Abstract
The aim of this study was to evaluate the use of renal systolic time intervals measured by electrocardiographic gated Doppler ultrasonography for predicting adverse cardiac events. This longitudinal observation study enrolled 205 patients. Renal systolic time intervals, including pre-ejection period (PEP) and ejection time (ET), and ratio of renal PEP to ET, were measured by electrocardiographic gated Doppler ultrasound. The 14 adverse cardiac events identified in this population included 9 cardiac deaths and 5 hospitalizations for heart failure during an average follow up of 30.9 months (25th–75th percentile: 30–33 months). Renal PEP (hazard ratio = 1.023, P = 0.001), renal ET (hazard ratio = 0.975, P = 0.001) and renal PEP/ET (per 0.01 unit increase, hazard ratio = 1.060, P < 0.001) were associated with poor cardiac outcomes. The addition of renal PEP/ET to a Cox model containing important clinical variables and renal resistive index further improved the value in predicting adverse cardiac events (Chi-square increase, 9.996; P = 0.002). This study showed that parameters of intra-renal hemodynamics were potential predictors of adverse cardiac outcomes. However, the generalizability of these indicators need to be investigated in future large-scale studies.
Similar content being viewed by others
Introduction
Heart failure is an important clinical issue associated with high morbidity, high cardiovascular (CV) mortality and high health care costs1. In hospitalized patients with acute decompensated heart failure, deterioration of renal function is associated with adverse cardiac prognosis2. In clinical practice, cardiac and renal dysfunction have synergistic effects that aggravate poor cardiac and renal outcomes3. Although estimated glomerular filtration rate is widely used as a serum marker for evaluating renal function, parameters of renal Doppler ultrasound used to analyze intra-renal hemodynamics are independent indicators of CV outcome4. Renal resistive index (RI), which is an established indicator obtained from Doppler ultrasound spectra for intra-renal arteries, reflects renal vascular resistance and can predict decline in renal function, pathological change in renal parenchyma, and adverse CV events4,5,6,7. In fact, renal RI correlates with left ventricular diastolic dysfunction but not with ventricular ejection fraction (LVEF)8,9.
The impairment of left ventricular systolic function is an important predictor of heart failure10,11. In addition to LVEF, other widely used global cardiac performance and prognostic predictors in patients with heart failure include cardiac systolic time interval (STI), including pre-ejection period (PEP), ejection time (ET), and ratio of PEP to ET12,13,14. Prolonged cardiac PEP, prolonged PEP/ET, and short ET are associated with decreased LV systolic function15,16,17. Our recent study showed that renal STIs measured by Doppler ultrasonography were significantly associated with cardiac STIs, which suggests that renal STIs may be associated with adverse CV outcomes18. However, no studies have used a single imaging modality to investigate the relationship of intra-renal hemodynamics and adverse cardiac events. Therefore, we hypothesized that renal STI measured by real-time internal electrocardiographic gated Doppler ultrasonography can predict adverse CV outcomes.
Methods
Study subjects and design
This longitudinal observational study enrolled 252 participants who had received ultrasonographic examination in a regional hospital in Taiwan from June, 2012 to December, 2012. Excluded patients (n = 22) included those with atrial fibrillation, significant valvular heart disease, left bundle branch block, or inadequate image visualization. Patients were also excluded if they had any history of the following: unilateral or bilateral renal artery stenosis, unilateral or bilateral nephrectomy, end stage renal disease requiring renal replacement or renal transplantation therapy, acute kidney injury, or acute unilateral or bilateral hydronephrosis. Twenty-one patients were lost to follow up. Three died of malignancy, and one had traumatic brain hemorrhage. Figure 1 shows that 205 patients completed the study.
Ethics statement
The study methods were carried out in accordance with the approved guidelines. The study protocols were approved by the institutional review board committee of the Kaohsiung Medical University Hospital (KMUHIRB-E(I)-20150180). Written informed consent was obtained from all subjects.
