Abstract
Vlasouliolides A-D (1–4), four rare sesquiterpene lactone dimers, were isolated from Vladimiria souliei. The common structural characteristic of 1–4 is the C32 skeleton comprising two sesquiterpene lactone units linked by a C11-C13′ single bond with one acetyl connected to the C-13 position of one of the two sesquiterpene lactone units. The stereochemistries of 1–4 were assigned by a combination of NOESY correlations and Cu-Κα X-ray crystallographic analyses. Compounds 1–4 strongly inhibited the production of NO in LPS-stimulated RAW 264.7 cells. Furthermore, 1 and 2 inhibited the activation of NF-κB in LPS-induced 293T cells.
Similar content being viewed by others
Introduction
Naturally occurring sesquiterpene lactone dimers (SLDs) are a type of complex found in natural products with many pharmacological activities1,2. Since the first SLD absinthin was isolated from Artemisia absinthium in 19533, more than 160 SLDs have been obtained. To the best of our knowledge, most of the published SLDs have C30 cores derived from two C15 sesquiterpenoid units4,5,6.
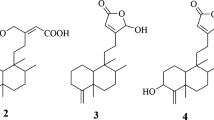
The genus Vladimiria, belonging to the family of Asteraceae, comprises approximately 12 species that are mainly distributed in the Sichuan Province, China7. Sesquiterpenes, as major constituents isolated from the Vladimiria species, possess various types of structures including guaianolide, carabrane, eudesmane and germacrane sesquiterpenes8,9,10,11,12. The plant of Vladimiria souliei, as a traditional Chinese medicine, has been used for relieving pain and stomach diseases since ancient times7,13. Additionally, the chemical constituents from the roots of V. souliei exhibited significant antimicrobial, antitumor and inhibitory effects on NO production activities10,13. In the course of our investigation on structurally novel SLDs from the family of Asteraceae, four rare SLDs with C32 cores were discovered from Vladimiria souliei, and they were designated vlasouliolides A-D (1–4) (Fig. 1).
Results and Discussion
Structure elucidation
Vlasouliolide A (1),  +3.77 (c 0.24, CH3COCH3), was obtained as a colorless orthorhombic crystal. Its molecular formula was determined to be C32H40O5 by positive HRESIMS at m/z 527.2763 ([M + Na]+, calcd. 527.2773). The 1H NMR spectrum of 1 (Supplementary Table S1) showed characteristic signals for one methyl singlet at δH 2.16 (3H, s, -Ac), two oxymethines at δH 3.91 (1H, t, J = 9.5 Hz, H-6′) and δH 4.17 (1H, t, J = 9.6 Hz, H-6), and four sets of terminal alkylene (δH 4.83, 1H, s, 4.76, 1H, s, H2-14; δH 4.88, 1H, s, 4.78, 1H, s, H2-14′; δH 5.29, 1H, d, J = 1.8 Hz, 5.06, 1H, d, J = 1.7 Hz, H2-15; δH 5.14, 1H, d, J = 1.9 Hz, 5.03, 1H, d, J = 1.7 Hz, H2-15′).
+3.77 (c 0.24, CH3COCH3), was obtained as a colorless orthorhombic crystal. Its molecular formula was determined to be C32H40O5 by positive HRESIMS at m/z 527.2763 ([M + Na]+, calcd. 527.2773). The 1H NMR spectrum of 1 (Supplementary Table S1) showed characteristic signals for one methyl singlet at δH 2.16 (3H, s, -Ac), two oxymethines at δH 3.91 (1H, t, J = 9.5 Hz, H-6′) and δH 4.17 (1H, t, J = 9.6 Hz, H-6), and four sets of terminal alkylene (δH 4.83, 1H, s, 4.76, 1H, s, H2-14; δH 4.88, 1H, s, 4.78, 1H, s, H2-14′; δH 5.29, 1H, d, J = 1.8 Hz, 5.06, 1H, d, J = 1.7 Hz, H2-15; δH 5.14, 1H, d, J = 1.9 Hz, 5.03, 1H, d, J = 1.7 Hz, H2-15′).
Analysis of the 13C NMR spectrum revealed the existence of 32 carbons (Supplementary Table S2), including 1 × CH3, 14 × CH2 (containing 4 sp2 alkylenes at δC 109.3, 109.6, 111.6, and 112.0), 9 × CH (containing 2 oxymethines at δC 82.4 and 84.5), and 8 × C (containing 3 carbonyl carbons at δC 177.5, 178.3 and 205.2). Detailed analysis of the NMR spectrum of 1 indicated that vlasouliolide A (1) should contain an acetyl due to the resonance signals at δC 205.2, 30.7, and δH 2.16 (3H, s, -Ac). Deducting the carbon resonances for the acetyl, the remaining 30 carbon resonances implied that two similar sesquiterpene lactone units existed.
