk fibroin solution in an experimental dry eye mouse model. We investigated changes in tear production, corneal irregularity score, corneal epithelial cell detachment, density of conjunctival goblet cells, and inflammatory factors in the lacrimal gland after instillation of silk fibroin in an experimental model of dry eye.
Results
Tear production changes of silk fibroin
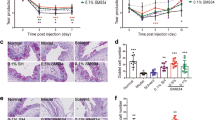
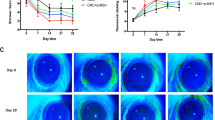
Compared with the PBS groups, tear production was increased in the silk groups (Fig. 1). Tear production was decreased to 76.2% in the desiccation stress for 10 days (DS 10D) group, compared with the control, and the PBS group exhibited increased tear production (3.0-fold) at 10 days, compared with the DS 10D group (P < 0.05). However, tear production was significantly increased by 4.0-fold in the 1 mg/mL and 5 mg/mL silk fibroin groups at 10 days, respectively, compared with the DS 10D group (P < 0.05). In addition, the tear volume in the 1 mg/mL and 5 mg/mL silk fibroin groups was significant increased to 1.3-fold at 10 days, compared with the PBS group (P < 0.05).
Silk fibroin induced alteration in corneal smoothness
After the desiccation stress did not change the shape of the white ring on the ocular surface in the PBS group. However, the shape of the white ring was improved on the ocular surface in the silk groups (Fig. 2a). The corneal irregularity scores were increased to 15-fold in the DS 10D group compared with the control, and a decrease to 13.3% in the PBS group was observed at 10 days compared with the DS 10D group (P < 0.05) (Fig. 2b). The corneal irregularities were decreased to 53.3% and 60% in the 1 mg/mL and 5 mg/mL silk fibroin groups at 10 days, respectively, compared with the DS 10D group (P < 0.05). Moreover, the corneal irregularities were significantly decreased to 46.2% and 53.8% at 10 days in the 1 mg/mL and 5 mg/mL silk fibroin groups, respectively, compared with the PBS group (P < 0.05).
Silk fibroin induced inhibition of epithelial cell detachment on the cornea
The corneal epithelial cells were stained with hematoxylin and eosin (H&E) (Fig. 3a). The detachment of corneal epithelial cells was increased to 16-fold in the DS 10D group compared with controls, and decreased to 93.8% in the silk groups, respectively, compared with the DS 10D group (P < 0.05) (Fig. 3b). Moreover, the detachment of corneal epithelial cells was decreased to 31.3% in the PBS group compared with the DS 10D group (P < 0.05). However, the number of detached corneal epithelial cells was significantly inhibited to 90.9% in the silk fibroin groups compared with the PBS group (P < 0.05).
Silk fibroin induced recovery of goblet cells on the conjunctiva
The goblet cells of the conjunctiva were stained with periodic acid Schiff (PAS) (Fig. 4a). The goblet cells densities of the conjunctiva decreased to 56.1% in the DS 10D group compared with the control (P < 0.05) (Fig. 4b). The goblet cell number of the conjunctiva increased to 1.7-fold and 2.1-fold in the 1 mg/mL and 5 mg/mL silk fibroin groups, respectively, compared with the DS 10D group (P < 0.05). However, the goblet cell densities of the conjunctiva increased to 1.2-fold in the PBS group compared with the DS 10D group (P < 0.05). Moreover, the densities of conjunctival goblet cells significantly increased to 1.4-fold and 1.7-fold at 10 days in the 1 mg/mL and 5 mg/mL silk fibroin groups, respectively, compared with PBS group (P < 0.05).