Renal Doppler ultrasonography study
Renal ultrasonographic examinations were performed with a CX50 machine (Philips Compact Xtreme System, USA). Renal RI, renal PEP, and renal ET were measured as described in our previous study18. Briefly, renal RI was measured as (peak systolic velocity – minimum diastolic velocity)/peak systolic velocity from arcuate arteries in intra-renal Doppler. Renal PEP was determined from the beginning of the electrocardiographic QRS complex to the foot of the intra-renal pulse Doppler signal. Renal ET was determined from the foot to the dicrotic notch of the intra-renal pulse Doppler signal. Three measurements were taken for the kidney on each side, and the mean value for each kidney was recorded for further analysis.
Collection of demographic, medical, and laboratory data
Baseline medical history and laboratory test values were collected from medical records. The eGFR was calculated by the equation used in the Modification of Diet in Renal Disease study19.
Definition of cardiac events
The cardiac events were defined as cardiac death and hospitalization for acute decompensated heart failure. Surviving patients were followed up until June, 2015.
Statistical analysis
Baseline data were presented as percentage or mean ± standard error. The predictors of cardiac events (cardiac death and hospitalization for heart failure) were analyses by Cox proportional hazards model. Time to cardiac events and covariates of risk factors were modeled using Cox proportional forward hazards model. In terms of their use for evaluating risk of adverse cardiac events, incremental values for renal PEP/ET were compared with conventional parameters by calculating the improvement in global Chi-square. Kaplan-Meier survival analysis with log-rank test was performed to determine the predictive role of renal PEP/ET in adverse cardiac outcomes. A P value less than 0.05 was considered statistically significant. Statistical analysis was performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
Results
A total of 205 participants enrolled in this study. Table 1 shows the clinical and renal Doppler ultrasonographic characteristics of these patients. The mean age was 64.4 ± 12.3 years, and 59.5% of participants were male. In renal Doppler parameters, the mean values for renal RI, renal PEP, renal ET, and renal PEP/ET were 0.69 ± 0.084 ms, 123.3 ± 23.7 ms, 304.8 ± 36.7 ms, and 0.41 ± 0.11, respectively. The variability coefficients of renal RI, renal PEP, renal ET, and renal PEP/ET were 0.12, 0.19, 0.12, and 0.27, respectively.
The follow-up period was 30.9 months (25th–75th percentile: 30–33 months). During the follow-up period, 14 cardiac events occurred, including 9 deaths and 5 hospitalizations for heart failure. Table 2 shows the results of a Cox proportional hazards regression analysis of cardiac events. Univariable analysis showed that increased cardiac events were significantly associated with the presence of diabetes mellitus and chronic heart failure, increased heart rate, increased serum glucose, decreased estimated glomerular filtration rate, decreased hemoglobin, use of diuretics and β blockers, increased renal RI, increased renal PEP (hazard ratio [HR] 1.023; 95% confidence interval [CI] 1.010 to 1.037; P = 0.001), decreased renal ET (hazard ratio = 0.975; 95% CI = 0.961 to 0.989; P = 0.001), and increased renal PEP/ET (per 0.01 unit increase, HR = 1.060; 95% CI = 1.034 to 1.088; P < 0.001).
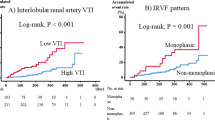
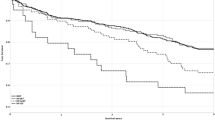
To find the appropriate cut-off value for using renal PEP/ET to predict adverse cardiac events, several models were constructed using different cut-off values. A comparison of Chi-square values showed that the renal PEP/ET >0.41 model was the best predictor of adverse cardiac events. Figure 2 compares Kaplan-Meier curves for cardiac event-free survival between renal PEP/ET  0.41 and renal PEP/ET >0.41 (log-rank P < 0.001).
0.41 and renal PEP/ET >0.41 (log-rank P < 0.001).