The planar structure of vlasouliolide A was constructed by comprehensive analyses of the 2D NMR spectra (Fig. 2A). Two similar long proton-bearing structural fragments, H2-3/H2-2/H-1/H-5/H-6/H-7/H2-8/H2-9 and H2-3′/H2-2′/H-1′/H-5′/H-6′/H-7′/H2-8′/H2-9′, as well as an extra short chain, H-7′/H-11′/H2-13′, were constructed based on interpretation of the 1H-1H COSY and HSQC-TOCSY spectra. The HMBC spectrum of 1 showed the key correlations of four typical terminal alkylene groups (Fig. 2A), including H2-14/C-1 and C-9; H2-15/C-3 and C-5; H2-14′/C-1′ and C-9′; and H2-15′/C-3′ and C-5′. Thus, subunits A and B were deduced to be two guaianolide moieties similar to dehydrocostus lactone14,15. Therefore, the structure of 1 should be composed of two guaianolide moieties and an acetyl moiety. In the HMBC spectrum of 1, the long correlations from H3-17 (δH 2.16, 3H, s) to C-13 (δC 46.4) as well as H2-13 (δH 2.86, 2.56, 1H, d, J = 19 Hz, respectively) to C-7 (δC 48.2) and C-12 (δC 178.3) indicated that the acetyl moiety was connected to the C-13 position of one guaianolide unit. Compared with dehydrocostus lactone, the C-11 position of subunit A was a quaternary carbon, while the C-13′ position of subunit B was an sp3 methylene. These changes implied that the two sesquiterpene lactone units should be linked directly via a C-C bond between C-11 and C-13′, and this assumption was verified by the HMBC correlations of H2-13′ (δH 2.23, 1.35, 1H, m, respectively) with C-11 (δC 46.1), C-12 (δC 178.3), C-11′ (δC 41.9) and C-12′ (δC 177.5). Thus, the planar structure of 1 was elucidated as shown in Fig. 1.
The relative configuration of 1 was characterized by interpretation of the NOESY spectrum (Fig. 2A). The similar NOESY correlations of H-1/H-7/H-5 and H-1′/H-7′/H-5′ in subunits A and B indicated that they are on the same face. In addition, the large coupling constant between H-6/H-7 and H-6′/H-7′ (J = 9.6, 9.5 Hz, respectively) implied that H-6/H-7 and H-6′/H-7′ were in the trans-form. Consequently, in subunit A, H-7 was arbitrarily assigned as having an α-orientation. The correlation between H-7 and H2-13 suggested that the side chain C13/C16/C17 should be located below the molecular plane. Thus, the H2-13′ was assigned as having a β-orientation, as indicated by the NOESY correlations of H-6 with H2-13′. It should be noted that H2-13′ exhibited NOESY correlations with H-7′. Combined with the NOESY correlation between H2-13 and H-11′, the relative configuration of H-11′ should be the α-orientation. Finally, the absolute configuration of 1 was confirmed to be 1R, 5R, 6S, 7S, 11S, 1′R, 5′R, 6′S, 7′S, 11′S by Cu-Κα X-ray crystallographic analysis (Fig. 2B).
Vlasouliolide B (2),  +20.41 (c 0.21, CH3COCH3), possessed the same molecular formula as that of 1 as determined by positive HRESIMS at m/z 527.2758 ([M + Na]+, calcd. 527.2773). Analysis of the NMR data (Supplementary Tables S1 and S2) indicated that compound 2 possesses the same planar structure as that of 1. The notable NOESY correlation of H2-13/H-11′/H-7′ in 2 instead of that H-11′/H-6′ in 1 implied that 2 was an 11′-epimer of 1. Comparing the NMR spectroscopic data of 2 with those of 1, an obvious downfield shift of Ha-13 from δH 2.86 in 1 to δH 3.36 in the 1H NMR was observed. As shown in Fig. 3A, the opposite arrangement of the two subunits led to the C-12′ carbonyl and H2-13 in 1 being far from each other. In contrast, the subunits A and B were arranged in the same direction in 2, resulting in the H2-13 being spatially adjacent to the carbon-oxygen double bond at C-12′. Thus, the intramolecular deshielding effect contributed to the downfield shift of Ha-13 from δH 2.86 in 1 to δH 3.36 in 2. Finally, the absolute configuration of C-11′ was confirmed as R by Cu-Κα X-ray diffraction (Fig. 3B).