Anti-inflammatory effects of silk fibroin
Sections of the lacrimal gland were immunostained for TNF-α, MMP-2, MMP-9, ICAM-1, and VCAM-1 (Fig. 5a). All inflammatory factors were overexpressed in the DS 10D group compared with the controls (Fig. 5b). The number of stained TNF-α cells decreased by 80% in the silk groups, compared with the DS 10D group (P < 0.05). Staining for ICAM-1 was decreased by 71.4% and 82.1% in the silk groups, respectively, compared with the DS 10D group (P < 0.05). The stained VCAM-1 cells were inhibited by 65% and 85% in the silk groups, respectively, compared with the DS 10D group (P < 0.05). MMP-2 expression was decreased by 76.9% and 84.6% in the silk groups, respectively, compared with the DS 10D group (P < 0.05). The stained MMP-9 cells were inhibited by 70.8% and 87.5% in the silk groups, respectively, compared with the DS 10D group (P < 0.05). However, all inflammatory factors exhibited no significant decrease in the PBS group compared with the DS 10D group. Moreover, the expression of ICAM-1, VCAM-1, MMP-2 and MMP-9 was reduced by 37.5%, 57.1%, 33.3% and 57.1% in the 5 mg/mL silk fibroin groups, respectively, compared with the 1 mg/mL silk fibroin group (P < 0.05).
Discussion
We confirmed the efficacy of silk fibroin in our experimental mouse model of dry eye. The silk fibroin exhibited increased tear production and reduced irregularity score of ocular surface in the dry eye. In addition, silk fibroin inhibited detachment of the corneal epithelial cells and recovered a number of conjunctival goblet cells in the dry eye model. Silk fibroin exhibited an anti-inflammatory effect by inhibiting the secretion of inflammatory factors by the lacrimal glands in the dry eye model.
Most dry eye treatments such as cyclosporine A, topical corticosteroids, tetracyclines, macrolides, and omega-3 and omega-6 fatty acids are known12,13,14,15,16,17,18,19,20,21. Cyclosporine and corticosteroids are anti-inflammatory agents that regulate the pro-inflammatory cytokines in the conjunctiva and lacrimal glands. Topical cyclosporine has been reported to improve tear production, decrease apoptosis of the corneal epithelial cells, and increase the number of conjunctival goblet cells through the inhibition of T-cell activation. However, cyclosporine appears to cause eye pain, irritation, and soreness symptoms in patients with dry eye, and long-term use of corticosteroids leads to side effects22. Tetracyclines and macrolides are antibiotics, which do not have an observed direct anti-inflammatory effect in dry eye. In addition, omega-3 and omega-6 fatty acids are unsaturated fatty acids, which help to supplement the lipid layer of tear film.
In a previous study, we investigated the a study on the effects of a therapeutic candidate agent in dry eye; we observed the effects of quercetin and chondrocyte-derived extracellular matrix in murine dry eye5,6. Quercetin is one of the flavonoid known to have antioxidant and anti-inflammatory effects; it decreased the detached epithelial cells of the cornea, reduced the number of conjunctival goblet cells, and exhibited anti-inflammatory effects in the lacrimal gland5. Chondrocyte-derived extracellular matrix is a new therapeutic agent that has demonstrated significantly suppressed neovascularization in a rabbit model of alkaline burn rabbit model and a pterygium mouse model as well as reduced the induction of inflammatory factors from the cornea or conjunctiva of a mouse model of dry eye6,43,44.
Silk is known to be useful in slowing tissue ingrowth into the biomaterial scaffold in vivo and in vitro, because of the slow rates of degradation27,28,29,30. In addition, there has been active research on the various formulations and preparation methods of the silk31,32,33. Silk has been studied in a variety of fields, such as bone tissue reconstruction, fabrication of artificial skin, acute tympanic membrane perforation, scaffolds for artificial esophagus, and segmental defects of the zygomatic arch45,46,47,48,49,50. In particular, silk membranes are suitable as a substrate for the restoration of damaged ocular surfaces to promote cellular attachment and growth in human limbal epithelial cells34,35.