Figure 3 shows how incremental change in renal PEP/ET is related to CV outcome. The clinical model consisted of variables potentially related to adverse CV outcomes in univariable analysis. These variables included presence of diabetes mellitus, chronic heart failure, serum glucose, estimated glomerular filtration rate, hemoglobin, and diuretic and β blocker use. In the clinical model, these variables were significant predictors of adverse cardiac events (Chi-square = 45.250, P < 0.001). However, prediction of adverse cardiac events did not significantly differ between the clinical model and the clinical model plus renal RI (Chi-square = 45.313, P = 0.802). Compared with the clinical model and the clinical model plus renal RI, the clinical model plus renal PEP/ET (Chi-square = 55.309) had significantly higher value in predicting adverse cardiac events (both P = 0.002).
Addition of the ratio of renal pre-ejection period (PEP) to ejection time (ET) significantly improved prediction of adverse cardiac events in the basic clinical model (diabetes mellitus, chronic heart failure, serum glucose, estimated glomerular filtration rate, hemoglobin, and diuretic and β blocker use) and in the basic clinical model with the addition of renal resistive index (RI) (both P = 0.002).
Discussion
This study showed that parameters of renal systolic time intervals derived by Doppler ultrasound are associated with adverse cardiac outcome. Notably, increased renal PEP/ET had significantly higher incremental prognostic value compared to conventional clinical parameters for predicting adverse cardiac events.
The LVEF is widely used to assess left ventricular systolic function17. An alternative parameter for evaluating cardiac systolic performance is STI20, Patients with decreased LVEF and left ventricular contractility have a long PEP, a shortened ET, and a long PEP/ET15,20,21. Various STIs measured by non-invasive sphygmography, phonocardiography, peripheral arterial waveform recordings, or echocardiography reportedly have significant correlations with LVEF16,17,22,23,24,25. Clinical applications of STI have been reported in various cardiac diseases, including heart failure, coronary artery disease, and hypertension14,26,27. The STI not only predicts global cardiac systolic function, it also predicts adverse CV events. Our previous studies showed that brachial PEP/brachial ET has a significant correlation with adverse CV outcomes in patients with hemodialysis and chronic kidney disease28,29. In the present study, renal STI derived by electrocardiographic gated renal Doppler ultrasound were associated with adverse CV outcomes. Compared to conventional clinical parameters, renal PEP/ET could be a superior predictor of cardiac prognosis.
Atherosclerotic reno-vascular disease is associated with an increased risk of death, cardiovascular events, and hospitalization30. Renal RI measured by Doppler ultrasound is a useful parameter for evaluating renal function and intra-renal vascular hemodynamics. Renal RI can be used not only to assess renal vascular resistance, but also to predict renal and CV outcomes31,32. Ennezat et al. demonstrated the use of renal RI as an independent predictor of re-hospitalization for heart failure in heart failure patients with preserved LVEF31. Ciccone et al. further showed that renal RI is an independent incremental predictor of disease progression in heart failure patients with reduced LVEF33. In the present study, renal RI showed significant association with CV outcome in the univariable analysis. However, renal RI did not have higher incremental prognostic value compared to conventional clinical parameters for predicting poor CV outcomes. The major difference between our study and previous studies of the relationship between renal RI and heart failure hospitalization was the study population. For example, Ennezat et al. and Ciccone et al. analyzed heart failure patients who had preserved or reduced LVEF. In contrast, we included patients referred for echocardiographic examinations, and only a small percentage (17.6%) of patients had a history of heart failure. Differences in the study population may partially explain the inconsistent results.
Study limitations
This study had several limitations. First, this observational study had a longitudinal design and was limited to a relatively small number of cases in a regional hospital, which might limit the generalizability of the results. Furthermore, the number (n = 14) of adverse cardiac events was too low to conduct meaningful multivariable analyses in the study. Second, most of our patients had been treated with medications for arterial hypertension. For ethical reasons, these medications could not be withdrawn. Hence, their effects on the present findings could not be completely excluded. Third, since the subjects of this study were already being evaluated for possible cardiac disease by echocardiography, the study was susceptible to selection bias, which also reduces the generalizability of the findings. Finally, patients with a history of renal artery stenosis, nephrectomy, end stage renal disease requiring renal replacement or renal transplantation therapy, acute kidney injury, and hydronephrosis were also excluded. Therefore, our results are inapplicable in these patients.