+20.41 (c 0.21, CH3COCH3), possessed the same molecular formula as that of 1 as determined by positive HRESIMS at m/z 527.2758 ([M + Na]+, calcd. 527.2773). Analysis of the NMR data (Supplementary Tables S1 and S2) indicated that compound 2 possesses the same planar structure as that of 1. The notable NOESY correlation of H2-13/H-11′/H-7′ in 2 instead of that H-11′/H-6′ in 1 implied that 2 was an 11′-epimer of 1. Comparing the NMR spectroscopic data of 2 with those of 1, an obvious downfield shift of Ha-13 from δH 2.86 in 1 to δH 3.36 in the 1H NMR was observed. As shown in Fig. 3A, the opposite arrangement of the two subunits led to the C-12′ carbonyl and H2-13 in 1 being far from each other. In contrast, the subunits A and B were arranged in the same direction in 2, resulting in the H2-13 being spatially adjacent to the carbon-oxygen double bond at C-12′. Thus, the intramolecular deshielding effect contributed to the downfield shift of Ha-13 from δH 2.86 in 1 to δH 3.36 in 2. Finally, the absolute configuration of C-11′ was confirmed as R by Cu-Κα X-ray diffraction (Fig. 3B).
Vlasouliolide C (3) was isolated as a colorless monoclinic crystal with  +30.23 (c 0.04, CHCl3). Its molecular formula was determined to be C32H42O5 by positive HRESIMS at m/z 529.2940 ([M + Na]+, calcd. 529.6728). The 1D NMR data (Supplementary Tables S1 and S2) of 3 disclosed a C32 skeleton similar to in 1 and 2. Comprehensive analysis of the 2D NMR spectra indicated that compound 3 possessed the same 13-acetyl-mokkolactone fragment (subunit A) as 1 and 2. Unlike the structures of 1 and 2, an eudesmane moiety existed as subunit B in the structure of 3, as deduced by the 1H-1H COSY correlations of H2-3′/H2-2′/H-1′, H-5′/H-6′/H-7′/H2-8′/H2-9′ and H-7′/H-11′/H2-13′ together with crucial HMBC correlations from H2-15′ to C-3′ and C-5′ as well as from CH3-14 to C-1, C-2, and C-9. Therefore, the structure of 3 was formed by an acetyl-substituted guaianolide moiety and eudesmane moiety. The HMBC correlations from H2-13′ (δH 2.30, 1.38) to C-11′ (δC 41.6), C-12′ (δC 178.8), C-11 (δC 46.2), C-12 (δC 178.3) and C-13 (δC 47.2) suggested that subunits A and B should also be directly linked by a C-11/13′ single bond (Fig. 4A).
+30.23 (c 0.04, CHCl3). Its molecular formula was determined to be C32H42O5 by positive HRESIMS at m/z 529.2940 ([M + Na]+, calcd. 529.6728). The 1D NMR data (Supplementary Tables S1 and S2) of 3 disclosed a C32 skeleton similar to in 1 and 2. Comprehensive analysis of the 2D NMR spectra indicated that compound 3 possessed the same 13-acetyl-mokkolactone fragment (subunit A) as 1 and 2. Unlike the structures of 1 and 2, an eudesmane moiety existed as subunit B in the structure of 3, as deduced by the 1H-1H COSY correlations of H2-3′/H2-2′/H-1′, H-5′/H-6′/H-7′/H2-8′/H2-9′ and H-7′/H-11′/H2-13′ together with crucial HMBC correlations from H2-15′ to C-3′ and C-5′ as well as from CH3-14 to C-1, C-2, and C-9. Therefore, the structure of 3 was formed by an acetyl-substituted guaianolide moiety and eudesmane moiety. The HMBC correlations from H2-13′ (δH 2.30, 1.38) to C-11′ (δC 41.6), C-12′ (δC 178.8), C-11 (δC 46.2), C-12 (δC 178.3) and C-13 (δC 47.2) suggested that subunits A and B should also be directly linked by a C-11/13′ single bond (Fig. 4A).