Dry eye has been reported to cause inflammation, as well as abnormality of the cornea and conjunctiva from destabilization of the tear film, such as the lipid layer, aqueous layer, and mucous layer on the ocular surface3,4,5,6,7,8,9,10,11. These results suggest that the silk fibroin recovered the aqueous layer of tear film through its anti-inflammatory effects in the lacrimal gland and improved the mucus layer of tear film by increasing the number of conjunctival goblet cells in our experimental mouse model of dry eye. In addition, this improved tear film inhibited corneal epithelial damage, contributing to increased tear volume and improvement of ocular surface irregularity by the dry eye. This indicates that the silk fibroin provided a good environment through tear film stabilization in the ocular surface of the dry eye, which resulted in the recovery of the cornea, conjunctiva, and lacrimal gland of the dry eye.
In conclusion, our study demonstrated that silk fibroin has multi-target therapeutic effects that regulate inflammation of the lacrimal gland as well as ameliorate corneal and conjunctival abnormalities such as decreased tear production, deterioration of ocular surface smoothness, detachment of corneal epithelial cells and reduction of number of conjunctival goblet cells in dry eye. Therefore, silk fibroin is a potential new drug with great efficacy for treating inflammation and stabilizating the tear film in dry eye disease.
Methods
Preparation of silk fibroin solution
Silk fibroin aqueous solution was derived from B. mori cocoons. The procedure for the preparation of a silk solution is normally comprises three steps. The first process is a degumming step. Cocoons were finely chopped and boiled in 0.02 M sodium carbonate (Na2CO3) for an hour to be degummed. The silk fibers were then washed with distilled water and dried. The extracted silk fibroin was then dissolved in a mixing solution (CaCl2: Ethanol: H2O = 1: 2: 8) at 98 °C for 1 hour, yielding a 15 W/V% solution. This solution was dialyzed in distilled water using a dialysis membrane (MWCO 12,000–14,000, Spectra/Por, Spectrum Labs, Rancho Dominguez, CA) for 3 days. The concentration of the silk fibroin solution was calculated to 6 wt%. In this study, final silk fibroin solution was diluted to 0.1% (1 mg/mL) and 0.5% (5 mg/mL). The silk fibroin solutions were then stored at 4 °C before use to avoid premature precipitation51.
Animals
The NOD.B10.H2b mice used in the experiment were purchased through Jackson Laboratory (Bar Harbor, ME). This study was progressed in accordance with the guidelines for the care and use of laboratory animals of the Inje University College of Medicine and the Association for Research in Vision and Ophthalmology statement. All experimental protocols were approved by the Institutional Animal Care and Use Committee of Inje University College of Medicine (Approval ID: 2014–029).
Experimental procedures of murine dry eye
Desiccation stress was created by low ambient humidity (30–40%) using an air draft from a fan for 18 hours per day, and 0.5 mg/0.2 mL hypodermic injection of scopolamine hydrobromide (Sigma-Aldrich, St. Louis, MO) into both hindquarters (one after the other) four times (9 AM, 12 PM, 3 PM, and 6 PM) per day for 10 days on more than 12-weeks-old male NOD.B10.H2b mice52,53. After the desiccation stress for 10 days, the scopolamine hydrobromide injections were discontinued and the mice were placed in an environment of normal humidity and temperature. The eye drops, PBS and 1 mg/mL or 5 mg/mL silk fibroin were administered five times (9 AM, 11 AM, 1 PM, 3 PM, and 5 PM) per day for 10 days (Fig. 6).
The experiment was divided into the following groups: control (normal, n = 5); DS 10D (untreated, desiccation stress for 10 days, n = 5); PBS (n = 5, 10 μL treated PBS in both eyes after the desiccation stress); silk fibroin 1 mg/mL (n = 5, 10 μL treated 1 mg/mL silk fibroin in both eyes after the desiccation stress); and silk fibroin 5 mg/mL (n = 5, 10 μL treated 5 mg/mL silk fibroin in both eyes after the desiccation stress). Mice in all groups were euthanized after the treatment period.