Conclusions
This study is the first to show that renal systolic time intervals were associated with adverse cardiac events and may be better than conventional clinical parameters for predicting cardiac prognosis. Hence, these parameters should be included in renal Doppler ultrasound examination to improve prognostication. However, the generalizability of these indicators need to be investigated in future large-scale studies.
Additional Information
How to cite this article: Lee, W.-H. et al. Renal systolic time intervals derived from intra-renal artery Doppler as a novel predictor of adverse cardiac outcomes. Sci. Rep. 7, 43825; doi: 10.1038/srep43825 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Roger, V. L. Epidemiology of heart failure. Circ Res 113, 646–659 (2013).
Nohria, A. et al. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol 51, 1268–1274 (2008).
Bock, J. S. & Gottlieb, S. S. Cardiorenal syndrome: new perspectives. Circulation 121, 2592–2600 (2010).
Bude, R. O. & Rubin, J. M. Relationship between the resistive index and vascular compliance and resistance. Radiology 211, 411–417 (1999).
Ikee, R. et al. Correlation between the resistive index by Doppler ultrasound and kidney function and histology. Am J Kidney Dis 46, 603–609 (2005).
Lee, M. K. et al. Significant Correlation between Brachial Pulse Pressure Index and Renal Resistive Index. Acta Cardiol Sin 31, 98–105 (2015).
Doi, Y. et al. Renal resistive index and cardiovascular and renal outcomes in essential hypertension. Hypertension 60, 770–777 (2012).
Tedesco, M. A., Natale, F., Mocerino, R., Tassinario, G. & Calabro, R. Renal resistive index and cardiovascular organ damage in a large population of hypertensive patients. J Hum Hypertens 21, 291–296 (2007).
Raff, U. et al. Renal resistive index in addition to low-grade albuminuria complements screening for target organ damage in therapy-resistant hypertension. J Hypertens 28, 608–614 (2010).
Meta-analysis Global Group in Chronic Heart, F. The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J 33, 1750–1757 (2012).
Ponikowski, P. et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 18, 891–975 (2016).
Lewis, R. P., Rittogers, S. E., Froester, W. F. & Boudoulas, H. A critical review of the systolic time intervals. Circulation 56, 146–158 (1977).
Reant, P. et al. Systolic time intervals as simple echocardiographic parameters of left ventricular systolic performance: correlation with ejection fraction and longitudinal two-dimensional strain. Eur J Echocardiogr 11, 834–844 (2010).
Weissler, A. M., Harris, W. S. & Schoenfeld, C. D. Systolic time intervals in heart failure in man. Circulation 37, 149–159 (1968).
Garrard, C. L. Jr., Weissler, A. M. & Dodge, H. T. The relationship of alterations in systolic time intervals to ejection fraction in patients with cardiac disease. Circulation 42, 455–462 (1970).
Tavakolian, K. Systolic Time Intervals and New Measurement Methods. Cardiovascular engineering and technology 7, 118–125 (2016).
Su, H. M. et al. A comparison between brachial and echocardiographic systolic time intervals. PLoS One 8, e55840 (2013).
Lee, W. H. et al. Systolic time intervals derived from electrocardiographic gated intra-renal artery Doppler waveform associated with left ventricular systolic function. Scientific reports 6, 29293 (2016).
Vickery, S., Stevens, P. E., Dalton, R. N., van Lente, F. & Lamb, E. J. Does the ID-MS traceable MDRD equation work and is it suitable for use with compensated Jaffe and enzymatic creatinine assays? Nephrol Dial Transplant 21, 2439–2445 (2006).