The relative configuration of subunit B was deduced by analysis of the NOESY correlations (Fig. 4A). H-11′, H-6′ and H-14′ were assigned as having the α-orientation, and H-5′, H-7′ and H-13′ were assigned as having the β-orientation, which were consistent with the biosynthetic precursor β-cyclocostunolide16. Similar to 1, the opposite arrangement of the two subunits was verified by the NOESY correlations of H-13′/H-6 and H-11′/H-13. The structure of 3 was finally elucidated as shown in Fig. 1, and the absolute configuration was assigned as 1R, 5R, 6S, 7S, 11S, 5′S, 6′S, 7′S, 10′S, 11′S by Cu-Κα X-ray crystallographic analysis (Fig. 4B).
Vlasouliolide D (4), a colorless orthorhombic crystal with  +55.21 (c 0.08, CH3COCH3), possessed the same molecular formula as that of 3 determined by positive HRESIMS at m/z 529.2923 ([M + Na]+ calcd. 529.6722). The 1D NMR data of 4 (Supplementary Tables S1 and S2) revealed that 4 was constructed from one acetyl, one dehydrocostus lactone, and one β-cyclocostunolide moieties, which were identical with those of 3. However, different from 3, the acetyl in 4 was located at the C-13 position of β-cyclocostunolide to form 13-acetyl-eudesmenolide (subunit A), which is supported by the key HMBC correlations from H2-13 (δH 2.79, 2.66, 1H, d, J = 17 Hz, respectively) to C-7 (δC 51.9), C-11 (δC 46.2) and C-12 (δC 178.9). Subunit B was also connected to subunit A via a C-11/13′ single bond. Therefore, the structure of 4 was determined as shown in Fig. 1. The absolute configuration of 4 was determined as 5S, 6S, 7S, 10R, 11S, 1′R, 5′R, 6′S, 7′S, 11′S by Cu-Κα X-ray crystallographic analysis (Fig. 5).
+55.21 (c 0.08, CH3COCH3), possessed the same molecular formula as that of 3 determined by positive HRESIMS at m/z 529.2923 ([M + Na]+ calcd. 529.6722). The 1D NMR data of 4 (Supplementary Tables S1 and S2) revealed that 4 was constructed from one acetyl, one dehydrocostus lactone, and one β-cyclocostunolide moieties, which were identical with those of 3. However, different from 3, the acetyl in 4 was located at the C-13 position of β-cyclocostunolide to form 13-acetyl-eudesmenolide (subunit A), which is supported by the key HMBC correlations from H2-13 (δH 2.79, 2.66, 1H, d, J = 17 Hz, respectively) to C-7 (δC 51.9), C-11 (δC 46.2) and C-12 (δC 178.9). Subunit B was also connected to subunit A via a C-11/13′ single bond. Therefore, the structure of 4 was determined as shown in Fig. 1. The absolute configuration of 4 was determined as 5S, 6S, 7S, 10R, 11S, 1′R, 5′R, 6′S, 7′S, 11′S by Cu-Κα X-ray crystallographic analysis (Fig. 5).
Biological activity assay
The roots of V. souliei are often used in traditional Chinese medicine for the treatment of digestive disorders and inflammatory diseases. Many sesquiterpenes isolated from V. souliei showed inhibitory activity against lipopolysaccharide (LPS)-induced nitric oxide (NO) production in murine RAW264.7 cells10. Excessive NO has been implicated in the pathological process of tissue damage following inflammation17. The inhibition of the overproduction of NO is an important therapeutic way to treat inflammatory diseases18. We investigated the anti-inflammatory activities of compounds 1–4 by a LPS-induced NO production assay in RAW 264.7 macrophages5. Compounds 1–4 exhibited significant inhibitory effects against NO production with IC50 values of 1.14, 2.53, 1.57 and 3.19 μM, respectively. Moreover, these compounds were not notably cytotoxic at the concentrations required for inhibiting NO production, as determined by an MTT assay. NF-κB is a transcription factor that controls immune responses and plays a pivotal role in the regulation of NO expression19. We conducted an NF-κB luciferase reporter assay in 293T cells to evaluate the impact of compounds 1–4 on the transcriptional activity of NF-κB20.
The NF-κB reporter luciferase construct and Renilla luciferase control vector were cotransfected in 293T cells for 24 h. Thereafter, the cells were left untreated or were treated with a 10 μM concentration of compound for an additional 1 h before LPS activation for 4 h. Compounds 1 and 2 displayed inhibition towards the NF-κB pathway, while 3 and 4 showed no effects in the reduction of NF-κB luciferase activity (Fig. 6A). We also examined the effects of compounds 1–4 on the expression of IκBα and P65 proteins in the NF-κB pathway21. RAW 264.7 cells pretreated with 1–4 at the indicated concentrations for 1 h were subjected to LPS stimulation before Western blot analysis. Compounds 1–4 had no inhibitory effects on the degradation of IκBα, while 1 and 2 can dose-dependently down-regulate the LPS-induced phosphorylation of the NF-κB p65 subunit (Fig. 6B). These data indicated that the NO inhibitory activities of 1 and 2 might be attributed to suppressing NF-κB activation, while those of 3 and 4 might not be due to this mechanism.