Tear production measurements
Measurement of tear volume was performed using phenol red–impregnated cotton threads (Zone-Quick; Oasis, Glendora, CA), as previously described54. The threads were put in the lateral canthus of mice using a forceps for 20 seconds. The threads that absorb tears changed to red, and the red threads were evaluated in a millimeter through a microscope (SZX7; Olympus Corp., Tokyo, Japan). The tear volume was converted from millimeter to microliter compared with a standard curve55,56. The tear volume was measured within 2 hours of the scopolamine injection and within 1 hour of the PBS or silk fibroin administration, and evaluated by the average value from both eyes5,6.
Measurement of the corneal smoothness and irregularity score
The corneal smoothness was measured after the euthanization of the mice, to obtain an image of the white ring under a fiber optic ring illuminator from a stereoscopic zoom microscope6. The smoothness of the cornea was measured within 2 hours of the scopolamine injection and within 1 hour of the PBS or silk fibroin administration, and evaluated by the average value from both eyes5,6. The corneal irregularity was evaluated by scoring the reflected distortion of the white ring of the epithelium of the cornea in the digital image, as previously described15. The irregularity scores of the cornea were evaluated according to the distorted quarters of the reflected ring, according to a five-point scale as follows: 0, no distortion; 1, 1/4 distortion; 2, 1/2 distortion; 3, 3/4 distortion; 4, 4/4 distortion; and 5, severe distortion and the shape of the ring is not recognized.
Histologic experiment
The eyes and adnexa of each groups were surgically extraction, and then fixed by 10% formalin. The fixed tissues were embedded using paraffin and optimal cutting temperature compound (Tissue-Tek, Sakura Fine Technical Co., Ltd., Tokyo, Japan). The embedded tissues were sectioned to 6 μm, and then H&E and PAS staining was performed. The sections of each group were evaluated oven a range of 0.1 mm2 in the cornea and inferior fornices of the conjunctiva. The stained slides of each group were imaged through a Virtual Microscope (NanoZoomer 2.0 RS, Hamamatsu, Japan). The detachment of corneal epithelial cells and density of conjunctival goblet cells were evaluated as the average value of three non-consecutive cross-section slides from four mice per group.
Immunohistochemistry
The eyes and adnexa were surgically excised, embedded in optimal cutting temperature compound, and flash frozen in liquid nitrogen. Six micrometer sections were excised with a cryostat. The sections were fixed with pre-cooled acetone for 5 minutes, and the primary antibodies to TNF-α (Abcam Inc., Cambridge, MA), MMP-2 (Abcam Inc., Cambridge, MA), MMP-9 (Lifespan Biosciences Inc., Seattle, WA), ICAM-1 (Bioss Inc., Woburn, MA), and VCAM-1 (Bioss Inc., Woburn, MA) were added and incubated for 1 hour at room temperature. After washing, the sections were incubated with secondary antibody (DAKO Corp, Glostrup, Denmark) for 30 minutes. Immunoreactions were visualized with diaminobenzidine chromogen, and the sections were counterstained with Mayer’s hematoxylin (Sigma-Aldrich) for 30 seconds at room temperature. Images of the sections were photographed with a Virtual Microscope (NanoZoomer 2.0 RS). The stained cells were evaluated oven a range of 0.1 mm2 in the lacrimal gland of each group.
Statistical analysis
All data were analyzed using SPSS version 18.0 (SPSS, Chicago, IL) for Windows and reported as mean ± standard deviation. The comparative analysis of differences between groups were performed by two-way analyses of variance (with Tukey’s test), and statistical significance was indicated by P < 0.05.
Additional Information
How to cite this article: Kim, C. E. et al. Effects of silk fibroin in murine dry eye. Sci. Rep. 7, 44364; doi: 10.1038/srep44364 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
07 April 2017
A correction has been published and is appended to both the HTML and PDF versions of this paper. The error has not been fixed in the paper.
07 April 2017
Scientific Reports 7: Article number: 44364; published online: 10 March 2017; updated: 07 April 2017 This Article contains an error in the Acknowledgements section: “This study was supported by a grant from the Korea Healthcare Technology R&D Project of the Ministry of Health and Welfare Affairs, Republic of Korea (Grant No.