Gillebert, T. C., Van de Veire, N., De Buyzere, M. L. & De Sutter, J. Time intervals and global cardiac function. Use and limitations. Eur Heart J 25, 2185–2186 (2004).
Veyrat, C., Larrazet, F. & Pellerin, D. Renewed interest in preejectional isovolumic phase: new applications of tissue Doppler indexes: implications to ventricular dyssynchrony. Am J Cardiol 96, 1022–1030 (2005).
Oh, J. K. & Tajik, J. The return of cardiac time intervals: the phoenix is rising. J Am Coll Cardiol 42, 1471–1474, doi: S0735109703010362 [pii] (2003).
Shapiro, M. et al. Diagnostic characteristics of combining phonocardiographic third heart sound and systolic time intervals for the prediction of left ventricular dysfunction. J Card Fail 13, 18–24 (2007).
Smorenberg, A. et al. Systolic time intervals vs invasive predictors of fluid responsiveness after coronary artery bypass surgery. Eur J Cardiothorac Surg 44, 891–897 (2013).
Polak, J. F., Alessi-Chinetti, J. M., Estes, J. M. & Patel, A. R. Left Ventricular Ejection Time Derived From the Common Carotid Artery Doppler Waveform: Association With Left Ventricular Ejection Fraction and Prediction of Heart Failure. J Ultrasound Med 34, 1237–1242 (2015).
Dodek, A., Burg, J. R. & Kloster, F. R. Systolic time intervals in chronic hypertension: Alterations and response to treatment. Chest 68, 51–55 (1975).
Lewis, R. P., Boudoulas, H., Welch, T. G. & Forester, W. F. Usefulness of systolic time intervals in coronary artery disease. Am J Cardiol 37, 787–796 (1976).
Chen, S. C. et al. A new systolic parameter defined as the ratio of brachial pre-ejection period to brachial ejection time predicts overall and cardiovascular mortality in hemodialysis patients. Hypertens Res 33, 492–498 (2010).
Chen, S. C. et al. A systolic parameter defined as the ratio of brachial pre-ejection period to brachial ejection time predicts cardiovascular events in patients with chronic kidney disease. Circ J 74, 2206–2210 (2010).
Weiner, D. E. et al. Kidney disease as a risk factor for recurrent cardiovascular disease and mortality. Am J Kidney Dis 44, 198–206 (2004).
Ennezat, P. V. et al. Renal resistance index and its prognostic significance in patients with heart failure with preserved ejection fraction. Nephrol Dial Transplant 26, 3908–3913 (2011).
Bige, N. et al. Renal arterial resistive index is associated with severe histological changes and poor renal outcome during chronic kidney disease. BMC Nephrol 13, 139 (2012).
Ciccone, M. M. et al. The renal arterial resistance index: a marker of renal function with an independent and incremental role in predicting heart failure progression. Eur J Heart Fail 16, 210–216 (2014).
Acknowledgements
This study was supported a grant from Kaohsiung Municipal Hsiao-Kang Hospital (kmhk-104-005), Kaohsiung Medical University, Kaohsiung, Taiwan.
Author information
Authors and Affiliations
Contributions
Wen-Hsien Lee, Po-Chao Hsu, and Ho-Ming Su drafted the manuscript. Chun-Yuan Chu, Szu-Chia Chen, Hung-Hao Lee, and Meng-Kuang Lee prepared tables and assisted with the statistical analysis. Chee-Siong Lee, Hsueh-Wei Yen, Tsung-Hsien Lin, and Po-Lin Kuo prepared the figures. Wen-Chol Voon, Wen-Ter Lai, Sheng-Hsiung Sheu, and Ho-Ming Su conceived of the study and participated in its design and coordination. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lee, WH., Hsu, PC., Chu, CY. et al. Renal systolic time intervals derived from intra-renal artery Doppler as a novel predictor of adverse cardiac outcomes. Sci Rep 7, 43825 (2017). https://doi.org/10.1038/srep43825
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep43825
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.