(A) The NF-κB luciferase reporter assay shows that compounds 1 and 2 displayed suppression of LPS-induced NF-κB activation in 293T cells. (Mean ± SD in three separate experiments. *p < 0.05; **p < 0.001). (B) The effects of compounds 1–4 on the phosphorylation of IκBα and p65 in the presence of LPS stimulation in RAW 264.7 cells.
In conclusion, sesquiterpene lactone dimers usually have a C30 framework biosynthetically derived from a Diels-Alder adduct of two homo or hetero sesquiterpene monomers. Vlasouliolides A-D (1–4) possessed a C32 skeleton derived from two sesquiterpene lactone molecules and an acetyl group. Commonly, in the chemical structures of 1–4, the acetyl was connected to the C-13 of one of the two sesquiterpene lactone units to form a C17 unit, and the C17/C15 units were further directly linked by a C11-C13′ single bond. This is the first report regarding C17/C15 sesquiterpene lactone dimers from nature with significant anti-inflammation activities, which might be potentially useful for the treatment of inflammatory diseases. The discovery of Vlasouliolides A-D may encourage further investigations by natural product chemists, synthetic chemists, and pharmacists.
Methods
General experimental procedures
Column chromatography (CC): silica gel H (10–40 μm) and silica gel (200–300 mesh) (Marine Chemical Factory, Qingdao, P. R. China); Sephadex LH-20 (Pharmacia Fine Chemicals, Piscataway, NJ, USA); RP-C18 gel (40−63 μm; Daiso, Co., Japan). TLC: silica gel plates (Yantai Jiang You Silicone Development Co., Yantai, P. R. China), visualization by spraying with 10% H2SO4 in EtOH. HPLC: Agilent 1260 series (Agilent Technologies, US) with a Zorbax SB-C18 (5 μm, 9.4 × 150 mm) column. NMR: Bruker Avance III-500 and Avance III-600 spectrometers (Bruker, Switzerland). MS: Agilent MSD-Trap-XCT (for ESI) and Agilent-6520 Q-TOF mass spectrometers (for HR-ESI). Melting point: X-4B digital display micro-melting apparatus (Shanghai Jingsong Instrument, Shanghai, P. R. China). Optical rotation: Rudolph Autopo V (Rudolph Research Analytical, Hackettstown, NJ). UV: Agilent 1260 series DAD detector (Agilent Technologies, US). CD: Brighttime Chirascan (Applied Photophysics Ltd, UK). IR: Thermo Scientific Nicolet 6700 (Thermo Scientific, USA). RAW 264.7 cells and 293T cells: ATCC (American type culture collection). Dulbecco’s modified Eagle’s medium and fetal bovine serum: Gibco Invitrogen (Carlsbad, CA, USA). LPS Griess reagent and MTT: Sigma-Aldrich (St. Louis, MO, USA).
Plant material
The roots of V. souliei were collected from the Sichuan province of China in October 2014 and authenticated by professor Bao-Kang Huang, Department of Pharmacognosy, School of Pharmacy, Second Military Medical University. A voucher specimen (No. 201412-VS) is deposited in the Department of Pharmacognosy, Second Military Medical University.
Extraction and isolation
The chipped and dried roots of V. souliei (20.0 kg) were extracted by maceration with 95% ethanol overnight at room temperature (3 × 60 L). After removal of the solvent, the ethanol extract (2.12 kg) was successively partitioned between water and petroleum ether (PE)/ethyl acetate (EtOAc) to give PE, EtOAc and water extracts. The EtOAc extract (0.626 kg) was segmented by silica gel column chromatography (PE/EtOAc, 50:1–0:1) to yield 17 fractions (Fr. 1–17). Fraction 7 (31.99 g) underwent chromatography over an RP-C18 medium-pressure column (MeOH/H2O, 10:90 to 90:10) to give 12 subfractions (Fr. 7.1–7.12). Subfraction 7.9 (2.5 g) underwent further chromatography over an RP-C18 medium-pressure column (CH3CN/H2O, 45:55) and was finally purified by semi-preparative RP-C18 HPLC (MeOH/H2O, 71:29) to produce 1 (42.9 mg) and 4 (5.2 mg). Subfraction 7.10 (1.89 g) underwent chromatography over an RP-C18 medium-pressure column using MeOH/H2O (55:45–100:0) as the elution solvent, and the 70–80% fraction was purified by semi-preparative RP-C18 HPLC (CH3CN/H2O, 45:55), yielding 2 (22.2 mg). Fraction 8 (5.40 g) underwent chromatography over an RP-C18 medium-pressure column using MeOH/H2O in a gradient (10:90–100:0) to yield eight subfractions (Fr. 8.1–8.8). Subfraction 8.5 (2.35 g) was purified by an RP-C18 medium-pressure column (MeOH/H2O, 55:45 to 100:0) followed by semi-preparative RP-C18 HPLC (CH3CN/H2O, 63:27) to produce 3 (10.6 mg). Overall, we obtained compounds 1 (42.9 mg), 2 (22.2 mg), 3 (10.6 mg) and 4 (5.2 mg).