References
Schiffman, R. M. et al. Utility assessment among patient with dry eye disease. Ophthalmology 110, 1412–1419 (2003).
Lemp, M. A. Report of the national eye institute/industry workshop on clinical trials in dry eyes. CLAO. J. 21, 221–232 (1995).
Situ, P., Simpson, T. L., Fonn, D. & Jones, L. W. Conjunctival and corneal pneumatic sensitivity is associated with signs and symptoms of ocular dryness. Invest. Ophthalmol. Vis. Sci. 49, 2971–2976 (2008).
Rolando, M. & Zierhut, M. The ocular surface and tear film and their dysfunction in dry eye disease. Surv. Ophthalmol. 45, S203–S210 (2001).
Oh, H. N., Kim, C. E., Lee, J. H. & Yang, J. W. Effects of quercetin in a mouse model of experimental dry eye. Cornea 34, 1130–1136 (2015).
Kim, C. E., Oh, H. N., Lee, J. H. & Yang, J. W. Effects of chondrocyte-derived extracellular matrix in a dry eye mouse model. Mol. Vis. 21, 1210–1223 (2015).
Calonge, M. et al. Dry eye disease as an inflammatory disorder. Ocul. Immunol. Inflamm. 18, 244–253 (2010).
Enríquez-de-Salamanca, A. et al. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. Mol. Vis. 16, 862–873 (2010).
Pinazo-Durán, M. D. et al. Effects of a nutraceutical formulation based on the combination of antioxidants and ω-3 essential fatty acids in the expression of inflammation and immune response mediators in tears from patients with dry eye disorders. Clin. Interv. Aging 8, 139–148 (2013).
Galbis-Estrada, C. et al. Patients undergoing long-term treatment with antihypertensive eye drops responded positively with respect to their ocular surface disorder to oral supplementation with antioxidants and essential fatty acids. Clin. Interv. Aging 8, 711–719 (2013).
Brignole, F. et al. Flow cytometric analysis of inflammatory markers in conjunctival epithelial cells of patients with dry eyes. Invest. Ophthalmol. Vis. Sci. 41, 1356–1363 (2000).
Limberg, M. B., McCaa, C., Kissling, G. E. & Kaufman, H. E. Topical application of hyaluronic acid and chondroitin sulfate in the treatment of dry eyes. Am. J. Ophthalmol. 103, 194–197 (1987).
Nepp, J. et al. The clinical use of viscoelastic artificial tears and sodium chloride in dry-eye syndrome. Biomaterials 22, 3305–3310 (2001).
Toda, I., Shinozaki, N. & Tsubota, K. Hydroxypropyl methylcellulose for the treatment of severe dry eye associated with Sjögren’s syndrome. Cornea 15, 120–128 (1996).
De Paiva, C. S. et al. Apical corneal barrier disruption in experimental murine dry eye is abrogated by methylprednisolone and doxycycline. Invest. Ophthalmol. Vis. Sci. 47, 2847–2856 (2006).
De Paiva, C. S. et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp. Eye. Res. 83, 526–535 (2006).
Li, D. Q. et al. Regulation of MMP-9 production by human corneal epithelial cells. Exp. Eye. Res. 73, 449–459 (2001).
Rashid, S. et al. Topical omega-3 and omega-6 fatty acids for treatment of dry eye. Arch. Ophthalmol. 126, 219–225 (2008).
Sadrai, Z. et al. Effect of topical azithromycin on corneal innate immune responses. Invest. Ophthalmol. Vis. Sci. 52, 2525–2531 (2011).
Solomon, A. et al. Doxycycline inhibition of interleukin-1 in the corneal epithelium. Invest. Ophthalmol. Vis. Sci. 41, 2544–2557 (2000).
Yüksel, B., Bozdag, B., Acar, M. & Topaloğlu, E. Evaluation of the effect of topical cyclosporine A with impression cytology in dry eye patients. Eur. J. Ophthalmol. 20, 675–679 (2010).