Spectroscopic data
Vlasouliolide A (1) Colorless orthorhombic crystals in EtOH/H2O. m.p.: 169–173 °C;  +3.77 (c 0.24, CH3COCH3); UV (CH3OH/H2O) λmax 210 nm; IR (KBr) νmax 3089, 2931, 2854, 1770, 1714, 1639, 1446, 1400, 1367, 1334, 1209, 1178, 1126, 995, 890 cm−1; 1H- and 13C-NMR data, see Tables S1 and S2; ESIMS m/z 527.4 ([M + Na]+), 503.2 ([M − H]−); positive HRESIMS m/z 527.2763 ([M + Na]+, calcd. 527.2773).
+3.77 (c 0.24, CH3COCH3); UV (CH3OH/H2O) λmax 210 nm; IR (KBr) νmax 3089, 2931, 2854, 1770, 1714, 1639, 1446, 1400, 1367, 1334, 1209, 1178, 1126, 995, 890 cm−1; 1H- and 13C-NMR data, see Tables S1 and S2; ESIMS m/z 527.4 ([M + Na]+), 503.2 ([M − H]−); positive HRESIMS m/z 527.2763 ([M + Na]+, calcd. 527.2773).
Vlasouliolide B (2) Colorless orthorhombic crystals in EtOH/H2O. m.p.: 171–173 °C;  +20.41 (c 0.21, CH3COCH3); UV (CH3CN/H2O) λmax 210, 254, 290 nm; IR (KBr) νmax 3081, 2931, 2854, 1760, 1712, 1641, 1455, 1365, 1313, 1213, 1164, 995, 885 cm−1; 1H- and 13C-NMR data, see Tables S1 and S2; ESIMS m/z 527.3 ([M + Na]+), 539.3 ([M + Cl]−); positive HRESIMS m/z 527.2758 ([M + Na]+, calcd. 527.2773).
+20.41 (c 0.21, CH3COCH3); UV (CH3CN/H2O) λmax 210, 254, 290 nm; IR (KBr) νmax 3081, 2931, 2854, 1760, 1712, 1641, 1455, 1365, 1313, 1213, 1164, 995, 885 cm−1; 1H- and 13C-NMR data, see Tables S1 and S2; ESIMS m/z 527.3 ([M + Na]+), 539.3 ([M + Cl]−); positive HRESIMS m/z 527.2758 ([M + Na]+, calcd. 527.2773).
Vlasouliolide C (3) Colorless monoclinic crystals in CHCl3/MeOH. m.p.: 211–218 °C;  +30.23 (c 0.04 CHCl3); UV (CH3CN/H2O) λmax 210 nm; IR (KBr) νmax 3086, 2929, 2849, 1763, 1719, 1649, 1444, 1380, 1336, 1260, 1195, 1159, 1103, 1019, 991, 911, 891 cm−1; 1H- and 13C-NMR data, see Tables S1 and S2; ESIMS m/z 529.4 ([M + Na]+), 541.5 ([M + Cl]−); positive HRESIMS m/z 529.2940 ([M + Na]+, calcd. 529.2930).
+30.23 (c 0.04 CHCl3); UV (CH3CN/H2O) λmax 210 nm; IR (KBr) νmax 3086, 2929, 2849, 1763, 1719, 1649, 1444, 1380, 1336, 1260, 1195, 1159, 1103, 1019, 991, 911, 891 cm−1; 1H- and 13C-NMR data, see Tables S1 and S2; ESIMS m/z 529.4 ([M + Na]+), 541.5 ([M + Cl]−); positive HRESIMS m/z 529.2940 ([M + Na]+, calcd. 529.2930).
Vlasouliolide D (4) Colorless orthorhombic crystals in EtOH/H2O. m.p.: 172–182 °C;  +55.21 (c 0.08 CH3COCH3); UV (CH3CN/H2O) λmax 210 nm; IR (KBr) νmax 2933,1772,1712, 1648, 1457, 1367, 1205, 1176, 1130, 995, 892 cm−1; 1H- and 13C-NMR data, see Tables S1 and S2; ESIMS m/z 529.4 ([M + Na]+), 505.3 ([M − H]−); positive HRESIMS m/z 529.2923 ([M + Na]+, calcd. 529.2930).
+55.21 (c 0.08 CH3COCH3); UV (CH3CN/H2O) λmax 210 nm; IR (KBr) νmax 2933,1772,1712, 1648, 1457, 1367, 1205, 1176, 1130, 995, 892 cm−1; 1H- and 13C-NMR data, see Tables S1 and S2; ESIMS m/z 529.4 ([M + Na]+), 505.3 ([M − H]−); positive HRESIMS m/z 529.2923 ([M + Na]+, calcd. 529.2930).
Measurement of LPS-Induced NO Production
RAW 264.7 cells were seeded in 96-well culture plates at 5 × 105 cells/well at 37 °C for 6 h in DMEM medium. The cells were pretreated with different concentrations of samples for 12 h and then incubated for 16 h with or without 1 μg/mL LPS. The nitrite concentration in the culture supernatant was measured using Griess reagent (1% sulfanilamide, 0.1% N-1-naphthylenediamine dihydrochloride and 2.5% phosphoric acid). The absorbance was measured at 540 nm using a microplate reader after incubation for 15 min. The nitrite levels in the samples were calculated from a standard curve created using known concentrations of sodium nitrite.
MTT assay
RAW 264.7 cells were seeded in 96-well plates at 5 × 105 cells/well for 6 h and treated with different concentrations of compounds for 24 h. Thereafter, MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] was added to each well at a final concentration of 0.5 mg/ml and incubated at 37 °C for 3 h. The amount of MTT formazan was determined by dissolving it in dimethyl sulfoxide (DMSO) and measuring its absorbance at 495 nm using a microplate reader.
Dual-Luciferase reporter gene assay
293T cells were seeded in 24-well plates at 2 × 106 cells/well for 6 h. After being cotransfected with expression plasmids for NF-κB firefly luciferase and TK-Renilla luciferase for 24 h, the cells were treated with 10 μM concentrations of compounds 1–4 for an additional 1 h. Thereafter, the cells were stimulated with 1 μg/ml LPS for another 6 h and then lysed in lysis buffer. Luciferase activities were measured by the dual-luciferase reporter gene assay system (Promega). NF-κB firefly luciferase activity was normalized to the Renilla luciferase activity.
Antibodies and Western blot analysis
Antibodies for p-p65, p65, p-IκBα, IκBα and Gapdh were purchased from Cell Signaling Technology. For western blot analysis, RAW 264.7 cells were lysed in 2× SDS sample buffer (62.5 mM Tris-HCl, pH = 6.8, 2% SDS, 10% glycerol, 50 mM DTT, and 0.01% bromophenol blue) after treatment with the compounds and stimulation by LPS. Cell lysates were fractionated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) after denaturation treatment and subjected to immunoblot analysis with GAPDH as the loading control.
Additional Information
How to cite this article: Chen, L.-P. et al. Vlasouliolides A-D, four rare C17/C15 sesquiterpene lactone dimers with potential anti-inflammatory activity from Vladimiria souliei. Sci. Rep. 7, 43837; doi: 10.1038/srep43837 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Wang, G. W. et al. Inula sesquiterpenoids: structural diversity, cytotoxicity and anti-tumor activity. Exper Opin Inv Drug. 23, 317–45 (2014).
Zhang, J. P. et al. The genus Carpesium: a review of its ethnopharmacology, phytochemistry and pharmacology. J Ethnopharmacol. 163, 173–91 (2015).
Zhan, Z. J., Ying, Y. M., Ma, L. F. & Shan, W. G. Natural disesquiterpenoids. Nat. Prod. Rep. 28, 594–629 (2011).
Liao, S. G. & Yue, J. M. Dimeric Sesquiterpenoids (eds Kinghorn, A. D., Falk, H., Gibbons, S., Kobayashi ) 1–112 (Switzerland, 2016).