Barabino, S., Chen, Y., Chauhan, S. & Dana, R. Ocular surface immunity: homeostatic mechanisms and their disruption in dry eye disease. Prog. Retin. Eye. Res. 31, 271–285 (2012).
Panilaitis, B. et al. Macrophage responses to silk. Biomaterials 24, 3079–3085 (2003).
Meinel, L. et al. The inflammatory responses to silk films in vitro and in vivo . Biomaterials 26, 147–155 (2005).
Kim, H. J. et al. Influence of macroporous protein scaffolds on bone tissue engineering from bone marrow stem cells. Biomaterials 26, 4442–4452 (2005).
Wang, Y. et al. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials 29, 3415–3428 (2008).
Mori, H. & Tsukada, M. New silk protein: modification of silk protein by gene engineering for production of biomaterials. J. Biotechnol. 74, 95–103 (2000).
Horan, R. L. et al. In vitro degradation of silk fibroin. Biomaterials 26, 3385–3393 (2005).
Meinel, L. et al. The inflammatory responses to silk films in vitro and in vivo . Biomaterials 26, 147–155 (2005).
Mauney, J. R. et al. Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials 28, 5280–5290 (2007).
Altman, G. H. et al. Silk-based biomaterials. Biomaterials 24, 401–416 (2003).
Um, I. C., Kweon, H. Y., Park, Y. H. & Hudson, S. Structural characteristics and properties of the regenerated silk fibroin prepared from formic acid. Int. J. Biol. Macromol. 29, 91–97 (2001).
Li, M. et al. Structure and properties of silk fibroin-poly (vinyl alcohol) gel. Int. J. Biol. Macromol. 30, 89–94 (2002).
Chirila, T., Barnard, Z., Zainuddin, Z. & Harkin, D. Silk as substratum for cell attachment and proliferation. Mater. Sci. Forum. 561–565, 1549‒1552 (2007).
Chirila, T. et al. Bombyx mori silk fibroin membranes as potential substrata for epithelial constructs used in the management of ocular surface disorders. Tissue Eng. Part A 14, 1203‒1211 (2008).
Chirila, T. et al. Evaluation of silk sericin as a biomaterial: in vitro growth of human corneal limbal epithelial cells on Bombyx mori sericin membranes. Prog. Biomater. 2, 14 (2013).
Bray, L. J., Suzuki, S., Harkin, D. G. & Chirila, T. V. Incorporation of exogenous RGD peptide and inter-species blending as strategies for enhancing human corneal limbal epithelial cell growth on bombyx mori silk fibroin membrane. J. Funct. Biomater. 4, 74–88 (2013).
Hogerheyde, T. A. et al. Assessment of freestanding membranes prepared from Antheraea pernyi silk fibroin as a potential vehicle for corneal epithelial cell transplantation. Biomed. Mater. 9, 025016 (2014).
Bray, L. J. et al. Human corneal epithelial equivalents constructed on Bombyx mori silk fibroin membranes. Biomaterials 32, 5086–5091 (2011).
Bray, L. J. et al. A dual-layer silk fibroin scaffold for reconstructing the human corneal limbus. Biomaterials 33, 3529–3538 (2012).
Madden, P. W. et al. Human corneal endothelial cell growth on a silk fibroin membrane. Biomaterials 32, 4076–4084 (2011).
Shadforth, A. M. et al. The cultivation of human retinal pigment epithelial cells on Bombyx mori silk fibroin. Biomaterials 33, 4110–4117 (2012).
Lee, H. S., Lee, J. H. & Yang, J. W. Effect of porcine chondrocyte-derived extracellular matrix on the pterygium in mouse model. Graefes. Arch. Clin. Exp. Ophthalmol. 252, 609–618 (2014).
Lee, H. S., Lee, J. H., Kim, C. E. & Yang, J. W. Anti-neovascular effect of chondrocyte-derived extracellular matrix on corneal alkaline burns in rabbits. Graefes. Arch. Clin. Exp. Ophthalmol. 252, 951–961 (2014).