Qin, J. J. et al. Neojaponicone A, a bioactive sesquiterpene lactone dimer with an unprecedented carbon skeleton from Inula japonica . Chem. Commun. 47, 1222–1224 (2011).
Yang, Y. X. et al. Carpedilactones A-D, four new isomeric sesquiterpene lactone dimers with potent cytotoxicity from Carpesium faberi . Org. Lett. 16, 4216–4219 (2014).
Editorial commission of Traditional Chinese Medicine, State Administration Of Traditional Chinese Medicine (Ed.). Traditional Chinese Medicine. Shanghai Science & Technology Press, Vol. 7, p. 815–817 (Shanghai, 1999).
Tan, R. X., Jakupovic, J., Bohlmann, F., Jia, Z. J. & Schuster, A. Sesquiterpene lactones from Vladimiria souliei. Phytochemistry. 29, 1209–1212 (1990).
Xu, J. et al. Two carabrane-type sesquiterpenes from Vladimiria souliei . Phytochem Lett. 2, 204–206 (2009).
Xu, J. et al. Sesquiterpenes from Vladimiria souliei and their inhibitory effects on NO production. Fitoterapia. 82, 508–511 (2011).
Chen, J. J. et al. Sesquiterpenoids from the roots of Vladimiria muliensis . J. Asian Nat. Prod. Res. 17, 1–8 (2015).
Wei, H., Ma, G., Peng, Y., He, C. & Xiao, P. Chemical Constituents of the Roots of Dolomiaea souliei . Chem. Nat. Compd. 37, 1249–53 (2014).
Zhou, L. Z., Jiang, J. H., Li, Y. P. & Chen, Y. G. Chemical and Bioactive Studies on Tibetan Medicines Plants of Vladimiria Genus . Yunnan Chemical Technology (2010).
Rigby, J. H. & Wilson, J. A. Z. Total synthesis of guaianolides: (±)-dehydrocostus lactone and (±)-estafiatin. J. Am. Chem. Soc. 106, 8217–8224 (1984).
Saori Yuuya et al. Guaianolides as Immunomodulators. Synthesis and Biological Activities of Dehydrocostus Lactone, Mokko Lactone, Eremanthin, and Their Derivatives. J. Nat. Prod. 62, 22–30 (1999).
Wei, H. et al. Chemical constituents of Dolomiaea souliei . China J. Chin. Mater. Med. 37, 1249–1253 (2012).
Sakurai, H. et al. Nitric oxide production and inducible nitric oxide synthase expression in inflammatory arthritides. J. Clin. Invest. 96, 2357–2363 (1995).
Tan-no, K. et al. Anti-inflammatory Effect of Propolis through Inhibition of Nitric Oxide Production on Carrageenin-Induced Mouse Paw Edema. Biol. Pharm. Bull. 29, 96–99 (2006).
Duarte, J., Francisco, V. & Perez-Vizcaino, F. Modulation of nitric oxide by flavonoids. Food Funct. 5, 1653–1668 (2014).
Ono, R., Kaisho, T. & Tanaka, T. PDLIM1 inhibits NF-κB-mediated inflammatory signaling by sequestering the p65 subunit of NF-κB in the cytoplasm. Sci. Rep. 5, 18327 (2015).
Yu, C., Qi, D., Sun, J. F., Li, P. & Fan, H. Y. Rhein prevents endotoxin-induced acute kidney injury by inhibiting NF-κB activities. Sci. Rep. 5, 11822 (2015).
Acknowledgements
This work was supported by the Professor of Chang Jiang Scholars Program, the National Nature Science Foundation of China (81102335, 81230090, 81473109, 81502957), the National High-Tech Research and Development Program of China (863 Program, 2014AA022201-03), the Scientific Foundation of Shanghai China (13401900103), the Shanghai Engineering Research Center for the Preparation of Bioactive Natural Products (16DZ2280200) and the China Postdoctoral Science Foundation funded project (2015M572740).
Author information
Authors and Affiliations
Contributions
Chen, L.P. and Wu, G.Z. carried out the experimental work. Zhang, J.P., Ye, J., Liu, Q.X., Shen, Y.H. and Zhang, W.D. provided oversight. Li, H.L. conducted the experiments. Chen, L.P., Wu, G.Z. and Li, H.L. conceived the experiments and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Chen, LP., Wu, GZ., Zhang, JP. et al. Vlasouliolides A-D, four rare C17/C15 sesquiterpene lactone dimers with potential anti-inflammatory activity from Vladimiria souliei. Sci Rep 7, 43837 (2017). https://doi.org/10.1038/srep43837
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep43837
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.