Park, H. J. et al. Fabrication of 3D porous silk scaffolds by particulate (salt/sucrose) leaching for bone tissue reconstruction. Int. J. Biol. Macromol. 78, 215–223 (2015).
Sheikh, F. A. et al. 3D electrospun silk fibroin nanofibers for fabrication of artificial skin. Nanomedicine 11, 681–691 (2015).
Lee, J. H. et al. Clinical outcomes of silk patch in acute tympanic membrane perforatio. Clin. Exp. Otorhinolaryngol. 8, 117–122 (2015).
Chung, E. J., Ju, H. W., Park, H. J. & Park, C. H. Three-layered scaffolds for artificial esophagus using poly(ε-caprolactone) nanofibers and silk fibroin: An experimental study in a rat model. J. Biomed. Mater. Res. A. 103, 2057–2065 (2015).
Lee, J. M., Kim, J. H., Lee, O. J. & Park, C. H. The fixation effect of a silk fibroin-bacterial cellulose composite plate in segmental defects of the zygomatic arch: an experimental study. JAMA Otolaryngol. Head Neck Surg. 139, 629–635 (2013).
Lee, O. J. et al. Biodegradation behavior of silk fibroin membranes in repairing tympanic membrane perforations. J. Biomed. Mater. Res. A. 100, 2018–2026 (2012).
Yeo, J. H., Lee, K. G., Lee, Y. W. & Kim, S. Y. Simple preparation and characteristics of silk fibroin microsphere. Eur. Polym. J. 39, 1195–1199 (2003).
De Paiva, C. S. et al. Dry eye–induced conjunctival epithelial squamous metaplasia is modulated by interferon-gamma. Invest. Ophthalmol. Vis. Sci. 48, 2553–2560 (2007).
Yoon, K. C. et al. Tear Production and ocular surface changes in experimental dry eye after elimination of desiccating stress. Invest. Ophthalmol. Vis. Sci. 52, 7267–7273 (2011).
Villareal, A. L., Farley, W. & Pflugfelder, S. C. Effect of topical ophthalmic epinastine and olopatadine on tear volume in mice. Eye Contact Lens 32, 272–276 (2006).
Chen, Z. et al. Altered morphology and function of the lacrimal functional unit in protein kinase C alpha knockout mice. Invest. Ophthalmol. Vis. Sci. 51, 5592–5600 (2010).
Stewart, P. et al. Effect of experimental dry eye on tear sodium concentration in the mouse. Eye Contact Lens 31, 175–178 (2005).
Acknowledgements
This study was supported by a grant from the Korea Healthcare Technology R&D Project of the Ministry of Health and Welfare Affairs, Republic of Korea (Grant No. HI15C1142).
Author information
Authors and Affiliations
Contributions
J.W.Y. and C.H.P. designed the experiment; C.H.P. and Y.K.Y. provided silk fibroin solutions; C.E.K. and J.H.L. performed the experiments of dry eye mouse model; J.W.Y., C.E.K. and J.H.L. analysed the data; J.W.Y. and C.E.K. writing the manuscript; J.W.Y., C.H.P., C.E.K. and Y.K.Y. wrote the manuscript; J.W.Y. and C.H.P. supervised the research. All the authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Kim, C., Lee, J., Yeon, Y. et al. Effects of silk fibroin in murine dry eye. Sci Rep 7, 44364 (2017). https://doi.org/10.1038/srep44364
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep44364
This article is cited by
-
Evaluation of the effect of process parameters on the protein content of silk fibroin
Polymer Bulletin (2023)
-
Impact of silk hydrogel secondary structure on hydrogel formation, silk leaching and in vitro response
Scientific Reports (2022)
-
Effect of Silk Fibroin on Neuroregeneration After Traumatic Brain Injury
Neurochemical Research (2019)
-
RGN-259 (thymosin β4) improves clinically important dry eye efficacies in comparison with prescription drugs in a dry eye model
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.